Abstract
Background/Aims
Patients with cirrhosis hospitalized in the intensive care unit (ICU) have a high risk for acute-on-chronic liver failure (ACLF) and short-term mortality. A major factor in the pathogenesis of ACLF is systemic inflammation, the assessment of which includes the use of surrogate markers, such as neutrophil-to-lymphocyte ratio (NLR). This study aimed to assess the accuracy of NLR in predicting the outcome of patients with cirrhosis and ACLF hospitalized in the ICU.
Materials and Methods
This was a retrospective observational study on patients with cirrhosis with acute decompensation hospitalized in the ICU of a Romanian tertiary care center. ACLF was defined according to the CANONIC criteria, and NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count.
Results
A total of 70 patients were included, of whom 70% were men with a mean age of 62±6.2 years. ACLF was diagnosed in 58 (82.9%) patients who presented with higher in-hospital mortality rates than patients without ACLF (84.5% vs. 33.3%, p=0.001). The mean NLR value was 11.7±9.5, higher in non-survivors than in survivors (12.6±9.8 vs. 8.6±7.8, p=0.170). NLR had a poor accuracy in predicting the outcome in patients without ACLF (area under the curve [AUC]=0.611) but a better accuracy in patients with ACLF (AUC=0.776). Patients with cirrhosis and a high NLR had higher levels of bilirubin; higher Child-Turcotte-Pugh score; and higher incidence of ascites, coagulation, and circulatory failure, presenting a poor outcome. Receiver operating characteristic analysis showed a good accuracy for predicting mortality for the Child-Turcotte-Pugh score (AUC= 0.864) and NLR (AUC=0.732).
Conclusion
NLR is a promising and cost-effective method for the prediction of a poor outcome in critically ill patients with cirrhosis hospitalized in the ICU and shows greater accuracy in those with ACLF.
Keywords: Acute-on-chronic liver failure, liver cirrhosis, hospital mortality, inflammation
INTRODUCTION
Acute decompensation of liver cirrhosis (LC) severely impairs the prognosis of patients with LC and is associated with a low short-term survival (3 to 5 years) (1). However, prognosis of patients with LC is sometimes worsened by known or unknown precipitating factors, and such evolution has led to the development of a recent syndrome, namely acute-on-chronic liver failure (ACLF). This syndrome was first defined by the Asia Pacific Association for the Study of the Liver in 2009 as an acute hepatic insult manifesting in jaundice and coagulopathy complicated within 4 weeks by ascites and/or encephalopathy (2). Later, in 2014, short-term mortality was added to the definition (3).
The CANONIC study was the first large multicenter study to accurately establish and validate the diagnostic criteria for ACLF on the basis of the presence of acute decompensation in the presence of organ failure (OF) (1). The authors modified the sequential OF assessment (SOFA) score to better assess the prognosis of critically ill patients with LC, thus developing the chronic liver failure (CLIF)-SOFA and then the simplified CLIF consortium OF (CLIF-C OF) scores. Since then, numerous attempts were made toward the better characterization of this syndrome to identify the risk factors associated with the high short-term mortality rates of patients diagnosed with ACLF. Recent data suggest that exacerbated systemic inflammation plays a key role in the development and progression of the syndrome in the setting of both proven and unproven bacterial infections (4). The mechanism underlying the exacerbated systemic inflammation in patients with cirrhosis and with ACLF is not completely known, but 2 main pathways leading to dysregulated inflammation have been proposed (4). First pathway is the bacterial translocation and subsequent activation of toll-like receptors (TLRs) (5) and NOD-like receptors (6) in immune cells by the pathogen-associated molecular patterns (PAMPs) (7), such as bacterial lipopolysaccharides (8), which would generate a systemic inflammatory response. The second pathway is the release of damage-associated molecular patterns (7) by altered hepatocytes in the setting of acute or chronic liver injury, which produces similar effects, as these molecules also bind to the TLRs (5) and other receptors leading to the stimulation of immune cells. Prolonged activation of these pathways leads to a persistent pro-inflammatory status in patients with cirrhosis (4). Moreover, in patients with advanced LC and ACLF, there is an impaired response to PAMPs through immune exhaustion and endotoxin tolerance, thus leading to a cirrhosis-induced immunodeficiency (9). The consequence of this imbalance is the impairment of defense against microbial threats as well as an increased susceptibility for OF development (10). Although inflammation plays an important prognostic role in patients with ACLF (1), the clinical evaluation of the inflammatory status is difficult (11), mainly because of the lack of availability of routine cytokine assessment outside research facilities. Surrogate markers are therefore needed. Moreau et al. (1) have shown that white blood cell (WBC) count and C-reactive protein (CRP) levels varied progressively according to ACLF grade, with the highest values found in patients with ACLF grade 3 who presented poor short-term prognosis. Thus, the authors suggested that CRP levels and WBC counts can be used as markers of inflammation and unfavorable prognosis in patients with LC. Lymphocyte-to-monocyte ratio has been proposed as another surrogate marker of inflammation, having been recently proven to be a promising predictor factor for mortality in patients with hepatitis B virus (HBV)-LC as well as decompensated LC (12, 13). Other scoring systems, already used in the intensive care unit (ICU), such as acute kidney injury network score and SOFA, have been assessed for accuracy in predicting mortality in critically ill patients with cirrhosis and have proven to be useful in the assessment of outcomes in these patients (14). A novel surrogate marker of inflammation is the neutrophil-to-lymphocyte count (NLR), a marker which takes into account both the polymorphonuclear neutrophil count associated with inflammation and the lymphocyte count as a hallmark of immune impairment (13). Although it was first developed as an outcome predictor for patients with advanced neoplasia, cardiovascular diseases, and postoperative sepsis (15, 16), NLR has proven its utility as a prognostic tool in decompensated patients with cirrhosis (17), with and without ACLF (11, 18).
This study aimed to assess the utility of NLR in the prediction of short-term mortality in patients with cirrhosis with and without ACLF hospitalized in the ICU in a tertiary care center in Eastern Romania.
MATERIALS AND METHODS
Patients
We conducted a retrospective observational study on consecutive patients with LC hospitalized in the ICU of the St. Spiridon University Hospital, Iasi, Romania, between January and June 2017. The inclusion criteria included age over 18 years; LC diagnosed on clinical or biochemical tests or abdominal ultrasound; and endoscopic evidence of portal hypertension or acute decompensation in accordance with the definition proposed by Moreau et al. (1) as recently developed ascites (less than 2 weeks), hepatic encephalopathy (HE) (in patients without previous cognitive impairment), upper or lower gastrointestinal (GI) bleeding, bacterial infections, including spontaneous bacterial peritonitis, urinary tract infection, and pneumonia (1). Exclusion criteria included incomplete records, malignancy, hematological disease, pregnancy, human immunodeficiency virus infection, and liver transplantation.
Methods
Analysis of the patients’ electronic records was performed, and their demographic as well as clinical data were collected. Information on the results of abdominal ultrasound and upper GI endoscopy was obtained. Laboratory tests from venous blood obtained within 12 hours of admission (complete blood count, serum transaminase levels, total and direct bilirubin, albumin, creatinine, urea, and prothrombin time) were retrieved. NLR was calculated by dividing absolute neutrophil count by absolute lymphocyte count. CLIF score was calculated using the online CLIF-C ACLF calculator ( http://www.efclif.com/scientific-activity/score-calculators/clif-c-aclf ) according to the formula 10*[0.33*CLIF-OFs+0.04*age+0.63*Ln(white-cell count)-2].
Organ failures and ACLF definitions
OFs and ACLF were defined following the criteria published by Moreau et al. (1), and ACLF grades were established accordingly. Liver failure was diagnosed in patients with total bilirubin levels of more than 12 mg/dL, kidney failure was defined as an increase of serum creatinine level ≥2 mg/dL or the use of renal replacement therapy, cerebral failure was found in the presence of grade III or IV West Haven HE, coagulation failure was indicated by an International Normalized Ratio (INR) value over 2.5 and/or platelet count ≤20×109/L, respiratory insufficiency was defined as a partial pressure of arterial oxygen to FiO2≤200 or an SpO2 to FiO2 ratio ≤200, and circulatory failure was considered in patients with vasoactive drug therapy.
ACLF grade 1 included patients with kidney failure or other single OF with a creatinine level between 1.5–1.9 mg/dL and/or grade I–II HE. ACLF grade 2 was diagnosed in the setting of 2 OFs, and ACLF grade 3 included patients with cirrhosis with 3 or more OFs.
Statistical Analysis
IBM Statistical Package for Social Sciences (SPSS) version 22.0 (IBM, Armonk, NY, USA) was used for the statistical analysis. Continuous variables were expressed as mean±standard deviation or as median (interquartile range) accordingly. The Kolmogorov–Smirnov test was used to assess the distribution. The Student’s t-test was used to analyze continuous variables and the chi-squared or Fischer’s tests were used for the analysis of categorical variables. Continuous variables were further analyzed using the Pearson’s correlations. The accuracy for prediction of outcome was analyzed using receiver operating characteristic (ROC) analysis.
RESULTS
Group Characteristics
A total of 180 consecutive patients were screened, and after applying the exclusion criteria, 70 patients were included in the study. Their mean age was 62±6.2 years, and most participants were men (70%). The main etiology for LC was alcohol consumption (58.6%) followed by hepatitis C virus (HCV) infection (22.9%), cryptogenic (4.3%), alcohol consumption and HCV infection (5.7%), HBV and hepatitis delta virus infection (4.3%), alcohol consumption and HBV infection (2.9%), and HBV infection (1.4%). Model for end-stage liver disease (MELD) and Child-Turcotte-Pugh scores were 25.74±8.65 and 10.82±2.37, respectively. Spontaneous bacterial peritonitis was found in 12 patients (17.1%), 39 patients (55.7%) had bacterial infections, and 33 patients (47.1%) had active GI bleeding.
Acute-on-Chronic Liver Failure Analysis
ACLF was diagnosed in 58 patients (82.9%); of them, 2 patients (3.4%) had ACLF grade 1, 8 patients (13.8%) had ACLF grade 2, and 48 patients (82.8%) had ACLF grade 3. The in-hospital mortality for the entire group was 75.7%, significantly higher in patients with ACLF (84.5%) than in patients with cirrhosis without ACLF (33.3%). Patients with ACLF presented with significantly higher Child-Turcotte-Pugh and MELD scores as well as higher rates of ascites and bacterial infections than patients without ACLF, leading to significantly higher death rates (49 [84.5%] vs. 4 [33.3%], p=0.001) (Table 1).
Table 1.
Characteristics of patients with and without ACLF
| Variable | ACLF 58 patients (82.85%) |
No ACLF 12 patients (17.14%) |
p |
|---|---|---|---|
| Age (years) | 61.5±3 | 62.1±4 | 0.75 |
| WBC (×109/L) | 14.54±9.71 | 19.6±17.74 | 0.30 |
| PLT (×109/L) | 104.96±71.7 | 129.20±153.67 | 0.74 |
| Creatinine (mg/dL) | 1.74 (0.89–3.12) | 0.92 (0.71–2.4) | 0.37 |
| Sodium | 136.53±6.72 | 135±4.58 | 0.62 |
| CRP (mg/dL) | 4.78±3.9 | 4.85±4.15 | 0.97 |
| ALT (U/L) | 38 (21–94.5) | 39 (14.5–572) | 0.91 |
| AST (U/L) | 92 (43.5–211) | 31 (14–3085) | 0.35 |
| Bilirubin (mg/dL) | 3.7 (1.58–7.14) | 1.61 (0.76–2.19) | 0.68 |
| INR | 2.39±0.96 | 1.57±0.37 | 0.98 |
| HE, n (%) | 34 (58.6) | 4 (33.3) | 0.10 |
| Ascites, n (%) | 42 (72.4) | 2 (20) | 0.03 |
| SBP, n (%) | 12 (20.7) | 0 | 0.18 |
| Bacterial infections, n (%) | 36 (62.1) | 3 (25) | 0.01 |
| NLR | 11.53±9.7 | 13.04±8.53 | 0.66 |
| MELD score | 26.5±8.38 | 14.75±3.77 | 0.008 |
| Child-Turcotte-Pugh score | 11.03±2.3 | 7.75±0.95 | 0.007 |
| Death rate, n (%) | 49 (84.5) | 4 (33.3) | 0.001 |
ACLF: acute-on-chronic liver failure; NLR: neutrophil-to-lymphocyte count; WBC: white blood cells; PLT: platelets; CRP: C-reactive protein; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MELD: model for end-stage liver disease; HE: hepatic encephalopathy; SBP: spontaneous bacterial peritonitis; INR: international normalized ratio; Note: the bold values denote statistical significance.871
Analysis of Neutrophil-to-Lymphocyte Ratio
Mean NLR value for the entire cohort was 11.7±9.5 with a slightly higher value found in non-survivors than in survivors (12.6±9.8 vs. 8.6±7.8, p=0.170).
In the group with ACLF, a statistically significant difference between survivors and non-survivors was found in terms of NLR (5.6±4.7 vs. 12.5±9.9, p<0.05). However, in the group without ACLF, no significant differences were found. ROC analysis showed a poor accuracy of NLR in predicting outcomes in patients without ACLF (area under the curve [AUC]=0.611) (Figure 1). However, the accuracy improved when patients with ACLF were considered (AUC=0.776) (Figure 2), with an optimal cutoff of 5. We consequently divided the cohort into 2 groups, with NLR >5 and ≤5. No notable differences were found in CRP and WBC counts between the 2 groups of patients with cirrhosis. However, patients with ACLF and NLR over 5 had significantly higher levels of bilirubin, Child-Turcotte-Pugh score, as well as a higher incidence of ascites, coagulation failure, and circulatory failure rates. Mortality rate was significantly increased in patients with cirrhotics with ACLF and NLR over 5 (Table 2). There were significant correlations between NLR and MELD score (p=0.014) as well as ACLF coagulation score (p=0.008).
Figure 1.
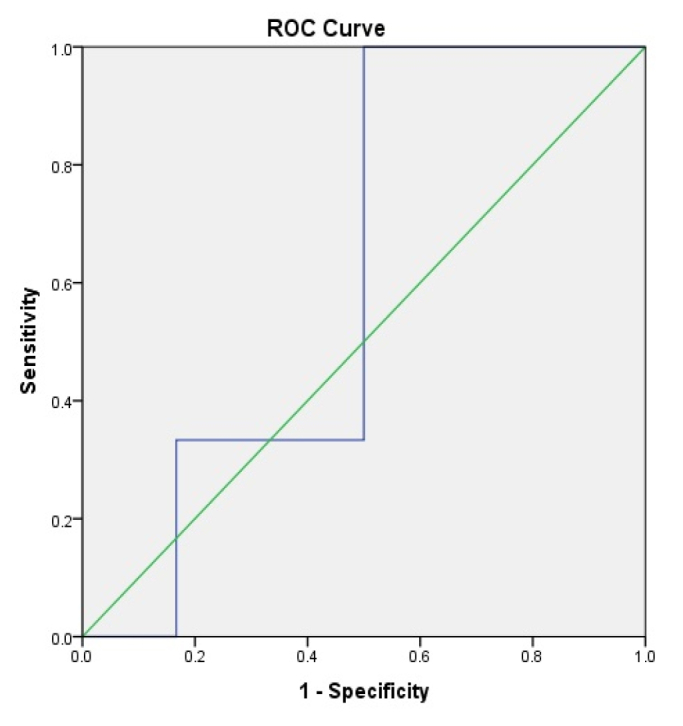
Accuracy of neutrophil-to-lymphocyte ratio for the prediction of mortality in patients without acute-on-chronic liver failure
Figure 2.
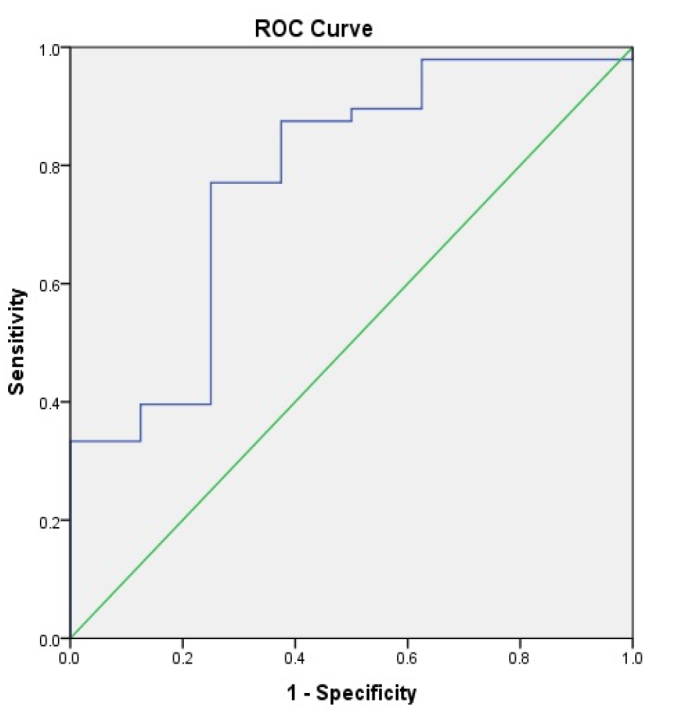
Accuracy of neutrophil-to-lymphocyte ratio for the prediction of mortality in patients with acute-on-chronic liver failure.
Table 2.
Clinical and laboratory differences between ACLF patients in relation to NLR values.
| Variable | NLR >5 | NLR ≤5 | p |
|---|---|---|---|
| Age (years) | 62.3±4 | 63.1±2 | 0.84 |
| WBC (×109/L) | 14.93±9.52 | 13.99±11.16 | 0.76 |
| PLT (×109/L) | 103.19±69.87 | 111.07±84.21 | 0.73 |
| Creatinine (mg/dL) | 2.31±1.73 | 2.33±2.52 | 0.97 |
| CRP (mg/dL) | 5.03±4.13 | 4.03±3.25 | 0.44 |
| ALT (U/L) | 39 (20–130) | 51 (24.5–237.25) | 0.75 |
| AST (U/L) | 98 (50.5–317) | 121 (47–1250) | 0.75 |
| Bilirubin (mg/dL) | 4.2 (1.34–8.64) | 2.3 (1.27–4.29) | 0.03 |
| INR | 2.45±0.78 | 2.24±1.43 | 0.50 |
| HE, n (%) | 27 (64.3) | 7 (50) | 0.34 |
| Ascites, n (%) | 34 (81) | 7 (50) | 0.03 |
| SBP, n (%) | 10 (23.8) | 1 (7.1) | 0.25 |
| Bacterial infections, n (%) | 26 (61.9) | 8 (57.1) | 0.75 |
| MELD score | 27.8±8.37 | 23±7.5 | 0.06 |
| Child-Pugh score | 11.4±2.36 | 10±1.7 | 0.04 |
| Liver failure, n (%) | 24 (57.1) | 6 (42.9) | 0.37 |
| Renal failure, n (%) | 40 (95.2) | 11 (78.6) | 0.09 |
| Cerebral failure, n (%) | 21 (50) | 10 (71.4) | 0.16 |
| Coagulation failure, n (%) | 19 (45.2) | 1 (7.1) | 0.01 |
| Circulation failure, n (%) | 38 (90.5) | 9 (64.3) | 0.03 |
| Respiratory failure, n (%) | 30 (71.4) | 10 (71.4) | 1 |
| Death rate, n (%) | 39 (92.9) | 9 (64.3) | 0.01 |
| Days to death (for the deceased patients) | 5.2±3.81 | 4.4±3.16 | 0.58 |
ACLF: acute-on-chronic liver failure; NLR: neutrophil-to-lymphocyte count; WBC: white blood cells; PLT: platelets; CRP: C-reactive protein; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MELD: model for end-stage liver disease; HE: hepatic encephalopathy; SBP: spontaneous bacterial peritonitis; INR: international normalized ratio; Note: the bold values denote statistical significance.
Analysis of Mortality
Overall in-hospital mortality of patients with cirrhosis hospitalized in the ICU was high with 53 (75.7%) deceased patients. Univariate analysis identified low platelet counts; high bilirubin levels; HE; presence of ascites; low systolic blood pressure; higher MELD and Child-Turcotte-Pugh scores; renal and cerebral coagulation and circulation; and respiratory failures as risk factors for death. Moreover, ACLF was found to be a risk factor for poor outcome, and ACLF grade 3 had the highest death rate (Table 3). In all patients, ROC analysis showed a good accuracy for predicting mortality for Child-Turcotte-Pugh score (AUC=0.864), followed by NLR (AUC=0.732), MELD score (AUC=0.718), and CLIF score (AUC=0.688) (Figure 3). In patients with ACLF, ROC analysis showed a good accuracy for predicting mortality for Child-Turcotte-Pugh score (AUC=0.832), followed by NLR (AUC=0.776), MELD score (AUC=0.630), and CLIF score (0.691) (Figure 4).
Table 3.
Mortality analysis of the main group.
| Variable | Deceased 53 patients (75.7%) |
Survivors 17 patients (24.3%) |
p |
|---|---|---|---|
| Age (years) | 62.1±3 | 61.4±4 | 0.65 |
| WBC (×109/L) | 14.98±10.52 | 14.77±10.55 | 0.95 |
| PLT (×109/L) | 94.92±70.57 | 157.75±96.73 | 0.01 |
| Creatinine (mg/dL) | 1.67 (0.9–3.27) | 1.22 (0.7–2.64) | 0.79 |
| CRP (mg/dL) | 4.85±4.06 | 4.55±3.25 | 0.81 |
| ALT (U/L) | 37.5 (20–100.25) | 38.5 (20–62.5) | 0.89 |
| AST (U/L) | 90 (42.75–252.75) | 67.5 (31.75–122.75) | 0.74 |
| Bilirubin (mg/dL) | 4.15 (1.99–9.15) | 1.44 (0.88–1.89) | 0.001 |
| INR | 2.41±0.83 | 2.04±1.37 | 0.38 |
| HE, n (%) | 34 (64.2) | 4 (23.5) | 0.003 |
| Ascites, n (%) | 38 (71.7) | 6 (40) | 0.02 |
| SBP, n (%) | 12 (22.6) | 0 | 0.05 |
| Bacterial infections, n (%) | 31 (58.5) | 8 (47.1) | 0.40 |
| SP (mmHg) | 97.5±20.64 | 113.5±19.5 | 0.01 |
| NLR | 12.6±9.81 | 8.63±7.8 | 0.17 |
| MELD score | 27±8.35 | 20.5±8.17 | 0.01 |
| Child-Turcotte-Pugh score | 11.4±2.18 | 8.41±1.5 | <0.001 |
| ACLF | 49 (92.5) | 9 (52.9) | 0.001 |
| ACLF grade 1 | 0 | 2 (22.2) | <0.001 |
| ACLF grade 2 | 3 (6.1) | 5 (55.6) | |
| ACLF grade 3 | 46 (93.9) | 2 (22.2) | |
| Liver failure, n (%) | 24 (49) | 8 (66.7) | 0.27 |
| Renal failure, n (%) | 46 (93.9) | 8 (66.7) | 0.02 |
| Cerebral failure, n (%) | 30 (61.2) | 2 (16.7) | 0.006 |
| Coagulation failure, n (%) | 21 (42.9) | 0 | 0.005 |
| Circulation failure, n (%) | 45 (91.8) | 4 (33.3) | <0.001 |
| Respiratory failure, n (%) | 41 (85.4) | 7 (50) | 0.01 |
NLR: neutrophil-to-lymphocyte count; WBC: white blood cells; PLT: platelets; CRP: C-reactive protein; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ACLF: acute-on-chronic liver failure; MELD: model for end-stage liver disease; HE: hepatic encephalopathy; SP: systolic blood pressure; SBP: spontaneous bacterial peritonitis; INR: international normalized ratio; Note: the bold values denote statistical significance.
Figure 3.
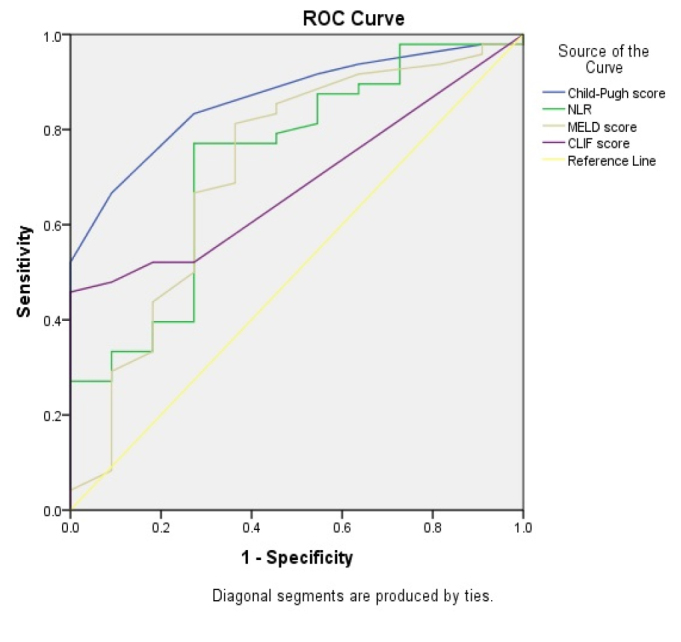
Accuracy of Child-Turcotte-Pugh score, neutrophil-to-lymphocyte ratio, model for end-stage liver disease score, and chronic liver failure score for the prediction of mortality in all patients.
Figure 4.
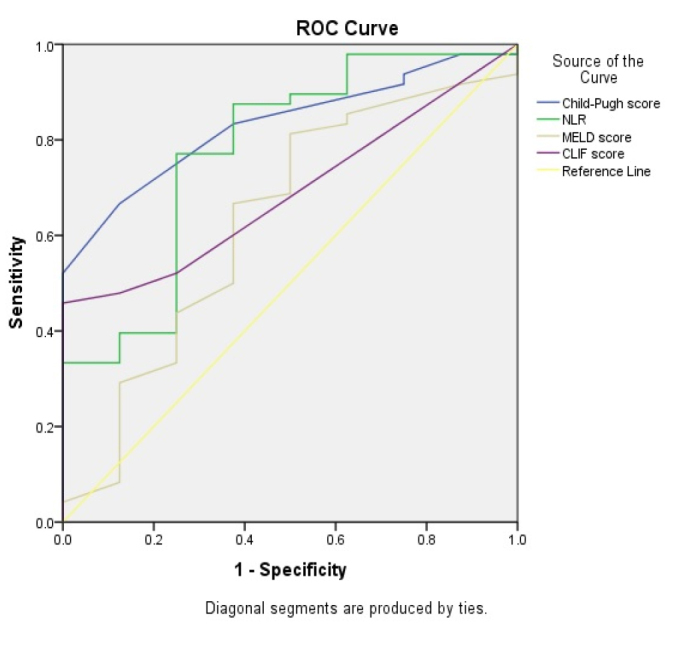
Accuracy of Child-Turcotte-Pugh score, neutrophil-to-lymphocyte ratio, model for end-stage liver disease score, and chronic liver failure score for the prediction of mortality in patients with acute-on-chronic liver failure.
DISCUSSION
In this study, we found that NLR was a promising tool for the evaluation of the risk of death in patients with decompensated LC and ACLF. Decompensated LC is responsible for many deaths in the ICU, and patients with ACLF have the worst prognosis, with death rates varying from 23.3% to 75.5% (1). Apparently, endotoxemia, a consequence of bacterial translocation, frequently found in patients with advanced liver disease, promotes systemic inflammation and leads to an elevated risk for OF and high mortality among these patients (19). Routine assessment of systemic inflammation includes the use of WBC count and CRP level. Although both these markers are observed to be elevated in patients with ACLF and to be associated with poor outcome (1), they can be influenced by various factors, such as GI bleeding, ongoing sepsis, and acute hepatitis and thus could be inadequate for the prediction of mortality in critically ill patients hospitalized in the ICU.
The accuracy of NLR in predicting mortality in patients with LC has been evaluated in several studies and found useful, especially in the setting of decompensated LC (17) and hepatocarcinoma (20) as well as in patients with low MELD score (18). More recently, Moreau et al. (11), in a retrospective observational cohort study, characterized the relation between NLR and early mortality in patients with decompensated LC, with and without ACLF. The authors have concluded that NLR calculated at admission had a prognostic value in patients with ACLF, showing a good potential for predicting mortality and that a value of NLR over 6.5 was associated with higher 90-day risk of death. Rice et al. (19), in a retrospective study including patients with cirrhosis hospitalized for non-elective reasons, analyzed the 1-year survival of these patients. The authors have concluded that NLR was associated with a high risk of death 1 year after the initial hospitalization.
Although NLR is considered to be a promising and cost-efficient method for predicting mortality in critically ill patients with cirrhosis, there is still debate over the most accurate cutoff value associated with high risk of poor outcome. Studies have reported cutoff values for NLR between 4.39 and 8 (11, 13, 19, 21–23), but an optimal cutoff value has not yet been established. In our study, patients with ACLF with an NLR value over 5 had a higher risk of in-hospital death and presented more frequently with coagulation and circulation failures as well as ascites. These patients had more severe liver disease, expressed by higher bilirubin levels and higher Child-Pugh score. We consequently concluded that a value of NLR over 5 was indicative for severe liver disease and poor outcome.
We found a positive correlation between NLR and MELD as well as ACLF coagulation scores, both markers used for the assessment of mortality in advanced liver disease (1). Several studies have already shown the potential use of NLR in predicting short-term (17) as well as medium-term mortality (19) in patients with LC. Furthermore, NLR has been recently found to be useful in evaluating the risk of death in patients with cirrhosis with low MELD scores (18). However, Moreau et al. (11) have found that the prognostic role of NLR was not dependent of MELD score in patients with ACLF, emphasizing the role of inflammation in the poor outcome of critically ill patients with cirrhosis, which is not currently assessed by the classic cirrhosis prognostic scores, such as the MELD score. NLR has been recently shown to vary progressively according to the severity of LC as well as ACLF grade (17). We found a high value for NLR in the entire cohort of critically ill patients with cirrhosis and a slightly higher value in the group with ACLF. Surprisingly, our results indicated that the Child-Turcotte-Pugh score showed a good accuracy for predicting mortality, which was followed by NLR, MELD, and CLIF scores, both in the entire cohort and in patients with ACLF. These findings could be explained by the higher incidence of HE found in our study than in the study of Moreau et al. (11). If HE is considered in the Child-Turcotte-Pugh score, this score could be more accurate in predicting the outcomes in these patients. Currently, there is a significant debate regarding the best prognostic score in critically ill patients with cirrhosis (24). Although the classic prognostic scores, such as the Child-Turcotte-Pugh or MELD scores, have proven their utility in predicting the outcomes of patients with cirrhosis; other scores, such as SOFA, and more recently, CLIF and NLR, have been found to be accurate for the prediction of mortality, especially in patients with advanced LC hospitalized in the ICU (11, 24, 25). However, some studies have found a similar predictive accuracy for death between the CLIF score and the traditional MELD and Child-Turcotte-Pugh scores, findings which could be explained by the low frequency of ICU hospitalization as well as the low rate of liver transplantation in their study group (26).
The predictive role of NLR in the assessment of urgent liver transplantation was assessed by Fang et al. (23), in a retrospective analysis of patients with HBV related ACLF, the authors have concluded that patients with an NLR value over 6 required emergency liver transplantation, as they showed the highest mortality rates. In our study, NLR showed a moderate accuracy in predicting outcome in patients with LC hospitalized in the ICU. However, the accuracy of NLR improved when only patients with ACLF were considered. These findings suggest that NLR has a better accuracy for predicting mortality in patients with severe LC and ACLF and therefore could be used in the stratification for the necessity of urgent liver transplantation in these patients.
Patients with ACLF hospitalized in the ICU have high mortality rates, and thus the need for accurate prognostic scores is high (25, 27, 28). We found high mortality levels both in patients with and without ACLF; however, as expected, there was a poor outcome associated with ACLF. Our results indicate that classic prognostic scores, such as Child-Turcotte-Pugh score, as well as novel ones, such as NLR, could be used in the stratification of prognosis in critically ill patients with cirrhosis hospitalized in the ICU.
This is one of the few studies to analyze the usefulness of NLR in the evaluation of critically ill patients with cirrhosis as well as one of the few studies to document the incidence of ACLF in Eastern Europe. We demonstrated that ACLF was frequently found in patients with LC hospitalized in the ICU and was associated with high mortality. NLR was found to be an adequate surrogate marker for outcome prediction in these patients.
Our study had several limitations, including a small sample size. The retrospective nature of the study was also a limitation in terms of the optimal documentation of patient evolution as well as the lack of medium- and long-term survival analysis. We also did not perform a complete analysis of the inflammatory status of patients with ACLF, and pro-inflammatory cytokines were not documented. This was owing to the lack of the routine use of these markers in our unit. From this perspective, a prospective investigation of these outcomes would be beneficial.
In conclusion, NLR is a promising and cost-effective method for the prediction of poor outcomes in critically ill patients with LC hospitalized in the ICU, more accurate in the case of patients with cirrhosis with ACLF. High NLR values are associated with high death rates among patients with ACLF but not in patients with LC, with “mere” acute decompensation. These patients could benefit from either urgent liver transplantation, or alternatively palliative care could be discussed. However, the optimal cutoff value for NLR is not yet clearly established, and results should be validated in large prospective studies.
MAIN POINTS.
Patients with liver cirrhosis and ACLF present a high risk for in-hospital mortality.
Systemic inflammation represents the main physiopathological driver for ACLF.
The neutrophil-to-lymphocyte count (NLR) is a marker which takes into account both the polymorphonuclear neutrophil count associated with inflammation and the lymphocyte count as a hallmark of immune impairment. NLR has been proven usefull in assessing the outcome of patients with advanced neoplasia, cardiovascular diseases, and postoperative sepsis.
NLR presented a good accuracy in predicting the outcome of cirrhotic patients with ACLF.
Footnotes
Ethics Committee Approval: This study was approved by the Ethics Committee of the “Grigore T. Popa” University of Medicine and Pharmacy Iasi, Romania (No 15046/19.7.2019).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.C., C.S., A.T.; Design - S.C., C.S., A.T.; Supervision - C.S., A.T.; Resource - C.V.S., A.M.S., T.C.; Materials - C.V.S., T.C; Data Collection and/or Processing - A.M.S., C.V.S.; Analysis and/or Interpretation - S.C., T.C.; Literature Search - A.M.S., C.V.S., T.C.; Writing - S.C., A.M.S., C.V.S.; Critical Reviews - C.S., A.T.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–37. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–82. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–71. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 4.Laleman W, Claria J, Van der Merwe S, Moreau R, Trebicka J. Systemic Inflammation and Acute-on-Chronic Liver Failure: Too Much, Not Enough. Can J Gastroenterol Hepatol. 2018;2018 doi: 10.1155/2018/1027152. 1027152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey S, Kawai T, Akira S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb Perspect Biol. 2014;7:a016246. doi: 10.1101/cshperspect.a016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–28. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Navajas JM. Inflammasome activation in decompensated liver cirrhosis. World J Hepatol. 2016;8:207–10. doi: 10.4254/wjh.v8.i4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albillos A, de la Hera A, González M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–17. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 9.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–96. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Noor MT, Manoria P. Immune Dysfunction in Cirrhosis. J Clin Transl Hepatol. 2017;5:50–8. doi: 10.14218/JCTH.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau N, Wittebole X, Fleury Y, Forget P, Laterre PF, Castanares-Zapatero D. Neutrophil-to-Lymphocyte Ratio Predicts Death in Acute-on-Chronic Liver Failure Patients Admitted to the Intensive Care Unit: A Retrospective Cohort Study. Shock. 2018;49:385–92. doi: 10.1097/SHK.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Feng G, Zhao Y, Zhang J, Feng L, Yang J. Association between lymphocyte-to-monocyte ratio (LMR) and the mortality of HBV-related liver cirrhosis: a retrospective cohort study. BMJ Open. 2015;5:e008033. doi: 10.1136/bmjopen-2015-008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai J, Wang K, Han T, Jiang H. Evaluation of prognostic values of inflammation-based makers in patients with HBV-related acute-on-chronic liver failure. Medicine (Baltimore) 2018;97:e13324. doi: 10.1097/MD.0000000000013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu KH, Jenq CC, Tsai MH, et al. Outcome scoring systems for short-term prognosis in critically ill cirrhotic patients. Shock. 2011;36:445–50. doi: 10.1097/SHK.0b013e31822fb7e2. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PH, Croal BL, Cuthbertson BH, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Yang F, Wang Y, et al. Prognostic nomogram incorporating neutrophil-to-lymphocyte ratio for early mortality in decompensated liver cirrhosis. Int Immunopharmacol. 2018;56:58–64. doi: 10.1016/j.intimp.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Kalra A, Wedd JP, Bambha KM, et al. Neutrophil-to-lymphocyte ratio correlates with proinflammatory neutrophils and predicts death in low model for end-stage liver disease patients with cirrhosis. Liver Transpl. 2017;23:155–165. doi: 10.1002/lt.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice J, Dodge JL, Bambha KM, et al. Neutrophil-to-Lymphocyte Ratio Associates Independently With Mortality in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2018;16:1786–91. doi: 10.1016/j.cgh.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 20.Goh BK, Kam JH, Lee SY, et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (≥10 cm) hepatocellular carcinoma. J Surg Oncol. 2016;113:621–7. doi: 10.1002/jso.24197. [DOI] [PubMed] [Google Scholar]
- 21.Biyik M, Ucar R, Solak Y, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2013;25:435–41. doi: 10.1097/MEG.0b013e32835c2af3. [DOI] [PubMed] [Google Scholar]
- 22.Wu W, Yan H, Zhao H, et al. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: Differentiate it from No-ACLF. Liver Int. 2018;38:248–57. doi: 10.1111/liv.13504. [DOI] [PubMed] [Google Scholar]
- 23.Fan Z, EnQiang C, Yao DL, et al. Neutrophil-lymphocyte ratio predicts short term mortality in patients with hepatitis B virus-related acute-on-chronic liver failure treated with an artificial liver support system. PLoS One. 2017;12:e0175332. doi: 10.1371/journal.pone.0175332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weil D, Levesque E, McPhail M, et al. Prognosis of cirrhotic patients admitted to intensive care unit: a meta-analysis. Ann Intensive Care. 2017;7:33. doi: 10.1186/s13613-017-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karvellas CJ, Garcia-Lopez E, Fernandez J, et al. Evaluation of the CLIF-C ACLF score in critically ill cirrhotic patients in intensive care units in Europe and North America: a multicenter cohort study. J Hepatol. 2017;66:S137. doi: 10.1016/S0168-8278(17)30544-5. [DOI] [Google Scholar]
- 26.Alexopoulou A, Vasilieva L, Mani I, Agiasotelli D, Pantelidaki H, Dourakis SP. Single center validation of mortality scores in patients with acute decompensation of cirrhosis with and without acute-on-chronic liver failure. Scand J Gastroenterol. 2017;52:1385–90. doi: 10.1080/00365521.2017.1369560. [DOI] [PubMed] [Google Scholar]
- 27.Engelmann C, Thomsen KL, Zakeri N, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 2018;22:254. doi: 10.1186/s13054-018-2156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meersseman P, Langouche L, du Plessis J, et al. The intensive care unit course and outcome in acute-on-chronic liver failure are comparable to other populations. J Hepatol. 2018;69:803–9. doi: 10.1016/j.jhep.2018.04.025. [DOI] [PubMed] [Google Scholar]


