Abstract
Background/Aims
This study aimed to explore the expression of long non-coding RNA MSC-AS1 in hepatocellular carcinoma (HCC) cells and its effect on the proliferation, migration, and apoptosis of HCC cells.
Materials and Methods
The expression of MSC-AS1 in HCC cell lines BEL7402, SMMC7721, Huh7, HepG2, MHCC97-H, and normal hepatocyte line L02 was detected by reverse transcriptase polymerase chain reaction. The HCC cells were divided into blank, negative control (NC)–small interfering RNA (siRNA) (transfected with negative siRNA), and MSC-AS1 siRNA (transfected with MSC-AS1 siRNA) groups. Cell counting kit-8 and colony formation assays were used to determine the proliferation, and cell apoptosis, migration, and invasion were detected by flow cytometry, wound healing, and transwell assays, respectively. Western blot was used to detect the expression of related proteins.
Results
The expression of MSC-AS1 in HCC cell lines was significantly higher than that in L02. In the MSC-AS1 siRNA group, the proliferation and colony formation of HCC cells were inhibited, whereas the apoptosis rate was significantly higher than that in the blank and NC-siRNA groups. The rate of wound healing and the number of invasion cells in the MSC-AS1 siRNA group were significantly lower than that in the blank and NC-siRNA groups.
Conclusion
MSC-AS1 was upregulated in HCC cells, and the downregulation of MSC-AS1 could inhibit cell proliferation, migration, and invasion and promote apoptosis of HCC cells.
Keywords: Carcinoma, hepatocellular, RNA, long noncoding, cells, apoptosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors of the digestive tract with high morbidity and mortality rates, which is a great menace to public health (1). The 2018 global cancer statistics has reported approximately 840 million newly diagnosed cases and 780 million deaths worldwide, of which approximately 75%–85% were because of HCC, and 50% of those cases and deaths were reported from China (2). Currently, the therapeutic effect and prognosis of HCC are not optimistic owing to recurrence and metastasis; therefore, an in-depth study of the mechanism of HCC cell proliferation and metastasis is of great significance for the treatment of HCC and the development of new therapeutic drugs.
Long non-coding RNA (lncRNA) is a type of non-coding RNA with a length longer than 200 nucleotides, and it regulates many gene expression levels through its interaction with other cellular macromolecules, including DNA, RNA, and proteins, at multiple levels (epigenetic, transcriptional, and post-transcriptional) (3). Multiple lncRNAs have been proven to be involved in cell proliferation, invasion, and prognosis of HCC, such as NEAT1 (4), UCA1 (5), CAMTA1 (6), and DILC (7). As a novel lncRNA, MSC-AS1 has been proven to be abnormally expressed in endometriosis, is associated with the pathogenesis of endometriosis (8), and might be a potential prognostic factor for HCC (9). However, there has been no detailed report about the role of MSC-AS1 in HCC and its effect on the biological behavior of HCC cells. This study aimed to explore the expression of MSC-AS1 in HCC cell lines and the effect of MSC-AS1 on the proliferation, apoptosis, and migration of HCC cells.
MATERIALS AND METHODS
Cell Culture
HCC cell lines BEL7402, SMMC7721, Huh7, HepG2, MHCC97-H, and human immortalized normal hepatocyte cell line L02 were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Roswell Park Memorial Institute-1640 medium (Thermo, USA) with 10% fetal bovine serum (FBS, Life Technologies, USA) and antibiotics, including 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, USA) at 37°C in a humidified incubator with 5% carbon dioxide (CO2). Cell passage was conducted at 80% confluence.
Groups and Transfection
MSC-AS1 small interfering RNA (siRNA) and negative control-siRNA (NC-siRNA) were purchased from GenePharma (Shanghai, China). The HCC cell lines Huh7 and MHCC97-H exhibiting the highest expression of MSC-AS1 were selected for further experiments. Huh7 and MHCC97-H cells at logarithmic phase were seeded into a 6-well plate (2×105/well) and cultured for 24 hours. The cells were grouped into the MSC-AS1-siRNA (transfected with MSC-AS1-siRNA), NC-siRNA (transfected with NC-siRNA), and blank (without any transfection) groups. When the cell confluence reached 60%, the cells were transfected according to the grouping and the instruction of liporfectamine 2000 (Invitrogen, USA). After transfection for 6–8 hours, the cells were further cultured in a new medium. The following experiments were conducted after transfection for 24–48 hours.
Quantitative Reverse Transcriptase Polymerase Chain Reaction
Total RNA was isolated using Trizol (Invitrogen, USA). SuperScript First Strand complementary DNA (cDNA) reverse transcription kit was used to synthesize cDNA according to the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (PCR) was performed using Applied Biosystem 7900HT (Thermo, USA) with the cDNA as template and GAPDH as internal reference. The special primer sequences was as follows: MSC-AS1, 5′-ACGTAGCCGTTCTCATAGCG-3′ (sense) and 5′-CCTTGGACGTGGCAGGTATT-3′ (anti-sense); GAPDH, 5′-GCTCTCGCTCCTCCTGTTC-3′ (sense) and 5′-ACGACCAAATCCGTTGACTC-3′ (anti-sense). The reaction conditions were 95°C for 10 minutes, 40 cycles of 95°C for 10 seconds, and 58°C for 20 seconds. The relative expression of MSC-AS1 was calculated by 2−ΔΔCt method.
Cell Counting Kit-8 and Colony Formation Assays
After transfection for 48 hours, the cell suspensions were seeded into 96-well plates at 1×104 cells/well, with 6 replicate wells per group. Cell counting kit-8 (CCK-8) solution (Beyotime, Shanghai, China) (10 μL) was added to each well at 0, 24, 48, and 72 hours. The absorbance at 450 nm was then measured after 2 hours incubation using a microplate reader (Thermo, USA), and the cell proliferation curves were plotted with time as the horizontal axis and light absorption as the vertical axis.
The transfected cells at logarithmic phase were seeded into the culture plate at a concentration of 200 cells/well. After 2 weeks of incubation with complete medium, the cells were washed with phosphate buffered saline (PBS), fixed with methanol for 15 minutes, and stained with Giemsa solution (Sigma, USA) for 15 minutes. The colonies that contained >50 cells were counted, and the total number of colonies in each group was quantified.
Flow Cytometry Assay
The cell apoptosis rates in MSC-AS1-siRNA, NC-siRNA, and blank groups were detected by flow cytometry (Becton Dickinson, USA) using double staining with Annexin V/propidium iodide (PI). The cells were collected, washed, with precooled PBS buffer and resuspended in binding buffer. Annexin V-fluorescein isothiocyanate (5 μL) was added, mixed, and stained for 15 minutes in the dark, and then PI was added, mixed, and stained for 5 minutes in the dark. The apoptosis rate was determined by CytoFLEX flow cytometry (Beckman, USA).
Wound Healing Assay
After transfection for 24 hours, the cells in the logarithmic phase were incubated into a 6-well plate (1×106 cells/well), and the cell confluence reached 80% after culturing for 24 hours. The wound was created by a 200 μL pipette, and the cell debris was washed with PBS. Fresh serum-free Dulbecco’s Modified Eagle’s medium (DMEM) was then added to each well for culture. The wound healing area was observed and photographed under an inverted microscope at 0 and 24 hours, and the rate of wound healing, which indicated the migration ability, was calculated by the Image J software using the following formula: wound healing rate=(scratch area at 0 hours−scratch area at 24 hours)/scratch area at 0 hours×100%. The experiment was repeated 3 times.
Transwell Assay
Matrigel was pre-melted, diluted with culture medium, and coated in the upper chamber of transwell chambers. The transfected cells were resuspended into serum-free DMEM, and the cell density was adjusted at 1×105 cells/mL. Cell suspension (200 μL) was added into the upper chamber, and 500 μL DMEM with 10% FBS was added into the lower chamber and cultured at 37°C with 5% CO2 for 24 hours. The cells that invaded into the lower chamber were fixed with methanol and stained with crystal violet. The invaded cells were observed and counted under a microscope from 5 random fields in each well.
Western Blot
Total protein was extracted from the cells using protein extraction kit, and the concentration was determined by the bicinchoninic acid assay. Protein (40 μg) was subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membrane and blocked by tris-buffered saline-Tween 20 with 5% skim milk. Primary antibodies: Ki67 (1:1000), PCNA (1:1000), MMP-9 (1:2000), MMP-2 (1:2000), Bax (1:1000), Bcl-2 (1:1000), and GAPDH (1:5000) (Abcam, USA) were incubated at 4°C overnight. Secondary antibodies were incubated at room temperature for 1 hour. After adding enhanced chemiluminescence detection reagent, the image was developed by Bio-Rad. The relative expression of target protein was calculated by the Image J software(National Institutes of Health, USA), with GAPDH as the internal reference.
Statistical Analysis
All data were analyzed using the Statistical Package for Social Sciences (SPSS) 19.0 (IBM Corp., Armonk, NY, USA). The data conformed to normal distribution by K-S test was denoted by x±s. The measurement data between the groups were compared using the t-test, and χ2 test was performed to detect the difference between 2 groups of enumeration data. p value <0.05, indicating the difference, was considered statistically significant.
RESULTS
Expression of MSC-AS1 in Hepatocellular Carcinoma Cell Lines
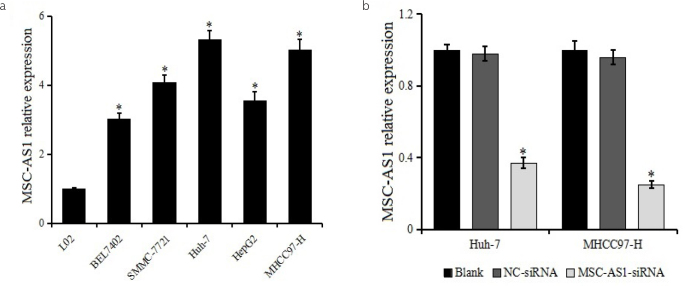
The relative expression of MSC-AS1 in HCC cell lines and normal hepatocyte cell line L02 was determined by reverse transcriptase (RT)-PCR (Figure 1a). The MSC-AS1 expression was upregulated in the HCC cell lines BEL7402, SMMC7721, Huh7, HepG2, MHCC97-H, which was higher than that in L02. Huh7 and MHCC97-H cell lines were selected for follow-up experiments because of the highest expression of MSC-AS1.
Figure 1. a, b.
MSC-AS1 expression in hepatocellular carcinoma (HCC) cell lines. (a) Expression of MSC-AS1 in HCC cell lines BEL7402, SMMC7721, Huh7, HepG2, MHCC97-H and normal hepatocyte line L02 is detected by quantitative reverse transcriptase polymerase chain reaction. *p<0.05 vs. L02 (b) Expression of MSC-AS1 in Huh7 and MHCC97-H after transfection. *p<0.05 vs. blank and negative control-small interfering RNA groups.
Detection of Transfection Effect
MSC-AS1 expression in Huh7 and MHCC97-H transfected with MSC-AS1 siRNA or NC-siRNA was detected by RT-PCR to detect the transfection effect. The result is shown in Figure 1b. The MSC-AS1 expression was significantly reduced following transfection with MSC-AS1 siRNA, indicating that MSC-AS1 siRNA inhibited the expression of MSC-AS1 in HCC cell lines Huh7 and MHCC97-H.
Effect of MSC-AS1 siRNA on the Proliferation of Hepatocellular Carcinoma Cells
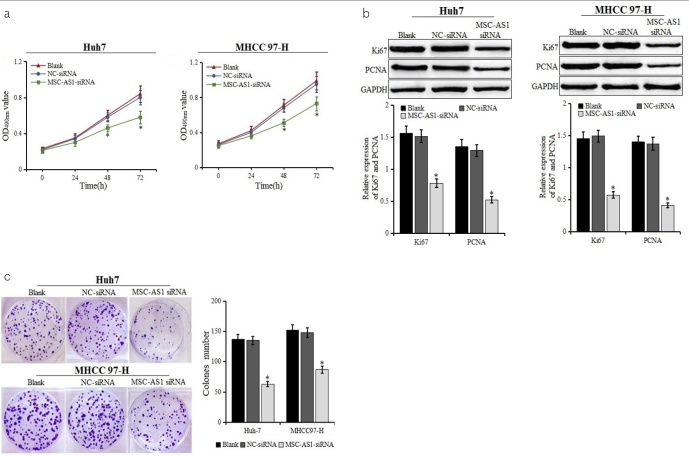
The effect of MSC-AS1 siRNA on the proliferation of HCC cells was determined by CCK-8 and colony formation assays (Figures 2a and 2c). After the transfection with MSC-AS1 siRNA for 24 hours, the proliferation of Huh7 and MHCC97-H were significantly inhibited (p<0.05), and there were smaller and fewer clones formed by Huh7 and MHCC97-H cells. Western blot was used to further detect the expression of proliferation markers Ki67 and PCNA (Figure 2b). It was found that the expression of Ki67 and PCNA in the MSC-AS1 siRNA group was significantly lower than those in the blank and NC-siRNA groups. The results suggested that the downregulation of MSC-AS1 could significantly inhibit the proliferation and colony formation of HCC cell lines Huh7 and MHCC97-H.
Figure 2. a–c.
Cell proliferation and colony formation after transfection. (a) Proliferation of Huh7 and MHCC97-H cells transfected with MSC-AS1 small interfering (si) RNA or negative control (NC)-siRNA examined using cell counting kit-8 assays. (b) Expression of cell proliferation marker Ki67 and PCNA detected by western blot.
*p<0.05 vs. blank and NC-siRNA groups. (c) Colony formation of Huh7 and MHCC97-H cells transfected with MSC-AS1 siRNA or NC-siRNA examined. *p<0.05 vs. blank and NC-siRNA groups
Effect of MSC-AS1 siRNA on the Apoptosis of Hepatocellular Carcinoma Cells
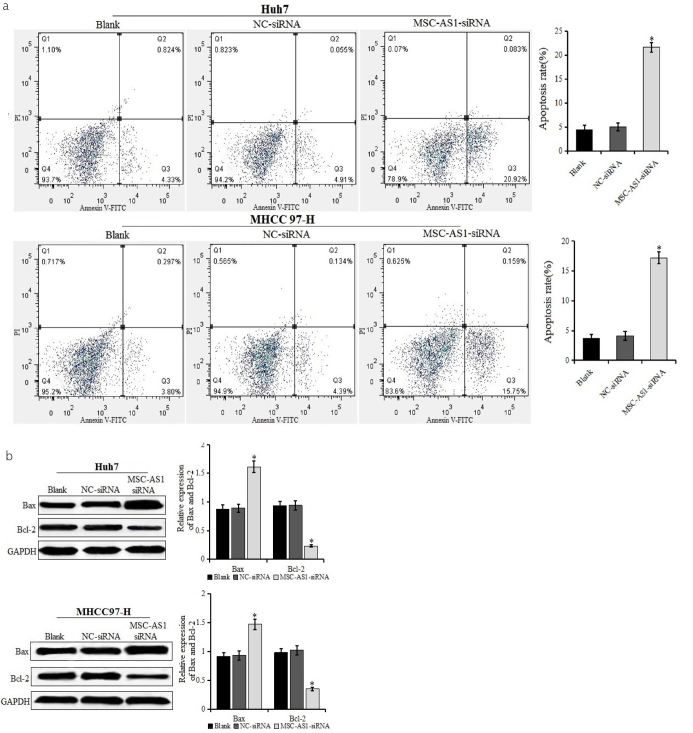
Annexin V/PI staining assay was used to detect the effect of MSC-AS1 siRNA on the apoptosis of HCC cell lines Huh7 and MHCC97-H (Figure 3a). The apoptosis rate in the MSC-AS1 siRNA group was significantly higher than that in the blank and NC-siRNA groups, and there was no significant difference between the blank and NC-siRNA groups. The results of western blot also showed that compared with the blank and NC-siRNA groups, the expression of Bax was upregulated, whereas the expression of Bcl-2 was decreased in the MSC-AS1 siRNA group, and the difference was statistically significant (Figure 3b). The results indicated that MSC-AS1 siRNA promoted the apoptosis of HCC cell lines Huh7 and MHCC97-H.
Figure 3. a, b.
Rate of apoptosis in cells after transfection. (a) Rate of apoptosis of Huh7 and MHCC97-H cells transfected with MSC-AS1 small interfering (si)RNA or negative control (NC)-siRNA examined using Annexin V/propidium iodide double staining by flow cytometry. (b) Expression of apoptosis-related factors Bax and Bcl-2 in Huh7 and MHCC97-H cells transfected with MSC-AS1 siRNA or NC-siRNA detected by western blot. *p<0.05 vs. blank and NC-siRNA groups
Effect of MSC-AS1 siRNA on Migration and Invasion of Hepatocellular Carcinoma Cells
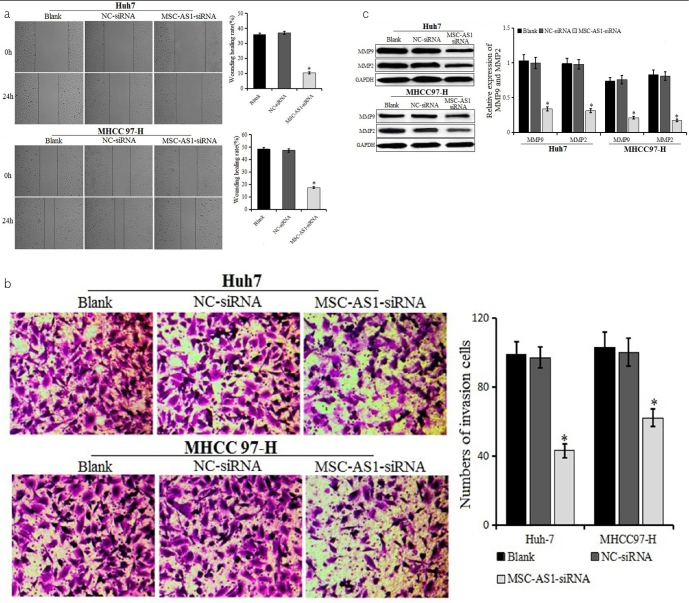
Wound healing and transwell assays were used to detect the effect of MSC-AS1 siRNA on the migration and invasion of HCC cells (Figure 4a and b). The rate of wound healing in the MSC-AS1 siRNA group was lower than that in the blank and NC-siRNA groups, and the number of invasion cells in the MSC-AS1 siRNA group was significantly lower than that in the blank and NC-siRNA groups, suggesting that MSC-AS1 siRNA could inhibit the migration and invasion of Huh7 and MHCC97-H cell lines. The marker proteins of migration and invasion, MMP2 and MMP9, respectively, were detected by western blot after transfection (Figure 4c). Compared with the blank and NC-siRNA groups, the expression of MMP2 and MMP9 was decreased in the MSC-AS1 siRNA group, with a significant difference. The results proved that the downregulation of MSC-AS1 could inhibit the migration and invasion of Huh7 and MHCC97-H cells.
Figure 4. a–c.
Migration and invasion of cells after transfection. (a) Migration of Huh7 and MHCC97-H cells transfected with MSC-AS1 small interfering (si)RNA or negative control (NC)-siRNA examined by wound healing test (100×). (b) Invasion of Huh7 and MHCC97-H cells transfected with MSC-AS1 siRNA or NC-siRNA examined by transwell assay (200×). (c) Expression of MMP9 and MMP2 in Huh7 and MHCC97-H cells transfected with MSC-AS1 siRNA or NC-siRNA detected by western blot. *p<0.05 vs. blank and NC-siRNA groups
DISCUSSION
HCC is a type of cancer with the presence of a highly malignant tumor, with high morbidity and mortality rates, and has an insidious onset and metastatic characteristics, which result in the poor treatment efficacy and prognosis (10). It has been reported that the 5-year survival rate of HCC was only 3%–5% (11). Therefore, finding new molecular therapeutic targets for diagnosis, treatment, and prognosis is critical to improve the poor prognosis of HCC.
It has been widely known that lncRNA is uniquely expressed in specific cancer types, and its abnormal expression is involved in the occurrence and development of tumors (12). Recently, the role of lncRNA and its possible molecular mechanism in the development of HCC has been widely studied; for example, Zhan et al. (13) have found that the expression of HOXA11-AS is upregulated in the HCC tissues and cell lines, and it promoted HCC proliferation and invasion and induced epithelial-mesenchymal transition (EMT) by downregulating miR-214-3p, suggesting that HOXA11-AS/miR-214-3p axis is involved in the development of HCC. Another study has indicated that HOXA11-AS, as an oncogene, promotes HCC cell proliferation and regulated cell cycle and apoptosis by suppressing the transcription of DUSP5 (14). Guo et al. (15) have found that PVT1 is upregulated in HCC and promotes propagation and inhibits apoptosis of HCC cells by recruiting EZH2 to stabilize the expression of MDM2 protein and restrain the expression of P53. High expression of HULC in HCC tissues promotes the proliferation and migration of HCC by targeting multiple micro RNAs and target genes, which may act as a novel biomarker for the diagnosis of HCC (16–18). MSC-AS1 is a newly discovered lncRNA, which might be involved into HCC. Zhang et al. (19) have analyzed The Cancer Genome Atlas (TCGA) HCC data and identified a spectrum of differentially expressed lncRNAs related to the EMT of HCC containing MSC-AS1, suggesting that it might be involved in the metastasis of HCC. Similarly, Gu et al. (9) have found that 6 lncRNAs (MSC-AS1, POLR2J4, EIF3J-AS1, PVT1, RMST, and SERHL) were abnormally expressed in HCC and predicted that these lncRNAs were significantly associated with the recurrence-free survival of HCC through analyzing the Gene Expression Omnibus and TCGA databases. However, there are no detailed reports on the relationship between MSC-AS1 and HCC, including the 2 reports. Sun et al. (20) have found that the expression of MSC-AS1 is upregulated in pancreatic ductal adenocarcinoma (PDAC) and correlated with poor prognosis demonstrating that MSC-AS1/miR-29b-3p axis regulated cell proliferation and induced apoptosis in PDAC cell lines by suppressing CDK14 in vitro. In this study, the expression of MSC-AS1 was more upregulated in HCC cell lines BEL7402, SMMC-7721, Huh7, HepG2, and MHCC97-H than in L02 cells, suggesting that MSC-AS1 might act as an oncogene in the development of HCC. The expression of MSC-AS1 in the Huh7 and MHCC97-H cells was the highest among the HCC cell lines, and both Huh7 and MHCC97-H cells might be related to the metastasis of HCC; hence, it was speculated that MSC-AS1 might be associated with the metastasis of HCC. Furthermore, the authors have found that the downregulation of MSC-AS1 could inhibit cell proliferation, migration, and invasion and induce apoptosis of HCC cells. Ki67 and PCNA, as the key proteins related to cell proliferation, had been used in the diagnosis of various tumor cell proliferations (21, 22). Western blot was used to analyze the expression of proteins related to proliferation, metastasis, and apoptosis and showed that MSC-AS1 siRNA can downregulate the expression of Ki67, PCNA, MMP2, MMP9, and Bcl2 and upregulate the expression of Bax. The results indicated that MSC-AS1 can promote the proliferation, migration, and invasion and inhibit apoptosis of HCC cells, which might play a carcinogenic role in HCC.
In conclusion, the expression of MSC-AS1 was upregulated in HCC cells; the downregulation of MSC-AS1 could inhibit the proliferation, migration, and invasion and promote apoptosis of HCC cells. However, this study was only conducted at a cell level in vitro and therefore had some limitations. The association between MSC-AS1 and clinicopathological parameters and prognosis of HCC was not analyzed, and the role of MSC-AS1 in vivo and the detailed molecular mechanism of MSC-AS1 in HCC also need to be further studied.
MAIN POINTS.
MSC-AS1 was up-regulated in hepatocellular carcinoma cells.
CCK-8 and colony formation assays showed that MSC-AS1 siRNA inhibited the cell proliferation, migration and invasion of HCC cells.
Down-regulation of MSC-AS1 promoted apoptosis of HCC cells.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – X.K., J.Z., Y.Z.; Design - X.K., J.Z., Y.Z.; Supervision - X.X., Y.Z.; Resource - X.K., J.Z.; Materials - X.K.; Data Collection and/or Processing - X.X., M.H.; Analysis and/or Interpretation - X.X., M.H.; Literature Search - X.K., J.Z.; Writing - X.K., J.Z.; Critical Reviews - X.X., M.H., Y.Z.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This study was supported by Shaanxi Province Social Development key project (Grant NO. 2018SF-295).
REFERENCES
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Kopp F, Mendell JT. Functional Classification and Experimental Dissection of Long Noncoding RNAs. CELL. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang L, Sun J, Pan Z, et al. Long non-coding RNA NEAT1 promotes hepatocellular carcinoma cell proliferation through the regulation of miR-129-5p-VCP-IkappaB. Am J Physiol Gastrointest Liver Physiol. 2017;313:G150–6. doi: 10.1152/ajpgi.00426.2016. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Ying HQ, He BS, et al. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6:7899–917. doi: 10.18632/oncotarget.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding LJ, Li Y, Wang SD, et al. Long Noncoding RNA lncCAMTA1 Promotes Proliferation and Cancer Stem Cell-Like Properties of Liver Cancer by Inhibiting CAMTA1. INT J MOL SCI. 2016;17:1617. doi: 10.3390/ijms17101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Sun W, Shen W, et al. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J HEPATOL. 2016;64:1283–94. doi: 10.1016/j.jhep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Wang Q, Zhang R, Zhang C, Lin J, Huang X. Identification of LINC01279 as a cell cycleassociated long noncoding RNA in endometriosis with GBA analysis. MOL MED REP. 2018;18:3850–8. doi: 10.3892/mmr.2018.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu JX, Zhang X, Miao RC, et al. Six-long non-coding RNA signature predicts recurrence-free survival in hepatocellular carcinoma. World J Gastroenterol. 2019;25:220–32. doi: 10.3748/wjg.v25.i2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waly RS, Yangde Z, Yuxiang C. Hepatocellular carcinoma: focus on different aspects of management. ISRN Oncol. 2012;2012 doi: 10.5402/2012/421673. 421673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu WB, Rao A, Vu V, Xu L, Rao JY, Wu JX. Management of centrally located hepatocellular carcinoma: Update 2016. World J Hepatol. 2017;9:627–34. doi: 10.4254/wjh.v9.i13.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt AM, Chang HY. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29:452–63. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan M, He K, Xiao J, et al. LncRNA HOXA11-AS promotes hepatocellular carcinoma progression by repressing miR-214-3p. J Cell Mol Med. 2018;22:3758–67. doi: 10.1111/jcmm.13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Li J, Liu X, et al. Long non-coding RNA HOXA11-AS promotes the proliferation HCC cells by epigenetically silencing DUSP5. Oncotarget. 2017;8:109509–21. doi: 10.18632/oncotarget.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J, Hao C, Wang C, Li L. Long noncoding RNA PVT1 modulates hepatocellular carcinoma cell proliferation and apoptosis by recruiting EZH2. Cancer Cell Int. 2018;18:98. doi: 10.1186/s12935-018-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong H, Ni Z, He J, et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017;36:3528–40. doi: 10.1038/onc.2016.521. [DOI] [PubMed] [Google Scholar]
- 17.Hu D, Shen D, Zhang M, et al. MiR-488 suppresses cell proliferation and invasion by targeting ADAM9 and lncRNA HULC in hepatocellular carcinoma. Am J Cancer Res. 2017;7:2070–80. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Li J, Wang X, Tang J, et al. HULC and Linc00152 Act as Novel Biomarkers in Predicting Diagnosis of Hepatocellular Carcinoma. Cell Physiol Biochem. 2015;37:687–96. doi: 10.1159/000430387. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Wang S, Liu W. EMT-related long non-coding RNA in hepatocellular carcinoma: A study with TCGA database. Biochem Biophys Res Commun. 2018;503:1530–6. doi: 10.1016/j.bbrc.2018.07.075. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Wang P, Yang W, Shan Y, Zhang Q, Wu H. The role of lncRNA MSC-AS1/miR-29b-3p axis-mediated CDK14 modulation in pancreatic cancer proliferation and Gemcitabine-induced apoptosis. Cancer Biol Ther. 2019;20:729–39. doi: 10.1080/15384047.2018.1529121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surowiak P, Pudelko M, Maciejczyk A, Dziegiel P, Wojnar A, Zabel M. The relationship of the expression of proliferetron--related antigens Ki67 and PCNA in the cells of ductal breast cancer with the differentiation grade. Ginekol Pol. 2005;76:9–14. [PubMed] [Google Scholar]
- 22.Kanitakis J, Narvaez D, Euvrard S, Faure M, Claudy A. Proliferation markers Ki67 and PCNA in cutaneous squamous cell carcinomas: lack of prognostic value. Br J Dermatol. 1997;136:643–4. doi: 10.1111/j.1365-2133.1997.tb02173.x. [DOI] [PubMed] [Google Scholar]