Abstract
Background/Aims
This study aimed to evaluate the real-life efficacy and tolerability of direct-acting antiviral treatments for patients with chronic hepatitis C (CHC) with/without cirrhosis in the Turkish population.
Material and Methods
A total of 4,352 patients with CHC from 36 different institutions in Turkey were enrolled. They received ledipasvir (LDV) and sofosbuvir (SOF)±ribavirin (RBV) ombitasvir/paritaprevir/ritonavir±dasabuvir (PrOD)±RBV for 12 or 24 weeks. Sustained virologic response (SVR) rates, factors affecting SVR, safety profile, and hepatocellular cancer (HCC) occurrence were analyzed.
Results
SVR12 was achieved in 92.8% of the patients (4,040/4,352) according to intention-to-treat and in 98.3% of the patients (4,040/4,108) according to per-protocol analysis. The SVR12 rates were similar between the treatment regimens (97.2%–100%) and genotypes (95.6%–100%). Patients achieving SVR showed a significant decrease in the mean serum alanine transaminase (ALT) levels (50.90±54.60 U/L to 17.00±14.50 U/L) and model for end-stage liver disease (MELD) scores (7.51±4.54 to 7.32±3.40) (p<0.05). Of the patients, 2 were diagnosed with HCC during the treatment and 14 were diagnosed with HCC 37.0±16.0 weeks post-treatment. Higher initial MELD score (odds ratio [OR]: 1.92, 95% confidence interval [CI]: 1.22–2.38; p=0.023]), higher hepatitis C virus (HCV) RNA levels (OR: 1.44, 95% CI: 1.31–2.28; p=0.038), and higher serum ALT levels (OR: 1.38, 95% CI: 1.21–1.83; p=0.042) were associated with poor SVR12. The most common adverse events were fatigue (12.6%), pruritis (7.3%), increased serum ALT (4.7%) and bilirubin (3.8%) levels, and anemia (3.1%).
Conclusion
LDV/SOF or PrOD±RBV were effective and tolerable treatments for patients with CHC and with or without advanced liver disease before and after liver transplantation. Although HCV eradication improves the liver function, there is a risk of developing HCC.
Keywords: HCV, treatment, direct-acting antiviral, Turkey
INTRODUCTION
Hepatitis C virus (HCV) infection is one of the leading causes of progressive liver disease, cirrhosis, and hepatocellular cancer (HCC). According to recent epidemiological studies, the global rate of HCV infection is approximately 3%, and it is still associated with 500,000 deaths annually (1, 2). Despite the availability of new treatment options, chronic hepatitis C (CHC) still remains to be one of the leading causes of cancer-related deaths worldwide (3).
The treatment of CHC has always been a challenge for hepatologists. For many years, the only available treatment was interferon and ribavirin (RBV). This regimen was not ideal as it was not well tolerated by most of the patients and yielded very low sustained virologic response (SVR) rates (40%–50%), especially in genotype 1 HCV infections. Interferon-free direct-acting antiviral (DAA) therapies have been a game changer in CHC treatment (3). As of 2020, DAA therapies are considered the first-line treatment option not only owing to their short treatment course and pan-genotypic effect but also because of excellent SVR rates (95%–100%) (4–6). Although hallmark studies, such as POSITRON and FISSION (SVR rates of 93%–97%), ELECTRON and LONESTAR (SVR rates of 95%–100%), SAPPHIRE-II (SVR rates of 86.7%–96.3%), and ALLY-I (SVR rates of 83%–100%), have reported high SVR levels with minimum side effects, real-world data from various parts of the world are still needed. The results of recently published real-world data from China, Taiwan, Brazil, and an East Asian cohorts were similar to those of the aforementioned hallmark studies with high SVR rates (96.6%,98.2%, 95.0%, and 96.4%, respectively) (7–10).
Epidemiological studies in Turkey have shown that CHC infection is seen in approximately 1% of the Turkish population and that genotype 1 is the predominant HCV type (92.1%), followed by genotypes 2, 3, and 4 (11). Ledipasvir/sofosbuvir (LDV/SOF) treatment was the first DAA regimen to be approved in Turkey (February 2015). It has been widely used after the social security system began reimbursing the medicine in June 2016.
Studies investigating the real-life effectiveness of DAA regimens in the Turkish population are limited (12–17). A recent study from Turkey on 1,224 CHC cases (9.7% were decompensated cirrhotic) investigated the real-life effectiveness of sofosbuvir-based DAAs±RBV for 12 or 24 weeks (n=808) and ombitasvir/paritaprevir/ritonavir±dasabuvir (OBV/PTV/r±DSV)±RBV for 12 weeks or 24 weeks (n=416) reported an overall SVR12 rate of 97.9% (12). Idilman et al. (17) have reported an overall SVR rate of 96% (per protocol) in 200 patients with CHC with genotype 1b infection treated with LDV/SOF±RBV. The largest study to date was undertaken by the Turkish clinical microbiology and infectious disease society, which analyzed real-life data of (OBV/PTV/r±DSV)±RBV treatment in 862 patients from multiple centers, reported an overall SVR rate of 99%. Although this study included all HCV genotypes, the rate of patients with cirrhosis was very low (n=73, 8.3%), with no decompensated cirrhosis cases (16).
Despite the excellent SVR rates achieved with DAA treatment, de novo development or recurrence of HCC during or after the treatment remains a major concern. Evidence regarding the risk of HCC in patients with HCV infection and cirrhosis treated with DAA therapy is still inconclusive. Although several earlier studies have reported high rates of HCC incidence during and after DAA regimens, recent studies did not confirm these results (18–22). The only study from Turkey investigating the effect of DAA treatment on HCC development was conducted by Idilman et al. (17) in 2019, and they found that virologic suppression decreased the risk of de novo HCC to 0.6% during a median follow-up period of 22 months.
In this study, we aimed to determine the treatment efficacy and safety profile of different DAA regimens in a large population cohort in Turkey, to analyze the factors associated with SVR, and to investigate the impact of DAA treatment on disease progression and de novo HCC occurrence, recurrence, and relapse during and after DAA treatment.
MATERIALS AND METHODS
Patient Population and Study Design
This was a retrospective-prospective observational study that enrolled 4,352 patients with CHC from 36 institutions in Turkey between June 2015 and January 2020. A total of 27 patients were lost to follow-up, 39 discontinued the treatment, 5 died, and 178 had insufficient data. Therefore, SVR analysis included 4,108 patients for per-protocol (PP) and 4,352 patients for intention-to-treat (ITT) analysis. This study was approved by the local ethics committee of Marmara University School of Medicine.
Data were collected upon enrolment 4, 8, 12, and 24 weeks into the treatment and 12 and 24 weeks post-treatment. Demographic, clinical, and laboratory parameters (blood count, creatinine, liver panel, prothrombin time/international normalized ratio, viral serology, HCV RNA level and genotype, model for end-stage liver disease [MELD] score data, liver biopsy findings, radiologic findings [for patients with HCC], data on previous treatment duration and treatment response, and data on liver and kidney transplantation history) were all entered into a central electronic database system. Data on tolerability and safety analyses, adverse events (AE), drug discontinuation rate owing to AEs, liver-related and all-cause mortality, and new occurrence and/or recurrence of HCC during treatment and follow-up were recorded for all patients. All the laboratory tests were performed using commercially available assays at the participating centers.
Hepatitis C virus RNA Assays and Genotyping
Serum HCV RNA levels were measured using the Cobas AmpliPrep/COBAS TaqMan HCV Test, v2.0 (Roche, CA, USA). The results were converted into log10 copies/mL using the conversion formulas provided by the manufacturers. The detection limit of this assay is 200 copies/mL. HCV genotypes and subtypes were determined by various methods because of the different set-up of each participating centers. Polymerase chain reaction DNA sequencing of the 5’UTR and NS5B regions of the HCV genome was mainly used for genotypic analysis.
Data Analysis and Definitions
Patients received 90 mg of LDV and 400 mg of SOF in a fixed-dose combination tablet once daily with or without RBV or OBV/PTV/r+DSV with or without RBV for 12 or 24 weeks per the physicians’ discretion. The decision on the duration and dosage of the treatment was made by the clinician according to the presence of treatment-related side effects and the starting/stopping rules imposed by the social security system. RBV was initially administered at a low dose of 600 mg/day, which was then increased to a maximum dose of 1,000–1,200 mg/day if tolerated by the patients.
SVR12 was defined as the absence of quantifiable HCV RNA levels in the serum 12 weeks after the end of the treatment. The definition of cirrhosis was based on the clinical, laboratory, and histological findings when available.
The secondary endpoints were defined as improvements in the Child-Pugh and MELD scores, tolerability of the treatments (AEs), and de novo occurrence or recurrence of HCC during treatment or follow-up.
Safety and tolerability analyses were based on the assessment of AE, serious AE (SAE), drug discontinuation owing to AEs, laboratory abnormalities, and deaths.
Statistical Analysis
Categorical data were expressed as absolute numbers and percentages and were compared using the chi-squared test or Fisher’s exact test as appropriate. Continuous variables were expressed as mean and standard deviations unless otherwise specified and were compared using the t-test or Mann-Whitney U test. Variables that had a p value of <0.2 in univariate analysis were entered into the Cox regression hazards model by forward logistic regression to determine the independent predictors of SVR12. The main population analyzed was an ITT population, and changes over time were analyzed by non-parametric tests. All statistical analyses were performed using the IBM Statistical Package for Social Sciences version 20.0 (IBM Corp.; Armonk, NY, USA).
RESULTS
Patient Characteristics
A total of 4,108 patients reached SVR12. All patients were Caucasian (mean age: 61.33±13.13 years; men/women: 1,755/2,353). The mean duration of chronic HCV infection was 6.76±5.24 years. The majority of patients (2.783; 70.1%) had previously received interferon-based treatments (16.1% relapse, 83.9% non-response cases). Most patients were infected with genotype 1 HCV (1a: 22.6%; 1b: 72.2%), followed by genotypes 2, 3, 4, 1b+4, and 1b+2. At the start of antiviral therapy, 39.2% of the patients had cirrhosis (1,159 compensated and 451 decompensated), 136 had undergone liver transplantation, 165 had chronic kidney disease, and 82 had undergone renal transplantation.
The patients were divided into 6 different treatment regimen groups; 1,731 received LDV/SOF, 365 received LDV/SOF+RBV, 1,765 received OBV/PTV/r+DSV, 207 received OBV/PTV/r+DSV+RBV, 32 received OBV/PTV/r+RBV, and 6 received OBV/PTV/r. Most patients were treated with LDV/SOF (44.59%) and OBV/PTV/r+DSV (41.09%).
The baseline mean serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and HCV RNA levels were 50.90±54.60 U/L, 51.40±136.90 U/L, and 5.64±1.51 log10 copies/mL, respectively. The mean MELD score was 7.51±4.54 for patients with cirrhosis. Liver biopsy results were available before treatment for 32% of the patients without cirrhosis. The mean histological activity index and fibrosis stage were 7.61±3.10 and 3.24±1.73, respectively. The characteristics of the study population are summarized in Table 1.
Table 1.
Baseline characteristics of the study population.
| Total (n=4,108) | |
|---|---|
| Age (years) | 61.33±13.13 (18–91) |
| Sex, male (n, %) | 1,755 (42.7) |
| Duration of infection (years) | 6.76±5.24 (0–32) |
| Treatment experienced, n (%) | 1,951/2,783 (70.1) |
| Indication for DAA treatment, n (%) | |
| Relapse | 316 (16.1) |
| Non-response | 1,635 (83.9) |
| Laboratory | |
| ALT (U/L) | 50.90±54.60 |
| AST (U/L) | 51.4±136.9 |
| Hgb (mg/dL) | 13.0±2.07 |
| Platelets (109/L) | 152,687±156,424 |
| Baseline HCV RNA log | 5.64±1.51 |
| Creatinine | 1.15±1.39 |
| INR | 1.12±0.31 |
| T. bilirubin | 0.95±0.29 |
| Albumin | 3.95±0.79 |
| Na | 138.9±3.52 |
| Baseline MELD score | 7.51± 4.54 |
| Genotype | |
| 1a | 927 (22.6) |
| 1b | 2,967 (72.2) |
| 2 | 40 (1.0) |
| 3 | 83 (2.0) |
| 4 | 77 (1.9) |
| 1b+4 | 12 (0.3) |
| 1b+2 | 2 (0) |
| History of HCC, n (%) | 82 (2.0) |
| HBV+HCV, n (%) | 32 (0.75) |
| Patients with liver biopsy, n (%) | 1,387 (33.7) |
| Grade | 7.61±3.10 |
| Stage | 3.24±1.73 |
| Non-cirrhotic | 2,498 (60.8) |
| Cirrhosis, n (%) | 1,610 (39.2) |
| Compensated | 1,159 (71.9) |
| Decompensated | 451 (28.1) |
| Chronic kidney disease (on HD), n (%) | 165 (4.0) |
| Kidney transplantation, n (%) | 82 (2.0) |
| Liver transplantation, n (%) | 136 (3.3) |
Results expressed as number (%) or median (range) unless specified otherwise
HD: hemodialysis; DAA: direct-acting antiviral; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HCV: hepatitis C; RNA: ribonucleic acid; Hgb: hemoglobin; INR: international normalized ratio; MELD: model for end-stage liver disease; HCC: hepatocellular carcinoma; HBV: hepatitis B; Na: sodium; T: total
Treatment Efficacy and Factors Affecting Sustained virologic response 12
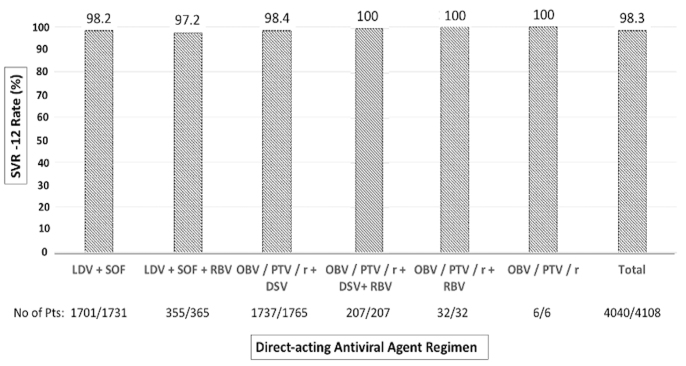
SVR12 was achieved in 92.8% of the patients (4,040/4,352) according to ITT analysis and 98.3% (4,040/4,108) according to PP analysis. Patients with SVR12 were slightly predominantly females (57.4% vs. 46.2%; p=0.108), older (61.95±12.70 vs. 57.11±16.90 years; p=0.078), and with a longer infection history (6.78±5.42 vs. 5.30±5.18 years; p=0.059) than patients who did not reach SVR12. The SVR12 rate was similar among patients with and without cirrhosis (98.2% vs. 98.3%, respectively; p= 0.106), among patients with compensated cirrhosis and decompensated cirrhosis (99.7% vs. 94.4%, respectively; p=0.06), and among treatment-naïve and treatment-experienced patients (68.0% vs. 70.1%, respectively; p=0.256). The SVR12 rates were 98.2% for LDV/SOF, 97.2% for LDV/SOF+RBV, 98.4% for OBV/PTV/r+DSV, and 100% for OBV/PTV/r+DSV+RBV, OBV/PTV/r+RBV, and OBV/PTV/r (Figure 1). The SVR rates did not differ according to HCV genotype (p>0.05): 98.4% for genotype 1 (98.0% for genotype 1b and 99.4% for genotype 1a), 100% for genotype 2, 98.7% for genotype 3, and 97.5% for genotype 4 (Figure 2).
Figure 1.
Sustained virologic response 12 rates of each direct-acting antiviral regimen.
Figure 2.
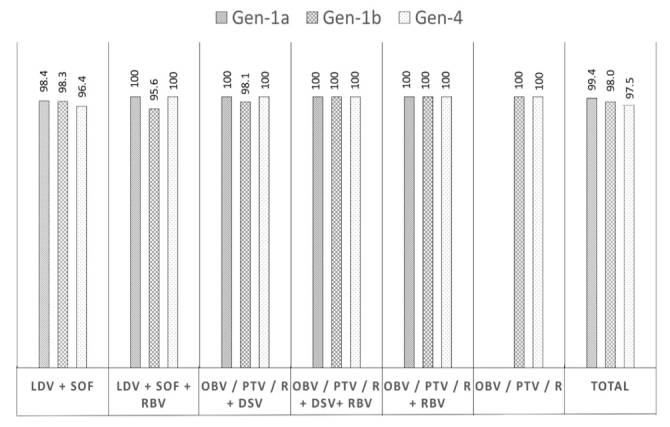
Sustained virologic response 12 rates of different genotypes.
The serum ALT levels and MELD scores significantly decreased from baseline to SVR12 (from 50.90±54.60 U/L to 17.00±14.50 U/L and from 7.51±4.54 to 7.32±3.40, respectively; p<0.05). Patients who did not achieve SVR12 had higher baseline HCV RNA levels (6.12±2.32 vs. 5.56±0.78 log10 IU/mL, p=0.043), serum ALT levels (53.11±71.35 vs. 49.90±12.72 U/L, p=0.05), and MELD scores (7.89±10.19 vs. 7.47± 4.07, p=0.035) than patients who achieved SVR12 (Table 2).
Table 2.
Characteristics of patients with and without SVR12.
| SVR12 (−) (n=68) | SVR 12 (+) (n=4,040) | Total (n=4,108) | p | |
|---|---|---|---|---|
| Age (years) | 57.11±16.9 (23–88) | 61.95±12.70 (18–91) | 61.33±13.13 (18–91) | 0.078 |
| Sex, male (%) | 24 (53.8) | 1,731 (42.6) | 1,755 (42.7) | 0.108 |
| Duration of infection (years) | 5.30±5.18 | 6.78±5.42 | 6.76±5.24 | 0.059 |
| Treatment experienced, n (%) | 17/25 (68.0) | 1934/2758 (70.1) | 1951/2783 (70.1) | 0.256 |
| Indication for DAA treatment | 0.310 | |||
| Relapse | 6 (35.2) | 310 (16.0) | 316 (16.1) | |
| Non-response | 9 (64.8) | 1,624 (84.0) | 1,635 (83.9) | |
| Laboratory | ||||
| Log10 HCV RNA (IU/mL) | 6.12±2.32 | 5.56±0.78 | 5.64±1.51 | 0.043* |
| ALT (U/L) | 53.11±71.35 | 49.90±12.72 | 50.90±54.60 | 0.050* |
| MELD score | 7.89± 10.19 | 7.47± 4.07 | 7.51± 4.54 | 0.035* |
| Genotype | 0.103 | |||
| 1a | 7 (21.0) | 920 (22.6) | 927 (22.6) | |
| 1b | 58 (75.0) | 2,909 (72.1) | 2,967 (72.2) | |
| 2 | 0 | 40 (0.99) | 40 (1.0) | |
| 3 | 2 (2.9) | 81 (2.05) | 83 (2.0) | |
| 4 | 1 (4.0) | 76 (1.85) | 77 (1.9) | |
| 1b+4 | 0 | 12 (0.29) | 12 (0.3) | |
| 1b+2 | 0 | 2 (0.04) | 2 (0) | |
| Non-cirrhotic | 40 (58.8) | 2,458 (60.9) | 2,498 (60.8) | 0.921 |
| Cirrhotic | 28 (41.2) | 1,582 (39.1) | 1,610 (39.2) | 0.061 |
| Compensated cirrhosis | 3 (11.8) | 1,156 (72.8) | 1,159 (71.9) | |
| Decompensated cirrhosis | 25 (89.2) | 426 (27.2) | 451 (28.1) | |
| Treatment regimen | 0.105 | |||
| LDV+SOF | 30 (44.5) | 1,701 (42.6) | 1,731 (44.59) | |
| LDV+SOF+RBV | 10 (16.4) | 355 (8.2) | 365 (9.43) | |
| OBV/PTV/r+DSV | 28 (40.1) | 1,737 (43.2) | 1,765 (41.09) | |
| OBV/PTV/r+DSV+RBV | 0 | 207 (5.2) | 207 (4.63) | |
| OBV/PTV/r+RBV | 0 | 32 (0.7) | 32 (0.22) | |
| OBV/PTV/r | 0 | 6 (0.1) | 6 (0.04) | |
p<0.05
Results expressed as number (%) or median (range) unless specified otherwise
DAA: direct-acting antiviral; SVR: sustained virologic response; LDV: ledipasvir; SOF: sofosbuvir, RBV: ribavirin; OBV: ombitasvir; PTV: paritaprevir; r: ritonavir; DSV: dasabuvir; ALT: alanine aminotransferase; HCV: hepatitis C virus; RNA: ribonucleic acid; MELD: model for end-stage liver disease
A total of 3 patients with SVR12 experienced a late relapse (virologic failure) 9.2±4.8 months after achieving SVR12. All 3 patients were infected with genotype 1, and 2 patients had compensated cirrhosis.
Univariate analysis showed that SVR12 was significantly associated with the baseline MELD score (p=0.035), serum ALT level (p=0.05), and HCV RNA level (p=0.043). Logistic regression analysis revealed that high baseline MELD scores (odds ratio [OR]: 1.92, 95% confidence interval [CI]: 1.22–2.38; p=0.023), HCV RNA levels (OR: 1.44, 95% CI: 1.31–2.28; p=0.038), and serum ALT levels (OR: 1.38, 95% CI: 1.21–1.83; p=0.042) were the factors associated with poor SVR12 rates (Table 3).
Table 3.
Poor prognostic factors for SVR12.
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
|
| |||
| p value | OR (95% CI) | p | |
| High MELD score | 0.035 | 1.92 (1.22–2.38) | 0.023 |
| High HCV RNA level | 0.043 | 1.44 (1.31–2.28) | 0.038 |
| High serum ALT level | 0.050 | 1.38 (1.21–1.83) | 0.042 |
| Longer duration of infection | 0.059 | 1.09 (1.03–1.35) | 0.101 |
| Decompensated cirrhosis | 0.061 | 1.15 (1.07–1.30) | 0.055 |
| Older age, years | 0.078 | 1.11 (1.05–1.23) | 0.108 |
| Treatment regimen | 0.105 | 1.03 (0.96–1.09) | 0.182 |
SVR: sustained virologic response; ALT: alanine aminotransferase; HCV: hepatitis C virus; RNA: ribonucleic acid; MELD: model for end-stage liver disease; OR: odds ratio; CI: confidence interval
De novo Development and Recurrence of Hepatocellular Cancer
A total of 82 patients had a history of HCC before receiving antiviral therapy. De novo HCC development was detected in 16 patients without a previous HCC history (in the 12th and 14th weeks of treatment in 2 patients and in the follow-up period in 14 patients). There was a male predominance in all the cases. The genotype distribution, treatment experience rate, type of treatment regimen, and mean age were similar in all patients (Table 4). The 2 patients with de novo HCC during treatment had achieved SVR12 with LDV/SOF and LDV/SOF+RBV. They showed no recurrence after a median follow-up of 8.5 months. A total of 14 patients with de novo HCC during follow-up were diagnosed 37.0±16.0 weeks after the antiviral treatment was discontinued. The SVR12 rate in this group was lower than that in patients without HCC (67.3% vs. 100% vs. 96.3%). None of the 82 patients had active HCC at the time of treatment. HCC recurrence was observed in 35 of the 82 patients with a history of previous HCC after a median follow-up of 9 months after treatment. The SVR12 rates were similar in patients with and without recurrence (96.9% vs. 96.1%, respectively) (Table 4).
Table 4.
Development and recurrence of HCC during and after treatment.
| Diagnosed before treatment (n=82) | Diagnosed during treatment (n=2) | Diagnosed after treatment (n=14) | |
|---|---|---|---|
| Age (year) | 62.10±12.1 | 60.05±3.10 | 61.92±8.54 |
| Sex, male (n, %) | 50 (61.2) | 2 (100) | 8 (57.1) |
| Duration of infection | 7.02±4.25 | 6.04±1.42 | 6.11±4.88 |
| Treatment experienced, n (%) | 71 (86.5) | 1 (50.0) | 11 (78.5) |
| Genotype | |||
| 1 | 79 (96.4) | 2 (100) | 13 (85.8) |
| 2 | 1 (1.2) | 0 | 1 (7.1) |
| 3 | 1 (1.2) | 0 | 1 (7.1) |
| 4 | 1 (1.2) | 0 | 0 (0) |
| Type of DAA regimen | |||
| LDV+SOF | 39 (47.8) | 1 (50) | 7 (50.0) |
| LDV+SOF+RBV | 4 (4.7) | 1 (50) | 1 (7.1) |
| OBV/PTV/r+DSV | 38 (46.3) | 0 | 6 (42.9) |
| Other | 1 (1.2) | 0 | 0 |
| SVR12 (+) | 79 (96.3) | 2 (100) | 12 (67.3) |
| Diagnosed after start of treatment (weeks) | NA | 13.0±1.41 | 37.0±15.98 |
Results expressed as number (%) or median (range) unless specified otherwise
DAA: direct-acting antiviral; SVR: sustained virologic response; HCC: hepatocellular cancer; LDV: ledipasvir; SOF: sofosbuvir; RBV: ribavirin; OBV: ombitasvir; PTV: paritaprevir; r: ritonavir; DSV: dasabuvir; NA: not applicable
Safety and Tolerability
A total of 957 AEs were reported by 819 patients (18.8%). Most were mild or moderate. No treatment-related SAEs were reported. The most common AEs were fatigue (551; 12.6%), pruritis (319; 7.3%), nausea (204; 4.7%), and headache (165; 3.8%). Increases in serum ALT and bilirubin levels and anemia were seen in 204 (4.7%), 66 (3.8%), and 134 patients (3.1%), respectively, and were mild. The overall discontinuation rate because of treatment was observed in 0.7% (30 patients) and was the highest in the OBV/PTV/r+RBV treatment group (12.2%) and lowest in the LDV/SOF group (0.1%). A total of 3 liver-related deaths were reported during the treatment period (2 in the LDV/SOF and 1 in the OBV/PTV/r+DSV groups). None were associated with treatment AEs. There were 32 patients with hepatitis B virus (HBV)+HCV co-infection in the study population. Of them, 5 experienced increased serum HBV DNA levels but none presented with HBV flare and needed antiviral treatment (Table 5).
Table 5.
Treatment-related adverse events and complications.
| LDV+SOF (n=1,843) | LDV+SOF+RBV (n=388) | OBV/PTV/r+DSV (n=1,864) | OBV/PTV/r+DSV+ RBV (n=217) | OBV/PTV/r+RBV (n=33) | OBV/PTV/r (n=7) | Total (n=4,352) | |
|---|---|---|---|---|---|---|---|
| Total AE (n) | 564 | 225 | 544 | 145 | 28 | 6 | 957 |
| Treatment-related SAE | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Early discontinuation (0–4 weeks) | 5 | 11 | 9 | 10 | 4 | 0 | 39 (0.89) |
| Discontinuation owing to treatment | 2 | 9 | 8 | 8 | 3 | 0 | 30 (0.68) |
| Liver-related deaths | 2 | 0 | 1 | 0 | 0 | 0 | 3 (0.06) |
| AEs | |||||||
| Fatigue | 223 | 63 | 207 | 48 | 8 | 2 | 551 (12.6) |
| Pruritis | 115 | 41 | 124 | 34 | 4 | 1 | 319 (7.32) |
| Increase in bilirubin | 81 | 19 | 87 | 13 | 3 | 1 | 204 (4.68) |
| Nausea | 64 | 17 | 69 | 12 | 2 | 1 | 165 (3.79) |
| Anemia | 30 | 58 | 16 | 23 | 7 | 134 (3.07) | |
| Increase in ALT level | 22 | 13 | 20 | 8 | 2 | 1 | 66 (1.51) |
| Headache | 8 | 4 | 5 | 1 | 1 | 19 (0.43) | |
| Hair loss | 5 | 3 | 4 | 1 | 13 (0.29) | ||
| Cough | 5 | 1 | 2 | 3 | 11 (0.25) | ||
| Chest pain | 2 | 3 | 3 | 1 | 9 (0.20) | ||
| Insomnia | 3 | 2 | 3 | 1 | 9 (0.20) | ||
| Dry nose | 3 | 1 | 2 | 1 | 7 (0.16) | ||
| Peripheral neuropathy | 1 | 1 | 2 (0.04) | ||||
| Vertigo | 1 | 1 | 2 (0.04) | ||||
| Acute pancreatitis | 1 | 1 (0.02) |
Results expressed as number (%) or median (range) unless specified otherwise
AE: adverse event, SAE: severe adverse event; LDV: ledipasvir; SOF: sofosbuvir; RBV: ribavirin; OBV: ombitasvir; PTV: paritaprevir; r: ritonavir; DSV: dasabuvir; ALT: alanine aminotransferase
DISCUSSION
In recent years, many studies on real-world DAA experiences have been published worldwide. However, real-world data from Turkey are still limited. In this multicenter study, real-world data on different DAA treatments in 4,108 patients with CHC were analyzed to determine their efficacy, safety, and tolerability. We found that the SVR12 rate was consistently high (98.3% according to the PP analysis) regardless of the HCV genotype, treatment regimen and duration, infection duration, and liver status. The antiviral treatments were well tolerated. These results are similar to those of phase III trials and other studies from Turkey and other parts of the world (5, 7–10, 23). In a recent meta-analysis of 25 studies involving a total of 5,158 patients, the SVR12 rates of patients with genotype 1 and genotype 4 HCV infection were 96.8% and 98.9%, respectively; the rates in patients with and without cirrhosis were similar (24). Our results are also in line with this meta-analysis and other studies (25–27). Patients with and without cirrhosis in our study showed similar SVR12 rates (98.2% vs. 98.3%). Moreover, the SVR12 rate was slightly lower in patients with decompensated cirrhosis than in those with compensated cirrhosis (94.4% vs. 99.7%), although multivariate logistic regression analysis did not show decompensated cirrhosis as an independent factor for SVR12 (p=0.055). The baseline HCV RNA level has been considered an important prognostic factor for HCV treatment since the interferon era. Studies on DAAs have shown that in patients with genotype 1 HCV infection in particular, both the baseline HCV RNA level and the baseline MELD score can be used as predictors of treatment response (28, 29). In this study, we found that high baseline HCV RNA levels, serum ALT levels, and MELD scores were predictors of poor SVR12 outcomes. The serum ALT levels and MELD scores improved when SVR12 was achieved. These results are consistent with those of other studies (28–30).
In our study, 82 patients had a history of HCC on enrolment, whereas 16 patients without a history of HCC later developed de novo HCC. Although the genotype distribution, treatment experience rate, treatment regimen, and mean age were similar, the SVR12 rate was the lowest in patients diagnosed after treatment (67.3% vs. 100% vs. 96.3%). HCC recurrence was observed in 35 of the 82 patients with a history of HCC after a median follow-up of 9 months. The SVR rates of patients with and without recurrence were similar (96.9% vs. 96.1%, respectively). The effect of DAA treatment on de novo HCC and recurrence is debatable. Although several studies have reported high rates of HCC incidence during and after DAA regimens in patients with cirrhosis, some recent studies did not confirm these results (18–22). Idilman et al. (17) have found that successful SVR reduced the risk of de novo HCC to only 0.6%. Our study reported similar results. We reported 12 new patients with HCC who achieved SVR12 (0.75%). In congruence with this finding, the rate of de novo HCC occurrence after treatment was higher in patients who had not achieved SVR12 (7.14%). This finding indicates that SVR reduces the risk of de novo HCC but not of recurrence. Therefore, patients with cirrhosis, especially those with failed treatments, should be monitored more closely for HCC development.
In this study, the treatments were generally well tolerated with side effects mostly being mild or moderate. No treatment-related SAEs were reported. The most common AEs were fatigue (12.6%), pruritis (7.32%), nausea (4.68%), and headache (3.79%). Increases in serum ALT and serum bilirubin levels and anemia were mild and were seen in 5%, 4%, and 3% patients, respectively. Acute pancreatitis was diagnosed in 1 patient, and 2 patients were diagnosed with peripheral neuropathy. The patient with pancreatitis was on LDV/SOF, and the patients with neuropathy were on LDV/SOF and OBV/PTV/r+DSV upon diagnosis. We were unable to establish the etiology for any patients. To the best of our knowledge, these were the first patients with AEs potentially related to DAA treatment. There were 32 patients with HBV+HCV co-infection in our study population. Of them, 5 exhibited increased serum HBV DNA levels but none presented with HBV flares. These findings provide further evidence that the treatments were generally well tolerated, but special attention must be paid to patients with cirrhosis and patients on RBV.
This study has considerable strengths, namely its multicenter design and large sample size, including high rates of patients with cirrhosis and patients with liver biopsy results. However, this study had some limitations. First, some patients in our cohort were analyzed retrospectively. Second, serum HCV RNA testing was performed in local laboratories with different standards. Finally, the follow-up duration was not long enough for complete assessment of the impact of DAA treatment on HCC. Despite these limitations, this study makes a significant contribution to the literature as it managed to analyze all the aspects of DAA treatment and is the largest study conducted in Turkey to date.
In conclusion, the overall SVR12 rate of the combination of LDV/SOF ± RBV and OBV/PTV/r+DSV ± RBV treatment regimens were high for both CHC and advanced liver disease patients before and after liver transplantation. The treatments were generally well tolerated. The SVR12 rate was associated with the baseline serum HCV RNA and serum ALT level, and MELD score. Virologic suppression was associated with improved liver function and a reduced risk of de novo HCC.
MAIN POINTS.
The sustained virologic response (SVR) 12 rate of different antiviral combination treatments in Turkish patients with chronic hepatitis C (CHC) was 98.3%.
The treatments were generally well tolerated.
The baseline serum hepatitis C virus (HCV) RNA level, serum alanine transaminase level, and model for end-stage liver disease score were the factors associated with SVR12.
HCV eradication was associated with clinical and laboratory improvements and a reduced risk of de novo hepatocellular cancer after treatment.
Acknowledgements
The authors are grateful for the contribution of the members of the TASL Viral Hepatitis Special Interest Group.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Marmara University School of Medicine Clinical Research Ethics Committee (March 2, 2018-09.2018.213)
Informed Consent: Written informed consent was obtained from the subjects who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.D., R.İ., M.D., U.S.A., H.T.K., E.Ü., E.Y., F.G., A.B., S.V., N.D., M.D., A.S., S.Y., A.K., A.Kefeli, F.G., K.Y., E.A., M.A., M.H., A.Ö., T.T., M.A., H.S., M.A., B.A., İ.Ş., H.A., A.U., K.I., S.Ö., Ç.B.U., Ş.G., S.G., K.N., İ.İ., S.K., D.D., L.D., H.S.G., A.M., A.M.C., H.D., R.A., S.A., Y.B., H.K., H.Ş., O.Ö., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.S., O.Ö., S.K., F.B., Z.K.; Design – B.D., U.S.A., F.G., O.Ö., M.Ç., Ş.P., T.A., S.Y., M.A., S.Ö., M.A., O.S., O.Ö., S.K., F.B., Z.K., R.İ.; Supervision – B.D., U.S.A., O.Ö., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.Ö., S.K., F.B., Z.K., R.İ.; Resource – B.D., U.S.A., O.Ö., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.Ö., S.K., F.B., Z.K., R.İ.; Materials – B.D., M.D., U.S.A., H.T.K., E.Ü., E.Y., F.G., A.B., S.V., N.D., M.D., A.S., S.Y., A.K., A.Kefeli, F.G., K.Y., E.A., M.A., M.H., A.Ö., T.T., M.A., H.S., M.A., B.A., İ.Ş., H.A., A.U., K.I., S.Ö., Ç.B.U., Ş.G., S.G., K.N., İ.İ., S.K., D.D., L.D., H.S.G., A.M., A.M.C., H.D., R.A., S.A., Y.B., H.K., H.Ş., O.Ö., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.Ö., S.K., F.B., Z.K., R.İ.; Data Collection and/or Processing – B.D., M.D., U.S.A., H.T.K., E.Ü., E.Y., F.G., A.B., S.V., N.D., M.D., A.S., S.Y., A.K., A.Kefeli, F.G., K.Y., E.A., M.A., M.H., A.Ö., T.T., M.A., H.S., M.A., B.A., İ.Ş., H.A., A.U., K.I., S.Ö., Ç.B.U., Ş.G., S.G., K.N., İ.İ., S.K., D.D., L.D., H.S.G., A.M., A.M.C., H.D., R.A., S.A., Y.B., H.K., H.Ş., O.Ö., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.Ö., S.K., F.B., Z.K., R.İ.; Analysis and/or Interpretation – B.D., R.İ., M.D., U.S.A., H.T.K., E.Ü., E.Y., F.G., A.B., S.V., N.D., M.D., A.S., S.Y., A.K., A.Kefeli, F.G., K.Y., E.A., M.A., M.H., A.Ö., T.T., M.A., H.S., M.A., B.A., İ.Ş., H.A., A.U., K.I., S.Ö., Ç.B.U., Ş.G., S.G., K.N., İ.İ., S.K., D.D., L.D., H.S.G., A.M., A.M.C., H.D., R.A., S.A., Y.B., H.K., H.Ş., O.Ö., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.Ö., S.K., F.B., Z.K.; Literature Search – B.D., R.İ., S.Y., A.M.C., M.D., H.T.K., E.Ü.; Writing – B.D., R.İ., M.D.,. U.S.A., H.T.K., E.Ü., E.Y., F.G., A.B., S.V., N.D., M.D., A.S., S.Y., A.K., A.Kefeli, F.G., K.Y., E.A., M.A., M.H., A.Ö., T.T., M.A., H.S., M.A., B.A., İ.Ş., H.A., A.U., K.I., S.Ö., Ç.B.U., Ş.G., S.G., K.N., İ.İ., S.K., D.D., L.D., H.S.G., A.M., A.M.C., H.D., R.A., S.A., Y.B., H.K., H.Ş., O.Ö., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.Ö., S.K., F.B., Z.K.; Critical Reviews – B.D., R.İ., U.S.A., F.B., M.D., M.Ç., Ş.P., T.A., S.Y., F.G., M.A., S.Ö., M.A., O.S., O.Ö., S.K., F.B., Z.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The present study was supported by The Turkish Association for the Study of The Liver (TASL).
REFERENCES
- 1.WHO Global Hepatitis Report. 2017 [Google Scholar]
- 2.Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22:833–8. doi: 10.1016/j.cmi.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Bertino G, Ardiri A, Proiti M, et al. Chronic hepatitis C: This and the new era of treatment. World J Hepatol. 2016;8:92–106. doi: 10.4254/wjh.v8.i2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panel A-IHG. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477–92. doi: 10.1093/cid/ciy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Alshuwayk O, Kwo PY. HCV treatment in 2020: How to translate highly effective therapies into elimination strategies. Hepatology Forum. 2020;2:72–4. doi: 10.14744/hf.2020.2020.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Wu FP, Wang WJ, et al. Real-life efficacy and safety of direct-acting antiviral therapy for treatment of patients infected with hepatitis C virus genotypes 1, 2 and 3 in northwest China. World J Gastroenterol. 2019;25:6551–60. doi: 10.3748/wjg.v25.i44.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CH, Liu CJ, Hung CC, et al. Glecaprevir/pibrentasvir for patients with chronic hepatitis C virus infection: Real-world effectiveness and safety in Taiwan. Liver Int. 2020;40:758–68. doi: 10.1111/liv.14295. [DOI] [PubMed] [Google Scholar]
- 9.Huang CF, Iio E, Jun DW, et al. Direct-acting antivirals in East Asian hepatitis C patients: real-world experience from the REAL-C Consortium. Hepatol Int. 2019;13:587–98. doi: 10.1007/s12072-019-09974-z. [DOI] [PubMed] [Google Scholar]
- 10.Lobato CMO, Codes L, Silva GF, et al. Direct antiviral therapy for treatment of hepatitis C: A real-world study from Brazil. Ann Hepatol. 2019;18:849–54. doi: 10.1016/j.aohep.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Tozun N, Ozdogan O, Cakaloglu Y, et al. Seroprevalence of hepatitis B and C virus infections and risk factors in Turkey: a fieldwork TURHEP study. Clin Microbiol Infect. 2015;21:1020–6. doi: 10.1016/j.cmi.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Ormeci N, Gulsen MT, Sezgin O, et al. Treatment of HCV infection with direct-acting antiviral agents. Real life experiences from the Euro-Asian region. Turk J Gastroenterol. 2020;31:148–55. doi: 10.5152/tjg.2020.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danis N, Toz H, Unal N, et al. Paritaprevir, ritonavir, ombitasvir, and dasabuvir treatment in renal transplant patients with hepatitis C virus infection. Turk J Gastroenterol. 2019;30:695–701. doi: 10.5152/tjg.2019.18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ormeci N, Sezgin O, Karaali R, et al. Effectiveness of fixed-dose combination of paritaprevir, ritonavir, ombitasvir, and dasabuvir in patients with chronic hepatitis C virus infection and chronic kidney diseases: real-life experiences. Eur J Gastroenterol Hepatol. 2019;31:534–9. doi: 10.1097/MEG.0000000000001334. [DOI] [PubMed] [Google Scholar]
- 15.Yaras S, Ucbilek E, Ozdogan O, Ates F, Altintas E, Sezgin O. Real-life results of treatment with ombitasvir, paritaprevir, dasabuvir, and ritonavir combination in patients with chronic renal failure infected with HCV in Turkey. Turk J Gastroenterol. 2019;30:331–5. doi: 10.5152/tjg.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aygen B, Demirturk N, Yildiz O, et al. Real-world efficacy, safety, and clinical outcomes of ombitasvir/paritaprevir/ritonavir +/− dasabuvir +/− ribavirin combination therapy in patients with hepatitis C virus genotype 1 or 4 infection: The Turkey experience experience. Turk J Gastroenterol. 2020;31:305–17. doi: 10.5152/tjg.2020.19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idilman R, Demir M, Aladag M, et al. Low recurrence rate of hepatocellular carcinoma following ledipasvir and sofosbuvir treatment in a real-world chronic hepatitis C patients cohort. J Viral Hepat. 2019;26:666–74. doi: 10.1111/jvh.13075. [DOI] [PubMed] [Google Scholar]
- 18.Alberti A, Piovesan S. Increased incidence of liver cancer after successful DAA treatment of chronic hepatitis C: Fact or fiction? Liver Int. 2017;37:802–8. doi: 10.1111/liv.13390. [DOI] [PubMed] [Google Scholar]
- 19.Kozbial K, Moser S, Schwarzer R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65:856–8. doi: 10.1016/j.jhep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Li DK, Ren Y, Fierer DS, et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. 2018;67:2244–53. doi: 10.1002/hep.29707. [DOI] [PubMed] [Google Scholar]
- 21.Ravi S, Axley P, Jones D, et al. Unusually High Rates of Hepatocellular Carcinoma After Treatment with Direct-Acting Antiviral Therapy for Hepatitis C Related Cirrhosis. Gastroenterology. 2017;152:911–2. doi: 10.1053/j.gastro.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 23.Kabakci Alagoz G, Karatayli SC, Karatayli E, et al. Hepatitis C virus genotype distribution in Turkey remains unchanged after a decade: performance of phylogenetic analysis of the NS5B, E1, and 5’UTR regions in genotyping efficiency. Turk J Gastroenterol. 2014;25:405–10. doi: 10.5152/tjg.2014.7083. [DOI] [PubMed] [Google Scholar]
- 24.Wedemeyer H, Craxi A, Zuckerman E, et al. Real-world effectiveness of ombitasvir/paritaprevir/ritonavir+/−dasabuvir+/−ribavirin in patients with hepatitis C virus genotype 1 or 4 infection: A meta-analysis. J Viral Hepat. 2017;24:936–43. doi: 10.1111/jvh.12722. [DOI] [PubMed] [Google Scholar]
- 25.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373:2618–28. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 27.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease. Gastroenterology. 2015;149:649–59. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Kim NJ, Locke CJ, Park H, Magee C, Bacchetti P, Khalili M. Race and Hepatitis C Care Continuum in an Underserved Birth Cohort. J Gen Intern Med. 2019;34:2005–13. doi: 10.1007/s11606-018-4649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verna EC, Morelli G, Terrault NA, et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol. 2020;73:540–8. doi: 10.1016/j.jhep.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Garg G, Dixit VK, Shukla SK, et al. Impact of Direct Acting Antiviral Drugs in Treatment Naive HCV Cirrhosis on Fibrosis and Severity of Liver Disease: A Real-Life Experience from a Tertiary Care Center of North India. J Clin Exp Hepatol. 2018;8:241–9. doi: 10.1016/j.jceh.2018.06.362. [DOI] [PMC free article] [PubMed] [Google Scholar]