Abstract
Objective:
To estimate the frequency of chronic joint pain after infection with chikungunya virus in a Latin American cohort.
Methods:
A cross sectional follow-up of a prospective cohort of 500 Chikungunya patients from Atlántico Department, Colombia clinically diagnosed with chikungunya during the 2014–2015 Colombian epidemic. Baseline and follow-up (20-months) symptoms were evaluated in serologically confirmed cases.
Results:
Among 500 patients enrolled, 485 cases were serologically confirmed with chikungunya. Patients were predominantly adults (age 49 ± 16 years), female, had a high school or less level of education and were of mestizo ethnicity. The most commonly affected joints were the small joints including the wrists, ankles and fingers. The initial joint pain lasted a median of 4 days (IQR 3–8). Sixteen percent of participants reported missing a median of 4 days (IQR 2–7) of school or work. After 20-months, one fourth of the participants had persistent joint pain. A multivariable analysis indicated that significant predictors of persistent joint pain included college graduate status, initial symptoms of headache or knee pain, missed work, normal activities affected, 4 or more days of initial symptoms, and 4 or more weeks of initial pain.
Conclusions:
This is the first report to describe the frequency of chikungunya-related arthritis in the Americas after a 20-month follow-up. The high frequency of chronic disease highlights the importance of development of prevention and treatment interventions.
Introduction
Chikungunya virus (CHIKV) fever is a mosquito-borne illness that can lead to chronic joint pain and arthritis (1). Acute infection presents with fever, headache, muscle pain, rash, and joint pain. Prior outbreaks have been reported in Africa, Asia, Europe, and the Indian and Pacific Ocean islands (2). In 2013, CHIKV was found for the first time in the Caribbean basin and has now infected over 1.2 million people throughout the Americas (3). Studies prior to the American epidemics have reported 30% to 70% of CHIKV-infected patients have persistent joint pains months or years after their acute illness (1,3–14). Until now there have been no large-scale observational studies of the frequency of CHIKV arthritis in the Americas. It is estimated that approximately 48% of Latin American patients after a median of 20-months post-CHIKV infection will develop chronic chikungunya arthritis (15).
With the exception of the Andes Mountains region, most of Colombia has an elevation less than 1000 meters and is thus favorable for the proliferation of Aedes aegypti, the mosquito vector of Chikungunya in the Americas. Thus, much of the population is vulnerable to infection with this virus. From July 2014 to January 2015, 113,000 cases of chikungunya were reported in Colombia (16).
There has been little description of the frequency of chronic arthritis in the Americas. One study of 39 Colombian chikungunya patients, ranging from 6 to 65 weeks post-infection, found that 90% had persistent polyarthralgias or arthritis at the time of evaluation (17). The primary objective of the current study was to describe the frequency of persistent joint pain and disability in a Latin American cohort of chikungunya patients from Colombia. Our hypothesis is that chronic joint pain will be present in one third of our Latin American cohort, which is similar to findings reported from other outbreaks of the Asian strain of the virus at 18-months (5,18). Defining the frequency of chronic joint pain and disability after chikungunya infection is important to understanding the long-term impact of the American outbreak.
Patients and Methods
Study design.
Five hundred patients with clinically confirmed chikungunya infection were enrolled as part of a prospective cohort in January 2015. CHIKV diagnosis was serologically confirmed via IgM and IgG antibody capture enzyme-linked immunosorbent assay (see below). A baseline 33-item survey for demographic, exposure history, and symptoms was applied. A subsequent 56-item telephone survey was performed at a median of 20-months post-infection and included an assessment of the character and duration of persistent CHIKV arthritis symptoms, including swollen joint count, tender joint count, comorbidities, lost work or school attendance, and a global pain score during the last week from the Disease Activity Score-28 (DAS-28) (19), as well as therapies administered.
Setting.
Patients were referred into the study from Sabanalarga, Baranquilla, Juan de Acosta, Manatí, Luruaco, and Baranoa municipalities, located in the Atlántico Department, Colombia which is located on the Caribbean costal plane (20).
Participants.
Primary care providers referred patients with clinically suspected chikungunya for enrollment. Clinical chikungunya was defined by the Colombian Institute of Health as a patient presenting with fever greater than 38°C, severe joint pain or arthritis and acute onset of erythema multiforme, with symptoms not explained by other medical conditions, and residing or having visited a municipality with evidence of CHIKV transmission or located in a municipality within 30 kilometers of confirmed viral transmission (21). Clinically suspected cases of CHIKV were confirmed serologically for the purposes of this study.
Variables.
Demographic factors collected included age, gender, ethnicity, education level, and insurance status. Outcomes were assessed at the follow-up survey. The primary outcome was the percent individuals with self-reported persistent CHIKV-related joint pain at follow-up approximately 20-month post-infection. Secondary self-reported outcomes included the duration of initial joint pain as many individuals describe relapsing remitting symptoms after initial infection, percentage of individuals who missed work or school, the median days of missed work or school, the percentage whose symptoms impacted their capacity to continue normal activity, and an estimate of disease severity. The latter includes elements of the Disease Activity Score-28 (DAS-28) measure, including mean swollen joint count, mean tender joint count, and mean global pain score (19). The full DAS-28 could not be calculated because this measure includes C-reactive protein, and no follow up blood draw was performed. Potential effect modifiers include medical comorbidities such as chronic arthritis, gout, osteoarthritis, ischemic heart disease, kidney disease, lung disease, diabetes, hypertension, and depression. Finally, analysis includes the types of therapies used for CHIKV arthritis including aspirin, ibuprofen, acetaminophen, prednisone, methotrexate, medicinal plants or other modalities.
Anti-CHIKV IgG and IgM.
IgG and IgM levels were assayed using the Euroimmun (Luebeck, Germany) Anti-Chikungunya Virus ELISA (IgG / IgM) as per the manufacturer’s instructions. These assays provide a qualitative evaluation of the presence or absence of anti-CHIKV IgG and IgM.
Statistical methods.
All 500 cases were contacted for the follow-up telephonic survey. Excluded cases (n =15) had no serologic confirmation of chikungunya or were missing data for current joint pain status. Variable distributions were examined for normality and outliers. Continuous variables were log-transformed if necessary. Univariable associations between patient variables and the presence of persistent joint pain, and between initial symptoms and sex, were tested using chi-square or Fisher’s Exact test for categorical variables and t-test or the Kruskal-Wallis test for continuous variables. A multivariable logistic regression model for persistent joint pain was tested using as factors any baseline variable that was significantly associated with persistent joint pain. Backward selection was used, dropping predictors that had p> 0.20. SAS (version 9.3, Cary, NC) was used for data analysis with p< 0.05 considered significant except for values where Bonferroni correction was applied in Table 2 (p <0.002), Table 3 (p<0.0017), and Table 4 (p<0.00167) were considered significant.
Table 2. Initial chikugunya symptoms and treatment of serologically confirmed chikungunya patients, by persistent joint pain at median 20-month follow-up.
Significant p-values after Bonferroni-adjustment are in bold.
| Characteristic n (%) | All serologically confirmed cases (n= 485) | Persistent Joint Pain (n= 123) | No Persistent Joint Pain (n= 362) | p-value |
|---|---|---|---|---|
| Initial Symptoms | ||||
| Muscle pain | 471 (98%) | 119 (98%) | 352 (98%) | 0.99 |
| Weakness | 427 (88%) | 114 (93%) | 313 (86%) | 0.09 |
| Joint pain and/or inflammation | 476 (98%) | 120 (98%) | 356 (98%) | 0.70 |
| Rash | 409 (85%) | 104 (85%) | 305 (84%) | 0.99 |
| Fever | 376 (79%) | 99 (83%) | 277 (78%) | 0.28 |
| Headache | 354 (73%) | 101 (82%) | 253 (70%) | 0.009 |
| Lymphadenopathy | 343 (71%) | 90 (73%) | 253 (70%) | 0.52 |
| Cool extremities | 257 (53%) | 68 (55%) | 189 (52%) | 0.57 |
| Nausea or vomiting | 178 (37%) | 54 (44%) | 125 (35%) | 0.16 |
| Bruising | 76 (16%) | 22 (18%) | 54 (15%) | 0.42 |
| Hemorrhage | 11 (2%) | 1 (1%) | 10 (3%) | 0.21 |
| Nose bleed | 5 (1%) | 1 (1%) | 4 (1%) | 0.99 |
| Oral bleeding | 6 (1%) | 2 (2%) | 4 (1%) | 0.65 |
| Initial Rheumatic Symptoms during Acute Chikungunya Infection | ||||
| Wrist pain | 426 (90%) | 110 (92%) | 316 (89%) | 0.37 |
| Ankle pain | 412 (87%) | 113 (94%) | 299 (84%) | 0.0047 |
| Finger pain | 403 (84%) | 103 (86%) | 300 (84%) | 0.64 |
| Elbow pain | 395 (83%) | 111 (93%) | 284 (80%) | 0.0013 |
| Toe pain | 387 (81%) | 109 (92%) | 278 (78%) | 0.001 |
| Knee pain | 383 (80%) | 113 (94%) | 270 (76%) | <0.0001 |
| Hip pain | 342 (72%) | 106 (89%) | 236 (66%) | <0.0001 |
| Initial Chikungunya Virus Symptom Duration (days) | ||||
| <0.0001A | ||||
| Mean (sd) | 12.9 ± 30.6 | 14.2 ± 22.8 | 12.3 ± 33.3 | |
| Median (IQR) | 4 (3 – 8) | 5 (4 – 10) | 4 (3 – 7) | |
| Range | 1 – 365 | 1 – 90 | 1 – 365 | |
| Duration of Initial Joint Pain (weeks) | ||||
| <0.0001A | ||||
| Mean (sd) | 18.4 ± 32.4 | 45.3 ± 39.5 | 10.5 ± 25.1 | |
| Median (IQR) | 4 (2 – 16) | 40 (6 – 92) | 3 (2 – 8) | |
| Range | 0 – 365 | 0.6 – 104 | 0 – 365 | |
| Medications Used | ||||
| Acetaminophen | 478 (100%) | 122 (100%) | 356 (100%) | - |
| Ibuprofen | 36 (8%) | 11 (9%) | 25 (7%) | 0.47 |
| Prednisone | 5 (1%) | 2 (2%) | 3 (1%) | 0.61 |
| Medicinal Plants | 5 (1%) | 1 (1%) | 4 (1%) | 0.99 |
| Aspirin | 0 | 0 | 0 | - |
| Methotrexate | 0 | 0 | 0 | - |
Table 3. Follow-up Symptoms and Disability of Serologically Confirmed Chikungunya Patients, by Persistent Joint Pain at Median 20-Month Follow-up.
Significant p-values after Bonferroni-adjustment are in bold.
| Characteristic | All serologically confirmed cases (n= 485) | Persistent Joint Pain (n= 123) | No Persistent Joint Pain (n= 326) | p-value |
|---|---|---|---|---|
| Time since onset in months | 0.017 | |||
| Mean (sd) | 20.0 ± 1.3 | 20.2 ± 0. 8 | 19.9 ± 1.4 | |
| Median (IQR) | 19.7(19.4 – 20.8) | 19.8(19.4 –20.9) | 19.6(19.4–20.6) | |
| Range | 8.9 – 31.4 | 19.2 – 22.9 | 8.9 – 31.4 | |
|
Missed work or school during initial infection n (%yes) |
79 (16%) | 49 (40%) | 30 (8%) | <0.0001 |
| Days of missed work/school | 0.83 A | |||
| Mean (sd) | 5.5 ± 5.3 | 6.0 ± 6.3 | 4.7 ± 3.0 | |
| Median (IQR) | 4 (2 – 7) | 4 (2 – 7) | 3.5 (2 – 7) | |
| Range | 0 – 30 | 2 – 30 | 0 – 14 | |
|
Symptoms impacted capacity to continue normal activity? n(%yes) |
46 (10%) | 33 (27%) | 13 (4%) | <0.0001 |
|
Mean Swollen Joint Count m(sd) |
0.2 ± 0.6 | 0.5 ± 1.0 | 0.06 ± 0.3 | <0.0001A |
|
Mean Tender Joint Count m(sd) |
0.9 ± 1.8 | 2.9 ± 2.3 | 0.2 ± 0.8 | <0.0001 A |
|
Global pain score in the last week (from 0–100) mean ± sd |
45.8 ± 19.6 | 46.7 ± 20.2 | 41.5 ± 16.3 | 0.39 |
Used non-parametric Kruskal-Walllis test
Table 4. Initial chikungunya symptoms and baseline co-morbidities by gender.
Significant p-values after Bonferroni-adjustment are in bold.
| Characteristic n (%) | Female (n=388) | Male (n=95) | p-value |
|---|---|---|---|
| Initial Symptoms | |||
| Muscle pain | 377 (97%) | 93 (98%) | 0.99 |
| Weakness | 353 (91%) | 73 (77%) | <0.0001 |
| Joint pain and/or inflammation | 383 (99%) | 92 (97%) | 0.19 |
| Rash/itch | 339 (87%) | 69 (73%) | 0.0004 |
| Fever | 304 (80%) | 71 (76%) | 0.49 |
| Headache | 291 (75%) | 62 (65%) | 0.06 |
| Lymphadenopathy | 287 (74%) | 55 (58%) | 0.002 |
| Cool extremities | 216 (56%) | 40 (42%) | 0.018 |
| Nausea or vomiting | 157 (41%) | 21 (22%) | 0.0004 |
| Bruising | 69 (18%) | 7 (7%) | 0.012 |
| Hemorrhage | 8 (2%) | 3 (3%) | 0.46 |
| Nose bleed | 3 (1%) | 2 (2%) | 0.25 |
| Oral bleeding | 4 (1%) | 2 (2%) | 0.34 |
| Initial Rheumatic Symptoms | |||
| Wrist pain | 347 (91%) | 78 (85%) | 0.10 |
| Ankle pain | 331 (86%) | 80 (87%) | 0.89 |
| Finger pain | 325 (85%) | 77 (83%) | 0.62 |
| Elbow pain | 329 (86%) | 65 (71%) | 0.0005 |
| Toe pain | 312 (82%) | 74 (80%) | 0.78 |
| Knee pain | 306 (80%) | 76 (82%) | 0.72 |
| Hip pain | 286 (75%) | 55 (59%) | 0.0022 |
| Prior Comorbidities | |||
| Arthritis | 9 (2%) | 8 (9%) | 0.009 |
| Chronic foot pain | 8 (2%) | 6 (6%) | 0.04 |
| Gout | 7 (2%) | 5 (5%) | 0.07 |
| Osteoarthritis | 10 (3%) | 3 (3%) | 0.73 |
| Heart disease | 10 (3%) | 3 (3%) | 0.73 |
| Kidney disease | 10 (3%) | 2 (2%) | 0.99 |
| Lung disease | 17 (4%) | 2 (2%) | 0.39 |
| Diabetes | 31 (8%) | 3 (3%) | 0.10 |
| HTN | 47 (12%) | 10 (11%) | 0.66 |
| Depression | 15 (4%) | 5 (5%) | 0.58 |
P<0.00167 required
IRB Approval.
This study was approved by the ethics committee of the Universidad El Bosque under a protocol entitled “Surveillance of sentinel infectious events prevalent in Colombia” with a non-human subjects determination made by the George Washington University IRB for analysis of de-identified data. All participants received written informed consent and all samples were collected by qualified medical personnel.
Results
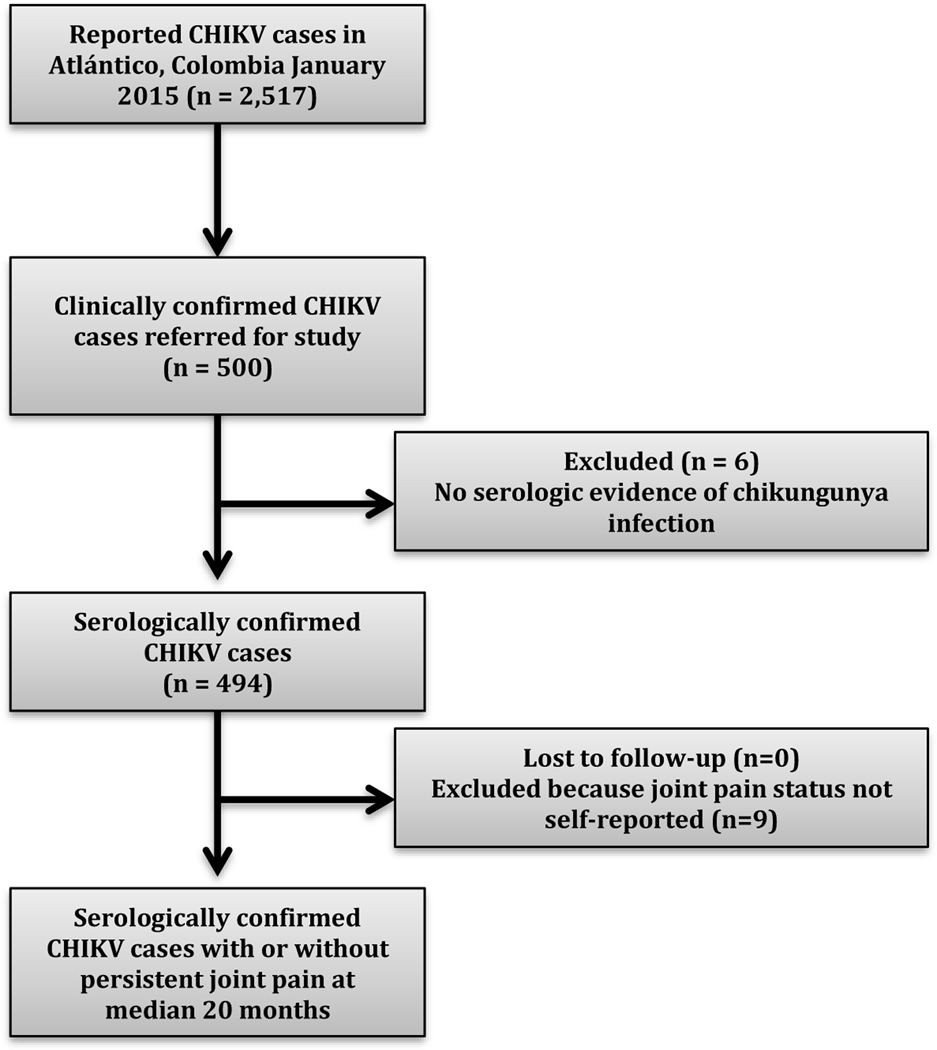
Five hundred participants with clinically suspected chikungunya infection were enrolled (Figure 1). Four hundred ninety-four cases were confirmed by ELISA with 483 acute cases (481 IgM + and IgG +, 2 IgM + and IgG-) and 11 convalescent patients (6 with equivocal IgM but IgG+ and 5 IgM – and IgG +). Six cases were negative for IgM and IgG antibodies and were excluded from the analysis. All 500 participants were reached for the follow-up telephone survey. However, nine participants did not report joint pain status and were excluded from the analysis (n = 485).
Figure 1.
Study flow diagram. There was a chikungunya virus (CHIKV) epidemic 2014 to 2015 in Atlántico Department, Colombia with 2,517 affected at the time of the study enrollment. 500 clinically confirmed cases were referred for the study, of which 494 were serologically confirmed. There was no attrition. All patients completed the follow-up telephonic survey at median 20-months post-infection.
At baseline (Table 1), the confirmed cases were predominantly adults with a mean age 49.1 years, female, and had a high school or less level of education. Almost all were of mestizo (i.e., mixed European, often Iberian, and indigenous Latin American ancestry) ethnicity and had health insurance. The most common baseline comorbidity was hypertension (12%) and only 17 patients reported prior arthritis at baseline (4%). The most commonly reported initial symptoms were weakness, rash, fever, headache and joint pain (Table 2). The most commonly affected joints were the small joints including the wrists, ankles, and fingers. The most commonly used medication to treat CHIKV-related joint pain was acetaminophen, which was utilized by every patient. Forty-six patients took ibuprofen, prednisone, or medicinal plants. The initial joint pain during acute infection lasted a median of 4 weeks, with interquartile range (IQR) of 3–8 weeks, with many patients with intermittent/persistent joint pain after the initial infection. Sixteen percent of participants reported missing school or work as a result of their CHIKV infection with a median of 4 days (IQR 2–7 days) lost (Table 3). When stratified by sex (Table 4), women were more likely to have chikungunya infection symptoms including weakness, rash, nausea or vomiting, and elbow pain.
Table 1.
Baseline demographic characteristics of serologically confirmed chikungunya patients, by persistent joint pain at median 20-month follow-up.
| Characteristic | All serologically confirmed cases (n= 485) | Persistent Joint Pain (n=123) | No Persistent Joint Pain (n= 362) | p-value |
|---|---|---|---|---|
| Age at baseline, mean (sd) | 49.1 ± 16.1 | 49.1 ± 17.1 | 49.2 ± 15.8 | 0.98 |
| Female Gender, n (%) | 388 (80%) | 109 (89%) | 279 (77%) | 0.004 |
| Ethnicity, n (%) | 0.40 | |||
| Mestizo | 451 (94%) | 115 (96%) | 336 (93%) | |
| Afro-Colombian | 4 (1%) | 0 (0%) | 4 (1%) | |
| White-Colombian | 26 (5%) | 5 (4%) | 21 (6%) | |
| Mean educational level, n (%) | 0.52 | |||
| High school or less | 377 (78%) | 96 (78%) | 281 (78%) | |
| Some college | 98 (20%) | 23 (19%) | 75 (21%) | |
| College graduate | 10 (2%) | 4 (3%) | 6 (2%) | |
| With medical insurance, n (%) | 461 (97%) | 117 (98%) | 344 (97%) | 0.53 |
| Prior comorbidity, n (%) | ||||
| Hypertension | 57 (12%) | 16 (13%) | 41 (12%) | 0.64 |
| Diabetes | 34 (7%) | 9 (7%) | 25 (7%) | 0.90 |
| Any type of arthritis | 17 (4%) | 7 (6%) | 10 (3%) | 0.16 |
| Disease of the lungs | 19 (4%) | 5 (4%) | 14 (4%) | 0.99 |
| Depression | 21 (4%) | 6 (5%) | 15 (4%) | 0.74 |
| Chronic Foot pain | 14 (3%) | 5 (4%) | 9 (3%) | 0.36 |
| Gout | 12 (3%) | 5 (4%) | 7 (2%) | 0.19 |
| Osteoarthritis | 13 (3%) | 4 (3%) | 9 (3%) | 0.75 |
| Ischemic Heart Disease | 13 (3%) | 4 (3%) | 9 (3%) | 0.75 |
| Disease of the Kidney | 12 (3%) | 3 (2%) | 9 (3%) | 0.97 |
After 20-months, one fourth of the participants (123/485) had persistent joint pain (Table 3). Among these patients, most had only one swollen joint but complained of tenderness in 3 other joints. They had a mean global pain score of 47 ± 20. Participants with persistent joint pain were more likely to be female (Table 1) and to have had more severe initial symptoms (Table 2). These patients reported greater joint involvement including the number of joints involved and the duration of initial joint symptoms. They were also more likely to report having missed work or school and to report that their normal activities were affected by the infection (Table 3).
In a model to examine factors that had independent associations with persistent joint pain, the area under the receiver operating characteristic curve (AUC) was 0.84, indicating good discrimination. Significant factors included being a college graduate, headache, knee pain, missed work, normal activities affected, 4 or more days of initial symptoms, and 4 or more weeks of initial joint pain (Table 5).
Table 5.
Multivariable Logistic Regression Model of Persistent Joint Pain.
| Baseline Factor | Adjusted OR | 95% Wald Confidence Limits | p-value | |
|---|---|---|---|---|
| College graduate | 5.53 | 1.13 | 27.17 | 0.0353 |
| Headache | 2.17 | 1.16 | 4.07 | 0.0157 |
| Knee pain | 4.69 | 1.91 | 11.51 | 0.0007 |
| Missed work | 5.23 | 2.87 | 9.52 | <0.0001 |
| Normal activities affected | 8.80 | 3.89 | 19.89 | <0.0001 |
| 4 or more days of initial symptoms | 2.69 | 1.57 | 4.60 | 0.0003 |
| 4 or more weeks of initial joint pain | 2.39 | 1.40 | 4.08 | 0.0014 |
Discussion
Key results.
We found the frequency of chronic joint pain after infection with chikungunya in a large Latin American cohort to be 25% at a median of 20-months post-infection. Significant predictors of persistent joint pain included being a college graduate, headache, knee pain, missed work, normal activities effected, 4 or more days of initial symptoms, and 4 or more weeks of initial joint pain.
Interpretation.
This is the first large-scale observational study of CHIKV arthritis in the Americas. The finding of chronic joint pain in one fourth of the patients infected with CHIKV approximately 2 years after initial infection has important implications for prediction of the magnitude of disability and health system costs after the Latin American epidemic. Prior predictions had over-estimated the expected frequency of CHIKV-related joint pain in Latin America indicating 48% of CHIKV-infected people were predicted to have chronic chikungunya arthritis 20 months after acute infection (15). These findings are consistent with findings reported from other outbreaks of the Asian strain of the virus at 18-months that showed approximately one-third of patients with persistent joint pain (5,18) and lower than reported findings in 15–18-month follow-up of patients affected by the East Central African strain that affected Le Réunion Island from 2005–2006 with reported persistent joint pain in 43% to 75% of CHIKV affected patients (9,12,13).
Significant predictors of persistent joint pain included factors that may indicate a more severe or prolonged initial infection such as missed work, normal activities affected, 4 or more days of initial symptoms, and 4 or more weeks of initial joint pain. The determination of the risk factors for persistent arthritis during initial infection enables early identification of patients who may require follow-up care. This finding is consistent with the findings of Hoarau et. al. and Sissoko et al. from Le Réunion Island (East Central African CHIKV strain outbreak) that showed that increased initial CHIKV viral load (22) and severe initial joint pain (9) was a predictor of persistent arthritis. However, in contrast to Hoarau et al. and Sissoko et al.’s findings, we did not find that older age was associated with an increased frequency of persistent CHIKV-related arthritis. This difference may be due to differences in CHIKV strains involved in the epidemics in Le Réunion Island as opposed to the Americas (9,22), significantly smaller cohort sizes in the Le Réunion cohorts (9,22), older mean age of the Le Réunion cohorts (9,22), and higher prevalence of underlying osteoarthritis comorbidity (26% in Sissoko et. al. versus 3% in our cohort).
Limitations.
A limitation of this study is the lack of a control group. It is possible that, over the course of 20 months, a few of the study participants might have developed joint symptoms and pain due to another etiology that they attributed to CHIKV infection. Furthermore, patients were not tested for other arboviral infections that may contribute to joint pain. While dengue (DENV) and Zika (ZIKV) are flaviviruses known to cause acute joint pain, Mayaro virus (MAYV) is an alphavirus, like CHIKV, known to cause similar chronic joint pain with known cross-reactivity between anti-Mayaro and anti-CHIKV antibodies (23). Patients with both CHIKV and MAYV viral arthritis almost universally report morning stiffness, which is a symptom of true inflammatory arthritis, even in the chronic phase of the disease. In comparison, DENV and ZIKV infections most commonly cause arthralgias rather than inflammatory arthritis which is an important distinction, as the alphaviruses preferentially invade and replicate within the synovium, whereas flaviviruses do not. During the CHIKV epidemic there was no known MAYV circulation in the Atlántico Department although MAYV is known to sporadically affect the Colombian Amazon region (24) so it is possible that a few of these cases could have been MAYV infections. Other limitations include the fact that self-reported joint pain was the primary outcome without serologic markers of inflammation and there was no formal assessment of validated quality of life measures.
Generalizability.
This study is the largest Latin American cohort of chikungunya patients to be followed at a median of 20-months. The study sample was Colombian and predominantly mestizo women. Nevertheless, it has important implications for future planning as this outbreak spreads.
Acknowledgements.
The support of multiple institutions made this publication possible including the Allied Research Society, Universidad El Bosque, George Washington University, and the Medical Faculty Associates. Specifically the authors would like to thank Martha Utrera, Ariel Gonzalez, April Barbour, Alan Wasserman, Donna Embersit, and Andreas Suhrbier for their support in this project.
Funding supported by The Rheumatology Research Foundation and Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessary represent the official views of these institutions.
Footnotes
Disclaimer. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.
References.
- (1).Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg 2010;104:392–399. [DOI] [PubMed] [Google Scholar]
- (2).Powers AM. Risks to the Americas associated with the continued expansion of chikungunya virus. J Gen Virol 2015;96:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Arboviral Disease Branch. Chikungunya virus-resources for healthcare providers. Center for Disease Control; 2016. Available from: https://www.cdc.gov/chikungunya/hc/resources.html. [Google Scholar]
- (4).Kularatne SA, Gihan MC, Weerasinghe SC, Gunasena S. Concurrent outbreaks of Chikungunya and Dengue fever in Kandy, Sri Lanka, 2006–07: a comparative analysis of clinical and laboratory features. Postgrad Med J 2009;85:342–346. [DOI] [PubMed] [Google Scholar]
- (5).Rahim AA, Thekkekara RJ, Bina T, Paul BJ. Disability with Persistent Pain Following an Epidemic of Chikungunya in Rural South India. J Rheumatol 2016;43:440–444. [DOI] [PubMed] [Google Scholar]
- (6).Gauri L, Thaned A, Fatima Q, Yadav H, Singh A, Jaipal H, et al. Clinical Spectrum of Chikungunya in Bikaner (North Western India) in 2006 and Follow up of Patients for Five Years. J Assoc Physicians India 2016;64:22. [PubMed] [Google Scholar]
- (7).Suhrbier A, Jaffar-Bandjee M, Gasque P. Arthritogenic alphaviruses—an overview. Nature Reviews Rheumatology 2012;8:420–429. [DOI] [PubMed] [Google Scholar]
- (8).Brighton S, Prozesky O, De La Harpe A. Chikungunya virus infection. A retrospective study of 107 cases. S Aft Med J 1983;63:313–5. [PubMed] [Google Scholar]
- (9).Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, et al. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS NTD 2009;3:e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ganu MA, Ganu A. Post-chikungunya chronic arthritis—our experience with DMARDs over two year follow up. J Assoc Physicians India 2011;59:83–86. [PubMed] [Google Scholar]
- (11).Chopra A, Anuradha V, Ghorpade R, Saluja M. Acute Chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol Infect 2012;140:842–850. [DOI] [PubMed] [Google Scholar]
- (12).Gérardin P, Fianu A, Malvy D, Mussard C, Boussaïd K, Rollot O, et al. Perceived morbidity and community burden after a Chikungunya outbreak: the TELECHIK survey, a population-based cohort study. BMC medicine 2011;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, et al. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis 2007;44:1401–1407. [DOI] [PubMed] [Google Scholar]
- (14).Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, et al. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on Reunion Island. Clin Infect Dis 2008;47:469–475. [DOI] [PubMed] [Google Scholar]
- (15).Rodriguez-Morales A, Cardona-Ospina J, Villamil-Gómez W, Paniz-Mondolfi A. How many patients with post-chikungunya chronic inflammatory rheumatism can we expect in the new endemic areas of Latin America? Rheumatol Int 2015;35:2091–2094. [DOI] [PubMed] [Google Scholar]
- (16).Randall S. Chikungunya in Colombia: 13,000 cases and counting. World wide outbreak. 2015. Available from: http://www.worldwideoutbreak.com/america/chikungunya-in-colombia-113000-cases-and-counting/. [Google Scholar]
- (17).Rodriguez-Morales AJ, Villamil-Gomez W, Merlano-Espinosa M, Simone-Kleber L. Post-chikungunya chronic arthralgia: a first retrospective follow-up study of 39 cases in Colombia. Clin Rheumatol 2016;35:831–832. [DOI] [PubMed] [Google Scholar]
- (18).Lanciotti RS, Lambert AJ. Phylogenetic Analysis of Chikungunya Virus Strains Circulating in the Western Hemisphere. Am J Trop Med Hyg 2016;94:800–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Prevoo M, Van’t Hof M, Kuper H, Van Leeuwen M, Van de Putte L, Van Riel P. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism 1995;38:44–48. [DOI] [PubMed] [Google Scholar]
- (20).The editors of Encyclopedia Britannica. Atlantico Department Colombia. Encylcopedia Britannica. 2017. Available from: https://www.britannica.com/place/Atlantico.
- (21).Ospina ML, Martínez Duran ME, Pacheco García OE, Quijada Bonilla H. Protocolo de Vigilancia en Salud Pública: Chikunguña. Instituto Nacional de Salud Vigilancia y Analysis del Riesgo en Salud Pública 2016;2:1–33. [Google Scholar]
- (22).Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol 2010;184:5914–5927. [DOI] [PubMed] [Google Scholar]
- (23).Fox JM, Long F, Edeling MA, Lin H, van Duijl-Richter MK, Fong RH, et al. Broadly neutralizing alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell 2015;163(5):1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rodriguez-Morales AJ, Paniz-Mondolfi AE, Villamil-Gomez WE, Navarro JC. Mayaro, Oropouche and Venezuelan Equine Encephalitis viruses: Following in the footsteps of Zika? Travel Med Infect Dis 2017;15:72–73. [DOI] [PubMed] [Google Scholar]