Abstract
Background/Aims
Psychosocial and psychiatric evaluations are crucial components of the assessment of a live donor candidate. The Live Donor Assessment Tool (LDAT) was developed for this purpose. This study aims to evaluate the validity and reliability of the Turkish version of LDAT.
Materials and Methods
132 live kidney or liver donor were referred to assess their psychosocial/psychiatric appropriateness for donation and were randomized for clinical evaluation as usual or with LDAT. The internal consistency of LDAT was measured by Chronbach’s alpha coefficient. Inter-rater reliability was measured by using Spearman’s correlation coefficient. The potential validity of LDAT was assessed by comparing LDAT scores to clinical decisions. The Mann-Whitney U test was used to compare LDAT scores across two clinically classified groups (acceptable/declined). Logistic regression was performed using LDAT scores to predict the clinical decision.
Results
The Turkish version of LDAT items demonstrate good internal consistency (α=0.773). Inter-rater reliability of LDAT demonstrated strong correlation (ICC=0.72). LDAT scores differentiated the accepted/declined groups, and strongly predicted the clinical decision. With a cut-off score of 60.5, LDAT was found to have high sensitivity and specificity.
Conclusion
The Turkish version of LDAT was found to be a valid and reliable tool. LDAT could be an appropriate tool to assess live donor candidates.
Keywords: Donation, live donors, organ, psychosocial, reliability, validity
INTRODUCTION
Organ failure is a life-threatening condition. Today, organ transplantation is a method used in the treatment of this condition. Cadaveric and healthy living individuals are both sources for solid organ transplantations. Owing to the shortage of cadaveric donors, live donor transplantation has become an important option and even constitutes a majority of transplantations in some countries. The rate of live donor transplantations is approximately 25%, although there is great variability across countries. For example, in Turkey up to 80% of liver and 60% of kidney transplantations are from live donors (1,2).
Live donor candidates are evaluated by a multidisciplinary team for suitability in all organ transplantation centers to improve postdonation outcomes (3). One crucial component of evaluation is psychiatric and psychosocial assessment, with challenging features such as a limited time to administer and the decision of domains to cover. The current literature demonstrates that although the postoperative psychosocial status of donor candidates was similar to that of the healthy population (nondonors), postoperative suicide or serious psychiatric complications may occur. Most of these negative conditions were shown to be associated with the preoperative psychiatric status of the donor (4). Some donors experience familial conflicts, frustration, a deterioration in body image, and feelings of being neglected and unappreciated (3). Therefore, it is recommended that individuals be evaluated carefully before transplantation (5).
Taken together, the path to well-being and minimal negative outcomes after donation for live donors, who potentially sacrifice their own well-being for another person, begins with an appropriate preoperative evaluation of donors. In terms of psychiatric or psychosocial assessment, there are many limitations. To fill the gap in this area, Iacoviello et. al (6) developed the Live Donor Assessment Tool (LDAT). LDAT demonstrated good validity and reliability in retrospective and prospective studies (6,7). In a recent multicenter study, further support for the validity and reliability of LDAT was provided (8). In the current study, we aimed to examine the reliability and validity of a Turkish version of LDAT.
MATERIALS AND METHODS
Study Design, Setting, and Recruitment Sites
The study participants were live potential kidney or liver donors referred to the consultation and liaison psychiatry for evaluation of psychiatric comorbidities and appropriateness for donation from a transplantation unit. All participants who fulfilled the following inclusion criteria were recruited: they were more than 18 years of age, potential donors for kidney or liver transplantation, and willing to participate in the study.
The study received approval from the local ethics committee (reference no: 17-1050-17). A written and informed consent was obtained from each participant after a description of study.
Measurements
Sociodemographic and Clinical Data Collection
Sociodemographic variables (age, sex, place of birth, education, work status, and marital status) and clinical information (comorbidity, substance, alcohol and nicotine use, history of psychiatric therapy, and current use of psychiatric medication) were obtained from all participants.
Live Donor Assessment Tool
The LDAT is a semistructured psychosocial assessment tool for potential organ donors. It was developed by Iacoviello et al. (6) for the use of mental health workers and other clinicians involved in the process of psychosocial evaluation for organ transplantation. LDAT contains 29 items scored 0 to 3 or 0 to 2 across 9 domains. These domains are motivation for donation, knowledge about donation, relationship with the recipient, support available to the donor, feelings about donation, postdonation expectations, stability in life, psychiatric issues, and alcohol and substance use. The total score can range from 0 to 82, with higher scores indicating greater psychosocial appropriateness for donation. With a cutoff of 59 in the 2015 study (6), LDAT showed 86.6% specificity to categorize low- or moderate-risk groups and 84.6% sensitivity for declined or high-risk groups. The tool was found to have good reliability and validity.
The translation of the tool into Turkish was performed by psychiatrists, who were blind to each other. Subsequently, another psychiatrist translated the Turkish version back to English. The back translation was then reviewed, and a consensus was established.
For the investigation of the validity and reliability of the Turkish version of LDAT, we conducted clinical evaluation as usual and with LDAT. The participants were randomized for the order of evaluation (first clinical evaluation or application of LDAT), and evaluators were blind to the clinical decision or LDAT. Psychiatric and psychosocial evaluation was conducted by an experienced liaison psychiatrist with 2 possible clinical decisions for organ donation. Live donor candidates were labeled as “acceptable” or “declined” for psychiatric and psychosocial reasons by clinical evaluation as usual. LDAT was applied to all participants by trained clinicians blinded to any clinical decision. Both evaluations were applied consecutively on the same day. To assess interrater reliability, LDAT was applied twice by 2 independent clinicians who were blind to each other’s LDAT.
Statistical Analysis
Descriptive values were provided as mean±standard deviation, number (percentage), or median (range), depending on the variable. Sociodemographic variables included information about smoking, alcohol and substance history, closeness to recipient, employment status, and candidacy for liver/kidney donation.
The reliability of LDAT was assessed in 2 ways. The internal consistency of LDAT was measured by calculating the Cronbach’s alpha coefficient. Intrarater reliability was measured with the intraclass correlation coefficient (ICC). Interrater reliability was analyzed by using Spearman’s correlation coefficients for participants with 2 available LDAT scores.
The potential validity of LDAT was assessed by comparing LDAT scores with clinical decisions. The Mann-Whitney U test was used to compare LDAT scores across 2 clinically classified groups (“acceptable” and “declined” groups). In addition, univariate logistic regression analysis was performed using LDAT scores to predict the clinically classified binary clinical decisions.
Sensitivity and specificity were determined using the receiver operating characteristic (ROC) curve; the best cutoff score of LDAT was determined by Youden’s J index. Areas under the curve for ROC were presented with a 95% confidence interval (CI).
All statistical analyses were performed using the Statistical Packages for the Social Sciences (SPSS) statistics for Windows version 22.0 (IBM Corp.; Armonk, NY, USA). The statistical significance threshold was established at p<0.05.
RESULTS
A total of 132 participants were enrolled in our study. Sociodemographic and clinical variables of the participants are presented in Table 1. The mean age of participants was 40.08±11.68 years; 54.5% of donor candidates were male, and the mean year of education was 10.27±4.5. Liver donor candidates were statistically significantly younger (t=6.507, p<0.001), and tended to be male (χ2=4.067, p=0.044) and more educated (t=−4.804, p<0.001) compared with kidney donor candidates. Most participants were married (71.2%), without any statistically significant difference between groups (χ2=2.213, p=0.137). Liver and kidney donor candidates were 72% (n=95) and 28% (n=37) of the entire sample, respectively. The rate of closeness of transplant recipients was as follows: daughter, 4.5% (n=6); son, 15.9% (n=21); mother, 16.7% (n=22); father, 22.7% (n=30); brother/sister, 12.9% (n=17); spouse, 9.8% (n=13); other relatives, 9.8% (n=13); and nonrelatives, 7.6% (n=10). No statistically significant differences were found between liver and kidney donor candidates when compared between 3 groups (first-degree relative, other relative, and nonrelative) (χ2=4.683, p=0.125). Employment status of the participants were as follows: full-time employed, 62.1% (n=82); housewife, 20.5% (n=27); retired, 6.8% (n=9); unemployed, 6.8% (n=9); and student, 3.8% (n=5). The rate of full-time employment was statistically significantly higher in liver donor candidates (χ2=3.985, p=0.046). In our participants, the rates for medical comorbidity, smoking, alcohol use, and drug use were 18.9% (n=25), 40.2% (n=53), 15.2% (n=20), 0.8%, and (n=1), respectively. The rate of psychiatric treatment, previous use of psychotropic drugs, and current use of psychotropic drugs of donor candidates were 17.4% (n=23), 11.4% (n=15), and 2.3% (n=3), respectively. We removed item 28, because none of the donor candidates reported problematic cannabis use. Therefore, all analyses were performed on a total of 28 LDAT items, with possible scores ranging from 0 to 79. In our participants, the LDAT mean score was 63.3±7.48 (range: 32–74). The mean LDAT score was 64.89±1.01 for kidney and 62.67±7.88 for liver donor candidates, without any significant difference between groups (t=1.537, p=0.127). In our participants, the rate of declined candidates was 9.1% (n=12), all of whom were liver donor candidates. No kidney donor candidates were declined for psychiatric and psychosocial reasons in our participants, with a significant difference by Fisher’s exact test compared with liver donor candidates (p=0.023).
Table 1.
Sociodemographic and clinical variables of participants.
| All candidates (n=132) | Liver donor candidates (n=95) | Kidney donor candidates (n=37) | χ2, t, p | |
|---|---|---|---|---|
| Age | 40.08±11.68 | 36.48±9.82 | 49.32±11.07 | t=6.507, p<0.001 |
| Gender (male/female) | 54.5%/45.5% | 60%/40% | 40.5%59.5% | χ2=4.067, p=0.044 |
| Relationship status | ||||
| Married | 71.2% | 68% | 81.1% | χ2=2.213, p=0.137 |
| Single | 28.8% | 32% | 18.9% | |
| Education (years) | 10.27±4.5 | 11.36±4.01 | 7.49±4.53 | t=−4.804, p<0.001 |
| Donor candidate | ||||
| Liver | 72% | |||
| Kidney | 28% | |||
| Closeness to recipient | ||||
| First-degree relative | 82.6% | 81.5% | 91.9% | χ2=4.683, p=0.125 |
| Other relative | 9.8% | 7.6% | 8.1% | |
| Nonrelative | 7.6% | 10.9% | - | |
| Employment status | ||||
| Full-time employed | 62.1% | 48.9% | 29.7% | χ2=3.985, p=0.046 |
| LDAT score | 63.3±7.48 (32–74) | 62.67±7.88 | 64.89±1.01 | t=1.537, p=0.127 |
| Clinical decision | ||||
| Declined | 9.1% (n=12) | 12.6% (n=12) | 0% (n=0) | χ2=5.121, p=0.023 |
LDAT: Live Donor Assessment Tool.
Mean±standard deviation and number (percentage) are given as appropriate.
For categorical variables χ2 and for parametric analyses, Student t tests are used as appropriate.
p<0.05 is significant.
A comparison of declined and accepted donor candidate groups according to age, sex, relationship status, education (years), closeness to recipient, employment status, solid organ, and LDAT scores is presented in Table 2.
Table 2.
Sociodemographic and clinical variables of declined and accepted candidates.
| Declined candidates (n=12) | Accepted candidates (n=120) | χ2, U, p | |
|---|---|---|---|
| Age | 36.17±10.26 (median=37, 22–55) | 40.48±11.78 (median=39, 19–70) | U=70.5, p=0.236 |
| Gender (male/female) | 58.3%/41.7% | 54.2%/45.8% | χ2=0.176, p=0.782 |
| Relationship status | |||
| Married/single | 50%/50% | 73.9%/26.1% | χ2=3.085, p=0.079 |
| Education (years) | 8.67±4.6 (median=10, 0–16) | 10.43±4.47 (median=11, 0–18) | U=573, p=0.255 |
| Closeness to recipient | |||
| First-degree relatives | 72.7% | 85.6% | χ2=5.123, p=0.08 |
| Other relatives | 0% | 8.5% | |
| Nonrelative | 27.3% | 5.9% | |
| Solid organ | |||
| Liver | 100% | 69.2% | χ2=5.141, p=0.02 |
| Kidney | 0% | 30.8% | |
| Employment status | |||
| Full-time employed | 58.3% | 42.01% | χ2=1.181, p=0.277 |
| LDAT score | 49.08±9.59 (median=31.5, 32–60) | 64.72±5.55 (median=66, 48–74) | U=84.5, p<0.001 |
LDAT: Live Donor Assessment Tool.
Mean±standard deviation, median (min-max), and number (percentage) are given as appropriate.
For categorical variables χ2 and for nonparametric analyses, Mann-Whitney U test are used as appropriate.
p<0.05 is significant.
Reliability of Live Donor Assessment Tool
Internal consistency of LDAT was good, with α=0.773 (0.6≤α≤ 0.7, “acceptable”; 0.7≤α≤0.9, “good”; α≥0.9, “excellent”) (9). Intrarater reliability with a 2-way random effects model, where both people effects and measures effects are random, was ICC of 0.72. The correlation analysis for the 21 participants who were enrolled in 2 independent LDAT evaluations by blind raters was r=0.642 (p=0.002) (Figure 1).
Figure 1.
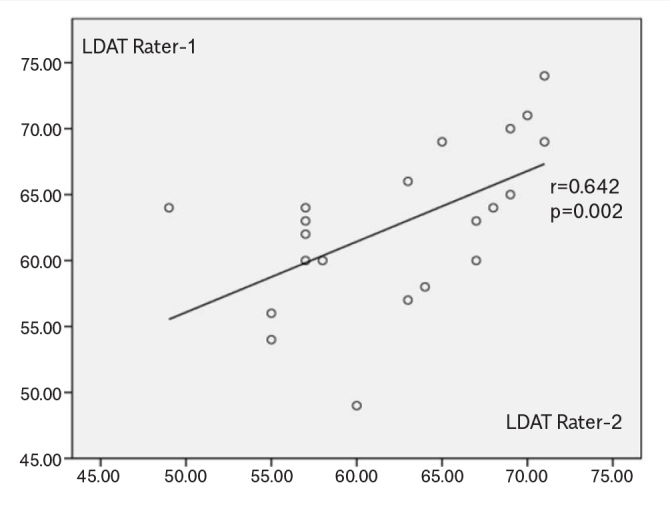
Scatter plot LDAT scores between raters.
Validity of Live Donor Assessment Tool
The rate of declined candidates was 9.1% (n=12). Owing to the skewed distribution of LDAT scores (Shapiro-Wilk test of departure from normality Shapiro-Wilk=0.151, p<0.001), we used the Mann-Whitney U test to compare clinical decision groups. The mean score of LDAT was 49.08±9.59 (median=51.5, 32–60) for declined candidates and 64.72±5.55 (median=66, 48–74) for accepted candidates, with statistically significant difference between groups (U=84.5, p<0.001).
A logistic regression analysis was performed to predict the clinical decision by using the LDAT score. The model was statistically significant (χ21=47.964, p<0.001), explained 54.3% (Nagelkerke R2) of the variance, and correctly classified 93.2% of cases (coefficients were B=−0.259, Exp(B)=0.772, CI=0.686–0.869).
Sensitivity and Specificity of Live Donor Assessment Tool
The area under the ROC curve was 0.941 (95% CI=0.895–0.988), and the cutoff value of 60.5 with a specificity of 79.2% and a sensitivity of 100% was determined by Youden’s J index. With a cutoff value of 59.5, the LDAT demonstrated a sensitivity of 84.2% and a specificity of 91.7%.
DISCUSSION
The increasing rate of live donor transplantations drives the need for a tool for psychosocial evaluation to assist in the decision making for transplantation centers. Recently, LDAT was developed to address the need to assess psychosocial factors in live donor candidates and can be used to quantify the psychosocial risk and assist in the decision of a live organ donor candidate (6). LDAT demonstrated good reliability and validity both in prospective and retrospective studies and also in a multicenter study (6–8). In the current study, the psychometric properties of the Turkish version of LDAT were investigated to assess validity and reliability.
The Turkish version of LDAT items demonstrates good internal consistency. The interrater reliability of LDAT demonstrated strong correlation. LDAT scores differentiated the accepted and declined groups and strongly predicted the clinical decision. With a cutoff score of 60.5, LDAT was found to have high sensitivity and specificity. Accordingly, the Turkish version of LDAT was found to be a reliable and valid tool.
There are several limitations in our study. First, our cross-sectional methodology prevents inference of conclusions about future psychiatric and/or medical morbidity/mortality. In a previous study, LDAT scores were reported to be significantly correlated to treatment adherence, but this was not assessed in the current study (7). Second, confirmatory factor analysis, an established method to assess validity, could not be used owing to the expected limitations of LDAT items. Third, liver donor candidates constitute the majority of our participants, which is slightly different from previous studies of LDAT and from the rates of live solid organ transplantations at other centers (7). Although the rate of declined candidates in this study is in line with previous studies, in this sample, all of the declined participants were liver donor candidates, which requires explanation. Perhaps the different sociodemographic profiles of candidates (such as younger age, higher education, male predominance, and employment status for liver donor candidates) contribute to this interesting finding. Fourth, we used mainly binary clinical decisions (accepted vs. declined) in this study, whereas 4-category clinical decisions were used in the previous study (7). We used binary clinical decisions because, in this hospital and in Turkey as a whole, 2-category clinical decisions are preferred. Fifth, although the raters of LDAT could be trained with easily accessible online education materials and the tool demonstrated strong interreliability, this finding should be interpreted cautiously. In this study, all raters of LDAT were experienced psychiatrists. Thus, the generalizability of these findings to other health practitioners such as nurses and social workers is unknown.
To the best of our knowledge, this is the first validity and reliability study of LDAT in a different language and culture than that in which it was developed, with promising results. LDAT should clearly contribute to the positive trend of live organ donation, an important source of donated organs for transplantation. LDAT may be used in routine clinical practice to assist clinicians’ decisions.
MAIN POINTS.
The Turkish version of the LDAT was found to be a valid and reliable tool.
This is the first validty and reliability study of LDAT outside the United States.
LDAT, with its proven validity and reliability across different countries and cultures, could be an appropriate tool to assist evaluation of liver donor candidates in other languages.
Footnotes
Ethics Committee Approval: Approval recieved from Ankara University Faculty of Medicine Human Research Ethical Commitee, 2017 (reference no: 17-1050-17).
Informed Consent: Written and informed consent was obtained from each participant after a brief description of study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.D., O.H., D.S.A., E.O.K.; Design - B.D, O.H.,D.S.A; Supervision A.F.K, A.T., K.K., M.K., D.B., H.K., B.M.I.; Resource H.K., B.D., D.B.; Materials – B.Ç., G.Ç, E.A.E, J.H.; Data Collection and/or Processing – O.H., D.S.A. E.A.E., J.H., B.Ç., G.Ç.; Analysis and/or Interpretation - B.D., O.H., B.D.E.; Literature Search – O.H., B.D., D.S.A.; Writing - B.D., O.H., B.Ç., E.O.K.; Critical Reviews – A.F.K., A.T., K.K., M.K., D.B., H.K., B.M.I..
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Turkey Organ Transplantation Foundation. 2002-2013 Organ Donation and Transplant Statistics. http://tonv.org.tr/admin/pages/files/TURKEY-2002-2013-ORGAN-DONATION-AND-TRANSPLANTATION-STATISTICS.pdf. Published 2014.
- 2.Organ Procurement and Transplantation Network. [Accessed January 2 2018]. https://optn.transplant.hrsa.gov/ Published 2018.
- 3.Massey EK, Timmerman L, Ismail SY, et al. The ELPAT living organ donor Psychosocial Assessment Tool (EPAT): from ‘what’ to ‘how’ of psychosocial screening - a pilot study. Transpl Int. 2018;31:56–70. doi: 10.1111/tri.13041. [DOI] [PubMed] [Google Scholar]
- 4.Trotter JF, Hill-Callahan MM, Gillespie BW, et al. Severe psychiatric problems in right hepatic lobe donors for living donor liver transplantation. Transplantation. 2007;83:1506–8. doi: 10.1097/01.tp.0000263343.21714.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad R, Kleiman A, Rambau S, et al. Psychosocial assessment of living kidney donors: What implications have temperament and character for decision-making? Compr Psychiatry. 2016;67:1–8. doi: 10.1016/j.comppsych.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Iacoviello BM, Shenoy A, Braoude J, et al. The Live Donor Assessment Tool: A Psychosocial Assessment Tool for Live Organ Donors. Psychosomatics. 2015;56:254–61. doi: 10.1016/j.psym.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Iacoviello BM, Shenoy A, Hunt J, Filipovic-Jewell Z, Haydel B, Rudow DL. A prospective study of the reliability and validity of the live donor assessment tool. Psychosomatics. 2017;58:519–26. doi: 10.1016/j.psym.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Kook Y, won A, Shenoy A, Hunt J, et al. Multicenter investigation of the reliability and validity of the live donor assessment tool as an enhancement to the psychosocial evaluation of living donors. Am J Transplant. 2019;19:1119–28. doi: 10.1111/ajt.15170. [DOI] [PubMed] [Google Scholar]
- 9.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. doi: 10.1007/BF02310555. [DOI] [Google Scholar]


