Abstract
Background/Aims
Nucleated red blood cell (NRBC) is an immature red blood cell, which can appear in the peripheral blood of newborns but not in normal adults. However, in the presence of hemorrhage, severe hypoxia, or severe infection, NRBCs may exist in adult blood and are associated with prognosis. The aims of this study were to establish a predictive model for the outcome of patients with severe acute pancreatitis (SAP) based on NRBCs.
Materials and Methods
Data from 92 patients with SAP were retrospectively collected for the study. We used chi-square automatic interaction detection (CHAID) to explore a prediction model of mortality in patients with SAP by NRBCs.
Results
During the 90-day follow-up, 11 participants (12.0%) died. The NRBC-positive rate of nonsurvivors was much higher than survivors (90.9% vs. 23.5%). Charlson Comorbidity Index (CCI), Acute Physiology and Chronic Health Evaluation II (APACHE II), Ranson score, and serum C-reactive protein were higher in nonsurvivors (5.0, 29.0, 6.0, and 140.0 g/L) than survivors (3.0, 13.0, 4.0, and 54.7 g/L). A CHAID model including NRBC, CCI, APACHE II score, and Ranson score showed that NRBCs differentiated well between nonsurvivors and survivors. All patients with SAP survived when they had a negative test result for NRBCs and CCI was below 7. All patients died when they had a positive test result for NRBCs and APACHE II score exceeded 30. Among patients whose NRBC test result was positive and APACHE II score was below 30, if the Ranson score was less than 5, the mortality rate was only 5.6%, whereas the mortality rate was 66.7% if the Ranson score exceeded 5. A validated population of 32 patients showed that the accuracy of the prediction model was 100%.
Conclusion
NRBC combined with CCI, APACHE II, and Ranson score can predict 90-day mortality of patients with SAP.
Keywords: Decision trees, erythroblasts, pancreatitis, mortality
INTRODUCTION
Severe acute pancreatitis (SAP) is a special type of acute pancreatitis (AP) with multiple complications and high mortality, accounting for 10% to 20% of the total AP (1). An estimated 70% to 80% of SAPs are caused by biliary tract diseases, alcoholism, and overeating. With the progress of surgical treatment of SAP, improved cure rate has been achieved; however, a high overall mortality rate up to 17% remains (2). Therefore, there is a certain clinical value to explore the risk factors in patients with SAP.
Nucleated red blood cells (NRBCs) are red blood cells in the early stage, which can appear in the periphery of newborns but not in adults. However, when erythropoiesis increases or bone marrow and blood barrier disorders such as proliferative anemia, hematological diseases, and malignant tumors occur, a positive test result for NRBCs in peripheral blood could be observed (3). In recent years, it has been found that the positive rate of NRBCs in critical patients is approximately 10% to 30%, which is related to an increased in-hospital mortality (4).
Chi-square automatic interaction detection (CHAID) is a common type of decision trees with the core idea of automatically grouping the multi-variables according to the significance of the chi-square test. CHAID can quickly and effectively discover the main factors. It can make it possible to not only process with the nonlinear and highly relevant data but also account for the missing values. Furthermore, CHAID is more effective than cross-contingency table analysis for classified or hierarchical data with more variables (5).
There are many risk factors for in-hospital mortality in critically ill patients, and the related indicators for predicting in-hospital outcomes can improve the prognosis of patients through certain interventions (4). Therefore, the purpose of this study is to construct a predictive model using NRBCs of outcomes of patients with SAP by decision tree–based CHAID, so as to provide a theoretical basis for clinicians to timely diagnose and treat patients with SAP, thereby improving their hospitalization outcomes.
MATERIALS AND METHODS
Research Aim and Outcome Measures
The aim of this research was to investigate the association of admission qualitative test of peripheral blood NRBCs and clinical outcomes in patients with SAP. The end point for this research was 90-day mortality.
Subjects and Design
A retrospective study was conducted in 92 patients with SAP in our hospital from January 2016 to February 2018. All candidates had to meet the SAP diagnostic criteria (6) of having the clinical manifestations and biochemical changes of AP and simultaneously one of the following: local complications (pancreatic necrosis, pseudocyst, and pancreatic abscess); organ failure; Ranson score ≥3; Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥8; Computed tomography (CT) grade of D or E. The exclusion criteria were as follows: patients with hemorrhagic diseases, tumors, end-stage renal disease, liver cirrhosis, hematological diseases, or blood transfusion in the recent 3 months were excluded. In addition, we excluded those lost to follow-up owing to transfer and incorrect contact method.
The participants were divided into survival group and nonsurvivor group according to their prognosis. All clinical data and laboratory indicators within 24 hours of admission were collected. After screening out the indicators by comparison between groups, decision tree–based CHAID was used to construct the outcome prediction model of patients with SAP. Clinical data and laboratory results of patients with SAP from March 2018 to November 2018 were further collected to validate the outcome prediction model. As a retrospective study, the institutional review board of Taizhou Hospital approved the study and waived the need for individual informed consent.
Specimen Collection
Fasting blood and urine samples were collected within 24 hours after admission. Fasting venous blood samples of patients with SAP were collected within 24 hours after admission, of which 2 mL was placed in the vacuum tube containing ethylenediaminetetraacetic acid-K2 for complete blood count (CBC) and NRBC qualitative test. Moreover, 5 mL was placed in the coagulant tube for 10 minutes at room temperature, followed by centrifugation for 5 minutes at 1000 g for serum separation, and the serum samples were used for the detection of C-reactive protein (CRP), procalcitonin (PCT), cancer antigen 199 (CA199), amylase (AMYL), and calcium (Ca); 5-mL urine collected in the morning was retained simultaneously for the determination of urinary AMYL.
Instruments and Reagents
CBC and NRBCs qualitative test were conducted according to the BC-6800 plus automatic blood cell counter (Mindray, Shenzhen, Guangdong, China). For qualitative test of NRBCs, if the result was positive, it had to be confirmed by microscopic examination. CRP was quantified by Immage 800 (Beckman Coulter, Brea, CA, USA). The performances of the PCT assays were conducted on the Cobas E601 (Roche, Switzerland). Serum AMYL, Ca, and urine AMYL were detected by the AU5800 automatic biochemical analyzer (Beckman Coulter, Brea, CA, USA). CA199 was detected by Architect-i2000 estradiol immunoassay (Abbott, Chicago, IL, USA). All tests were performed with corollary reagents.
Statistical Analysis
Continuous variables were expressed by medians and interquartile ranges. Categorical variables were expressed by frequencies and percentages. Comparisons of continuous variables between groups were performed using the Kruskal-Wallis test. Comparisons between groups for categorical variables were performed with a chi-square test. The outcome prediction model of patients with SAP was constructed by the decision tree–based CHAID. Tests were 2-tailed and performed at 5% significance levels. Statistical analyses were performed with the Statistical Package for Social Sciences (SPSS) Statistics for Windows version 19.0 (IBM Corp.; Armonk, NY, USA).
RESULTS
Baseline Data of the Patients
Comparisons between survivor patients with SAP (n=81) and nonsurvivor (n=11) on demographic and clinical characteristics are presented in Table 1. We did not find differences between survivor and nonsurvivor patients with SAP on age, sex, respiratory diseases, urinary system diseases, diabetes mellitus, surgical operation, bacteremia, and sepsis. However, we found that compared with survivor patients with SAP, nonsurvivors had a higher rate of cardiovascular and cerebrovascular diseases, mechanical ventilation, multiple infections, and septic shock and showed higher Charlson Comorbidity Index, Ranson score, and APACHE II scores.
Table 1.
Demographic and clinical characteristics of patients with SAP.
| Characteristic | Survivor (n=81) | Nonsurvivor (n=11) | p |
|---|---|---|---|
| Age (years) | 55.0 (41.5–70.0) | 63.0 (51.0–80.0) | 0.135 |
| Sex (male) | 52 (64.2%) | 5 (45.5%) | 0.322 |
| Complications | |||
| Respiratory diseases | 64 (79.0%) | 11 (100.0%) | 0.207 |
| Urinary system diseases | 41 (50.6%) | 9 (81.8%) | 0.104 |
| CCVD | 41 (50.6%) | 10 (90.9%) | 0.020 |
| Diabetes mellitus | 16 (19.8%) | 2 (18.2%) | 1.000 |
| CCI | 3.0 (2.0–5.0) | 5.0 (4.0–7.0) | 0.019 |
| Treatment | |||
| Surgical operation | 8 (9.9%) | 1 (9.1%) | 1.000 |
| Mechanical ventilation | 27 (33.3%) | 9 (81.8%) | 0.003 |
| Multiple infection | 48 (59.3%) | 10 (90.9%) | 0.049 |
| Bacteremia | 17 (21.0%) | 2 (18.2%) | 1.000 |
| Sepsis | 8 (9.9%) | 2(18.2%) | 0.342 |
| Septic shock | 9 (11.1%) | 5 (45.5%) | 0.011 |
| Ranson score | 4 (3–5) | 6 (4–6) | 0.003 |
| APACHE II score | 13.0 (10.0–18.0) | 29.0 (20.0–39.0) | 0.000 |
Data are presented as number (percentage) or median (interquartile range).
APACHE II: Acute Physiology and Chronic Health Evaluation II; CCI: Charlson Comorbidity Index; CCVD: cardiovascular and cerebrovascular disease; SAP: severe acute pancreatitis.
Laboratory Data of the Patients
Comparisons on laboratory indicators at admission of patients with SAP are presented in Table 2. We found that nonsurvivor patients with SAP showed higher CRP (p=0.021) and NRBC-positive rate (p<0.001) than survivors. However, we did not find statistically significant difference in inflammatory markers such as white blood cell count, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and PCT in nonsurvivor patients with SAP compared with survivors. Laboratory indicators related to SAP, for instance, serum AMYL, Ca, CA199, and urine AMYL, did not show any statistically significant difference.
Table 2.
Comparisons on laboratory indicators at admission of patients with SAP.
| Indicator | Survivor (n=81) | Nonsurvivor (n=11) | p |
|---|---|---|---|
| WBC (109/L) | 13.5 (9.9–18.2) | 12.5 (8.6–19.6) | 0.866 |
| NLR | 12.8 (7.7–20.6) | 16.7 (8.0–21.0) | 0.617 |
| MPV (fL) | 10.6 (9.8–11.4) | 9.8 (8.8–11.2) | 0.123 |
| PDW (%) | 13.9 (12.2–16.3) | 15.6 (13.7–16.1) | 0.441 |
| PLR | 236.4 (130.8–309.3) | 132.2 (115.0–448.0) | 0.497 |
| CRP (g/L) | 54.7 (25.6–89.3) | 140.0 (57.5–222.7) | 0.021 |
| PCT (ng/mL) | 1.06 (0.32–3.25) | 1.29 (0.28–8.59) | 0.674 |
| CRP/PCT | 81.9 (23.1–333.7) | 29.0 (3.5–82.0) | 0.055 |
| RBC (1012/L) | 4.56 (3.90–5.06) | 3.73 (3.54–4.93) | 0.136 |
| Hb (g/L) | 142 (122–156) | 117 (113–150) | 0.139 |
| RDW (%) | 13.7 (13.0–14.4) | 13.3 (12.5–14.0) | 0.233 |
| Serum AMYL (U/L) | 745 (324–1762) | 691 (403–1200) | 0.796 |
| Urine AMYL (U/L) | 1211 (323–2671) | 2708 (167–4110) | 1.000 |
| Ca (mmol/L) | 1.96 (1.75–2.06) | 1.96 (1.81–2.15) | 0.773 |
| CA199 (U/mL) | 19.4 (9.0–68.3) | 14.6 (12.6–88.7) | 0.783 |
| NRBC-positive rate | 19 (23.5%) | 10 (90.9%) | 0.000 |
AMYL: amylase; Ca: calcium; CA199: cancer antigen 199; CRP: C-reactive protein; Hb: hemoglobin; MPV: mean platelet volume; NLR: neutrophil-to-lymphocyte ratio; NRBC: nucleated red blood cell; PCT: procalcitonin; PDW: platelet distribution width; PLR: platelet-to-lymphocyte ratio; RBC: red blood cell; RDW: red cell distribution width; SAP: severe acute pancreatitis; WBC: white blood cell.
Bold text: p<0.05
Prediction Model of Outcomes of SAP Patients by CHAID
The prediction model of outcomes of patients with SAP by decision tree–based CHAID showed that 100% of SAP patients survived when they had a negative test result for NRBCs and CCI were lower than 7, whereas 100% died when NRBCs were positive and the APACHE II score was higher than 30. In patients with SAP with positive NRBC and simultaneously APACHE II score <30, if the Ranson score was lower than 5, the mortality rate was only 5.6%, and if the Ranson score was higher than 5, the mortality rate was up to 66.7% (Figure 1).
Figure 1.
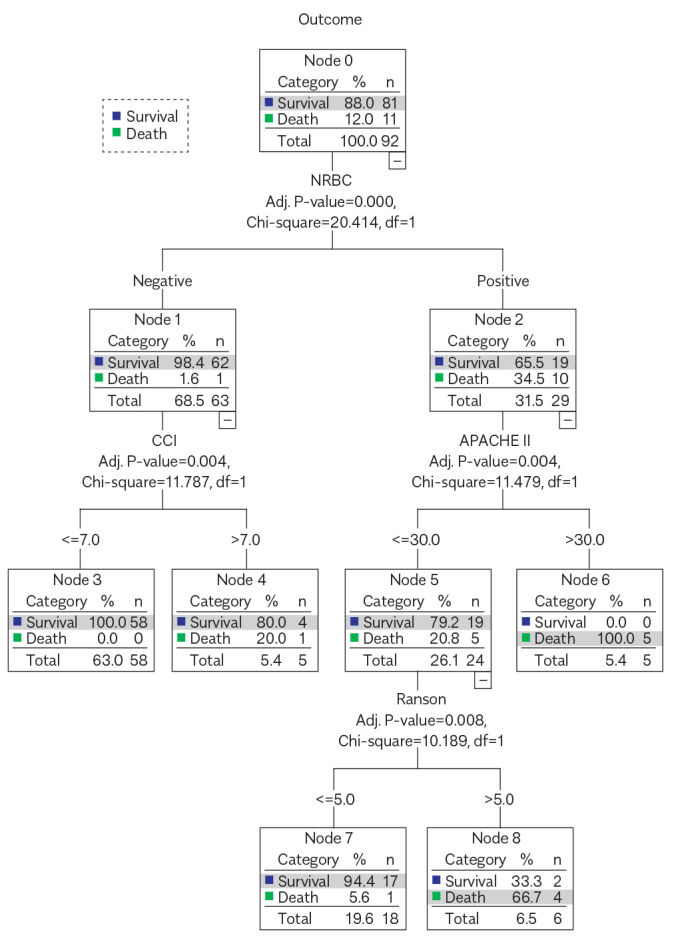
The depth of the decision tree was three levels. In the first level, patients were divided into two categories according to NRBC (parent node). In the second level, patients with a negative test result for NRBCs were divided into two categories according to CCI. According to APACHE II, patients with a positive test result for NRBCs were divided into two categories. Patients with APACHE II ≤ 30 in level 3 were divided into two categories according to Ranson score. Cutoff points of the second level and the third level were defined as child nodes, including CCI, APACHE II and Ranson score.
Outcome Verification
A total of 32 people were recruited to validate the outcome prediction model. In the verification group, 30 survived and 2 died (Table 3). The accuracy of the obtained prediction model was 100%.
Table 3.
Outcome discrimination of patients with SAP in a verified population.
| NRBC | Group | Survivor | Nonsurvivor | Total |
|---|---|---|---|---|
| NRBC negative | CCI ≤7 | 28 | 0 | 28 |
| CCI >7 | 1 | 0 | 1 | |
| Total | 29 | 0 | 29 | |
| NRBC positive | APACHE II ≥30 | 0 | 2 | 2 |
| APACHE II <30 and Ranson ≤5 | 1 | 0 | 1 | |
| APACHE II <30 and Ranson >5 | 0 | 0 | 0 | |
| Total | 1 | 2 | 3 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; CCI: Charlson Comorbidity Index; NRBC: nucleated red blood cell; SAP: severe acute pancreatitis.
DISCUSSION
To the best of our knowledge, this study is the first research on NRBCs from patients with SAP. The most interesting and novel findings of our study were that nonsurvivor patients with SAP showed a higher NRBC-positive rate than survivor patients.
Another highlight of this study is the use of decision tree–based CHAID to explore the predictive model for outcomes of patients with SAP. The traditional way of prognosis research is Cox regression model, which is an optimal method when the independent variables and dependent variables are linear. However, when they are nonlinear, the results obtained by Cox regression may be inaccurate. As a nonparametric and nonlinear research, decision tree–based CHAID can be conducted without considering the relationship between variables, and it is easier to identify interactions and subgroups. The potential advantage of decision tree–based CHAID is obtaining a better prognosis grouping via a combination of multiple factors (7).
The parent node shown in CHAID was the qualitative result of NRBCs. Patients with SAP could be divided into the NRBC-negative group and the NRBC-positive group. The 2 groups had different child nodes, including CCI in the NRBC-negative group and APACHE II score in the NRBC-positive group. Thus, the qualitative result of NRBCs was the most important factor on the outcome prediction in patients with SAP, followed by CCI and APACHE II scores, whereas the Ranson score was only helpful in predicting the outcome of patients with SAP with positive NRBC and APACHE II score <30.
Previous studies have found that the NRBC-positive rate in critical patients is approximately 10% to 30%, which was related to an increased in-hospital mortality (8–10). A novel finding of our current study was that the NRBC-positive rate of nonsurvivors was 90.9% compared with 23.5% of survivors. Another novel point of our study was about the association between 90-day mortality and qualitative test of NRBCs at admission.
NRBC is a newly discovered inflammatory marker. A series of immune-related biological processes such as phagocytosis, antigen presentation (11), and interleukin production (12, 13) have been reported to be associated with NRBCs. In vitro studies have shown that interleukin 6 could induce the differentiation of erythroid progenitor cells by induction of elevation of the erythropoietin, leading to an acute increase of NRBCs in the peripheral blood (14). Monterio et al. believed that the persistent existence of NRBCs in the peripheral blood of intensive care unit patients after treatment predicted a higher mortality rate (15). Even if symptoms were alleviated, patients should not be transferred to general wards. Desai et al. reported that the presence of NRBCs 3 weeks before death indicated that NRBC had a predictive value for prognosis (9). The conclusions of the abovementioned studies supported our findings.
Our study had some limitations. First, the small sample size could result in bias of the obtained results. Second, the retrospective property of this study could result in the failure to obtain the detailed information presented during the whole treatment and prognosis procedure. Third, the lack of the quantitative data of NRBCs could result in the failure to obtain the accurate cutoff value to discriminate the patients with good or poor outcome. Therefore, our data should be verified by a prospective study with a large number of patients in the near future.
In conclusion, the qualitative test of peripheral blood NRBCs combined with CCI and APACHE II score can predict outcomes of patients with SAP. This may be an efficient and accurate way for prognosis prediction in patients with SAP. Moreover, it can be helpful for the employment of early individualized treatment especially for patients with SAP with high mortality.
MAIN POINTS.
The presence of nucleated red blood cells (NRBCs) in peripheral blood is associated with mortality in patients with severe acute pancreatitis (SAP).
SAP patients had a positive test result for NRBCs and APACHE II score exceeded 30 were at high risk of death.
SAP patients with a negative test result for NRBCs and Charlson Comorbidity Index (CCI) below 7 had favorable prognosis of high survival rate.
Acknowledgements
We thank the Department of Gastroenterology of Taizhou hospital for their help in collecting SAP patients.
Footnotes
Ethics Committee Approval: Data collection and analysis of the cases in this study were approved by the ethics review committee of Taizhou hospital of Zhejiang Province. Decision date:December 19, 2019. Decision number: K20191203.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – C.X., J.W., G.L.; Design - C.X., J.W., G.L.; Supervision - G.L.; Resource - Y.Y.Y; Materials - Y.Y., G.L.; Data Collection and/or Processing - J.W., X.J.; Analysis and/or Interpretation - J.W., X.J.; Literature Search - J.W., X.J.; Writing - J.W.; Critical Reviews - C.X., G.L.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: This study was supported by grants from Taizhou Science and Technology Plan (1802ky18) (Zhejiang, China). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Yang L, Liu J, Xing Y, et al. Comparison of BISAP, Ranson, MCTSI, and APACHE II in Predicting Severity and Prognoses of Hyperlipidemic Acute Pancreatitis in Chinese Patients. Gastroent Res Pract. 2016;2016:1–7. doi: 10.1155/2016/8236367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susanta M, Subhadarshan MT, Kumar SP, Satyajit R, Rakesh S, Bikram R. Role of biomarkers in diagnosis and prognostic evaluation of acute pancreatitis. J Biomark. 2015;2015:1–13. doi: 10.1155/2015/519534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purtle SW, Horkan CM, Moromizato T, Gibbons FK, Christopher KB. Nucleated red blood cells critical illness survivors and postdischarge outcomes: A cohort study. Crit Care. 2017;21:154. doi: 10.1186/s13054-017-1724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stachon A, Segbers E, Holland-Letz T, Kempf R, Hering S. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: A prospective cohort study. Crit Care. 2007;11:R62. doi: 10.1186/cc5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi D, Takahashi O, Arioka H, Koga S, Fukui T. A prediction rule for the development of delirium among patients in medical wards: Chi-square automatic interaction detector (chaid) decision tree analysis model Am J Geriatr Psychiatry. 2013;21:957–62. doi: 10.1016/j.jagp.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG. Classification of acute pancreatitis--2012: Revision of the atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 7.Aviles-Jurado FX, Leon X. Prognostic factors in head and neck squamous cell carcinoma: Comparison of chaid decision trees technology and cox analysis. Head Neck. 2013;35:877–83. doi: 10.1002/hed.23058. [DOI] [PubMed] [Google Scholar]
- 8.Stachon A, Holland-Letz T, Kempf R. Poor prognosis indicated by nucleated red blood cells in peripheral blood is not associated with organ failure of the liver or kidney. Clin Chem Lab Med. 2006;44:955–61. doi: 10.1515/CCLM.2006.183. [DOI] [PubMed] [Google Scholar]
- 9.Desai S, Jones SL, Turner KL, Hall J. Nucleated red blood cells are associated with a higher mortality rate in patients with surgical sepsis. Surg Infect (Larchmt) 2012;13:360–5. doi: 10.1089/sur.2011.089. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro Junior JG, de Torres DO, da Silva MC, Ramos TMDB, Alves ML, Filho WJN. Nucleated red blood cells as predictors of all-cause mortality in cardiac intensive care unit patients: A prospective cohort study. PLoS One. 2015;10:e0144259. doi: 10.1371/journal.pone.0144259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passantino L, Altamura M, Cianciotta A, Patruno R, Passantino GF. Fish immunology. I. Binding and engulfment of candida albicans by erythrocytes of rainbow trout (salmo gairdneri richardson) Immunopharmacol Immunotoxicol. 2002;24:665–78. doi: 10.1081/IPH-120016050. [DOI] [PubMed] [Google Scholar]
- 12.Passantino L, Massaro MA, Jirillo F, Di Modugno D, Ribaud MR, Di Modugno G. Antigenically activated avian erythrocytes release cytokine-like factors: A conserved phylogenetic function discovered in fish. Immunopharmacol Immunotoxicol. 2007;29:141–52. doi: 10.1080/08923970701284664. [DOI] [PubMed] [Google Scholar]
- 13.Morera D, Roher N, Ribas L, Balasch JC, Carmen Doñate, Callol A. Rna-seq reveals an integrated immune response in nucleated erythrocytes. PLoS One. 2011;6:e26998. doi: 10.1371/journal.pone.0026998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stachon A, Bolulu O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock. 2005;24:34–39. doi: 10.1097/01.shk.0000164693.11649.91. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro JGM, Junior, Torres DOC, Clementino DSMCF, Holanda MCMD, Karina DSI, Mendon ADNME. Prognostic value of hematological parameters in patients with acute myocardial infarction: Intrahospital outcomes. PLoS One. 2018;13:e0194897. doi: 10.1371/journal.pone.0194897. [DOI] [PMC free article] [PubMed] [Google Scholar]


