Abstract
Purpose: Coronavirus disease 2019 (COVID-19) has been associated with acute liver injury in reports worldwide. But no studies to date have described hypoxic hepatitis (HH) in patients with COVID-19. We aim to identify the prevalence of and possible mechanisms of HH in COVID-19 patients in the Intensive Care Unit (ICU).
Methods: This retrospective study was conducted on 51 patients with confirmed SARS-CoV-2 infection in the ICU at Zhongnan Hospital of Wuhan University from December 21, 2019, to March 11, 2020. Information on clinical features of enrolled patients was collected for analysis.
Results: HH was observed in 5.88% of the ICU patients with SARS-CoV-2 infection. All HH patients were progressing to respiratory failure and peak alanine aminotransferase (ALT) values were 1665, 1414, and 1140 U/L during hospitalization, respectively. All patients with HH died as a result of the deterioration of multiple organ failure (MOF). The dynamic changes of ALT, aspartate transaminase (AST), and total bilirubin (TBIL) levels were more dramatic in HH groups. Levels of TBIL, C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6(IL-6) showed statistically significant elevation in HH cases compared with that in non-HH cases (P < 0.001). Besides, the median survival time of the HH group was significantly shorter than the non-HH group (P < 0.05).
Conclusions: In ICU, HH was not a rare condition in patients with severe COVID-19 and has a high mortality. The main causes of HH are respiratory and cardiac failure and may be associated with the immune-mediated inflammatory response. Clinicians should search for any underlying hemodynamic or respiratory instability even in patients with normal ALT levels on admission.
Keywords: 2019-nCoV, SARS-CoV-2, hypoxic hepatitis, ischemia, liver injury, multiple organ failure (MOF), COVID-19
Introduction
In December 2019, a range of viral pneumonia cases started to spread in the city of Wuhan, China. On 11 February 2020, this unknown etiology was officially named severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) (1), and the disease was named coronavirus disease 2019(COVID-19) by the World Health Organization. Some patients had different degrees of liver injury. Moreover, four relatively large-scale studies have reported the clinical characteristics of COVID-19 patients, including liver impairment (2–5). Although transaminase elevation is usually mild, severe liver injury has been reported (3–6). However, the reason for severe liver injury is still not clear.
Acute liver impairment can present as a life-threatening disorder and can be caused by a series of reasons, including drug-induced liver injury (DILI), acute viral hepatitis, HH, acute alcohol-induced liver injury, liver trauma, etc. Whether SARS-CoV-2 is hepatotropic like viral hepatitis A, B, C, and what roles does the virus plays in the course of liver injury is worth studying. What's more, other causes of acute liver injury, if not SARS-CoV-2, need to be identified.
HH is the most common cause of a significant but transient elevation in serum aminotransferase activities (S-AT) in most studies. In ICU, the prevalence of HH accounts for at least 1% of admission (7). Currently, the pathogenesis of HH is not very clear, but most scholars agree with the three mechanisms proposed by Dunn et al. (8) that lead to HH: hepatic ischemia caused by reduced blood flow to the liver, venous congestion caused by right heart failure, and arterial hypoxemia caused by decreased blood oxygen content. Thus, remarkedly hypoxemic conditions could result in HH. Severe COVID-19 cases have led to respiratory failure, which can decrease oxygen supply to the liver. For this reason, HH may be a possible cause of severe liver injury in COVID-19 patients. Up to date, there has been no study of HH in patients infected with SARS-CoV-2. In the largest cohort study of critically ill patients with HH, the 28-day mortality in ICU was 45.0%(9). Because of this high mortality, the prompt identification and treatment of HH are crucial to the prognosis. We aim to describe the prevalence of HH in COVID-19 patients in ICU, as well as possible mechanisms.
Materials and Methods
Patients
From December 21, 2019, to March 11, 2020, 51 patients with COVID-19 were admitted and treated to the ICU at Zhongnan Hospital of Wuhan University. Epidemiological, clinical, laboratory characteristics, treatment, and outcomes data were acquired from the electronic medical records. This retrospective study was approved by the Research Ethics Commission of Zhongnan Hospital of Wuhan University and the Ethics Committee of Zhejiang Provincial People's Hospital. Due to the retrospective nature of this study, the need for informed consent was waived.
Data Collection
Laboratory investigations included complete blood count, coagulation function, routine biochemical, and liver function tests. Parameters related to patient characteristics included sex, age, and comorbidities (diabetes mellitus, hypertension, coronary heart disease, heart disease, and chronic obstructive pulmonary disease). Parameters related to the episode of HH included cause, supportive therapy (vasopressor agents, mechanical ventilation, and extracorporeal membrane oxygenation), and specific drug use (ribavirin, remdesivir, oseltamivir, glucocorticoid, etc.).
Hypoxic Hepatitis
Patients who met all of the following criteria were diagnosed with HH based on a previous report (10) (i) a massive but transient elevated ALT level [more than 20-fold the upper limit of normal (ULN)], (ii) the presence of respiratory, cardiac or circulatory failure, and (iii) exclusion of other causes of liver injury. Liver biopsy was not required for the diagnosis of HH, in agreement with other studies showing that a histological confirmation is unwarranted and even inadvisable when the criteria listed above are met (11, 12).
Statistical Analysis
Statistical analysis was performed using the SPSS software package (version 21.0, SPSS Inc., IBM, Chicago, IL, USA). Categorical variables were described as frequency and percentages, and continuous variables as means ± standard deviation (SD) or median and interquartile range (IQR). Continuous variables were compared using independent group t-tests when the data were normally distributed; otherwise, the Mann-Whitney U test was used. Comparison of categorical variables was done using the chi-square test or the Fisher exact test if the cell counts were small. Dynamic changes of liver function indicators by the influence of hypoxic hepatitis were presented using locally weighted scatterplot smoothing (LOESS). Survival analysis was performed by the Kaplan-Meier and Log rank test. A P-value < 0.05(two-tailed) was considered to indicate significance.
Results
Characteristics of the Patients With COVID-19 in ICU
The 51 patients in ICU with COVID-19 were predominantly male (n = 35,68.63%), and aged ≤65 years (n = 32,62.75%). There underlying medical conditions described in Table 1, mainly included hypertension (n = 22,43.14%), diabetes mellitus (DM) (n = 7,13.73%), coronary heart disease (CHD) (n = 12,23.53%), chronic obstructive pulmonary disease (COPD) (n = 2,3.92%). None of the critical patients had a malignant tumor. The proportion of patients with liver impairment on admission was much lower than the hospitalization (n = 39, 76.47% vs. n = 21, 41.18%, P < 0.001). Antiviral therapy was performed in 38 (74.51%) patients and antibiotic therapy was performed in 16 (31.4%), while the combination of the two therapies was 11 (21.56%). What's more, the proportion of patients using two or more antiviral drugs is 23.53% (12/51) and glucocorticoid therapy is 82.35% (42/51). Mechanical ventilation was performed in 37 (72.55%) while extracorporeal membrane oxygenation (ECMO) was performed in 3 (5.88%) (Table 1).
Table 1.
Characteristics of the patients with COVID-19 in ICU (n = 51).
| Characteristics | Number (%) | |
|---|---|---|
| Age | ||
| ≤65 years | 32 (62.75) | |
| >65 years | 19 (37.25) | |
| Sex | ||
| Male | 35 (68.63) | |
| Female | 16 (31.37) | |
| BMI>25 (kg/m2) | 16 (31.37) | |
| Underlying medical conditions | ||
| Hypertension | 22 (43.14) | |
| DM | 7 (13.73) | |
| CHD | 12 (23.53) | |
| COPD | 2 (3.92) | |
| Cerebrovascular disease | 5 (9.80) | |
| Hepatopathy | 1 (1.96) | |
| Hemorrhage of digestive tract | 2 (3.92) | |
| Chronic kidney disease | 1 (1.96) | |
| Renal transplant | 1 (1.96) | |
| Malignant tumor | 0 | |
| Liver impairment | Value (mean ± SD) U/L | |
| On admission | 21 (41.18) | 53.13 ± 65.55 |
| ULN <ALT ≤20-fold ULN | 21 (41.18) | 53.13 ± 65.55 |
| ALT >20-fold ULN | 0 | |
| Hospitalization* | 39 (76.47)* | 70.54 ± 142.90 |
| ULN <ALT ≤20-fold ULN | 36 (70.59) | 118.23 ± 90.75 |
| ALT >20-fold ULN | 3 (5.88) | 1406.33 ± 262.58# |
| Treatment | ||
| Antibiotic therapy | 16 (31.37) | |
| Antiviral therapy | 38 (74.51) | |
| A combination of antibiotic and antiviral therapy | 11 (21.56) | |
| Multiple antiviral therapies (≥2) | 12 (23.53) | |
| Glucocorticoid therapy | 42 (82.35) | |
| Mechanical ventilation | 37 (72.55) | |
| ECMO | 3 (5.88) | |
Liver impairment ratio (hospitalization vs. on admission, P < 0.001).
Peak ALT level during hospitalization (HH patients vs. non-HH patients with liver impairment, P < 0.001).
ALT, alanine aminotransferase; BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ULN, upper limit of normal; ECMO: extracorporeal membrane oxygenation; SD, standard deviation; HH, hypoxic hepatitis.
An ALT level of >20-fold the ULN was found in only three patients, not on admission but during hospitalization. The peak value of ALT was 1665, 1414, and 1140 U/L respectively. The degree of ALT elevation in HH patients was significantly higher than that in non-HH patients with liver impairment (during hospitalization, 1406.33 ± 262.58 vs. 118.23 ± 90.75 U/L, P < 0.001). These cases were diagnosed as HH. The incidence of HH in our single-center cohort of ICU patients with COVID-19 was 5.88% (3/51). All cases deteriorated into respiratory failure. Also, viral hepatitis and autoimmune diseases were excluded based on negative results of serum marker tests.
Comparison of Clinical Characteristics Between HH and Non-HH Patients
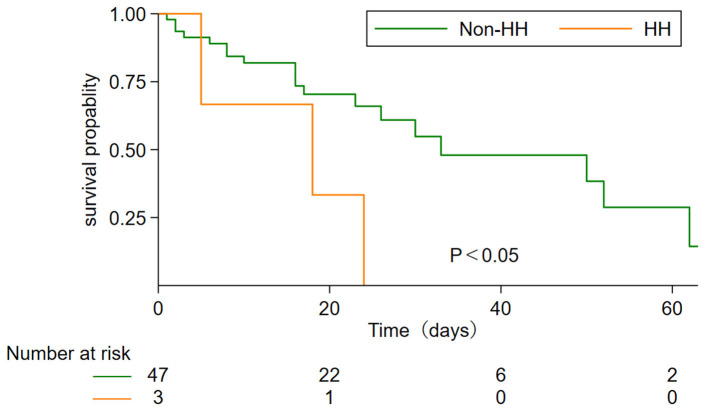
The clinical characteristics of the two groups were shown in Table 2. Mean age at diagnosis was (63.33 ± 7.64), which was slightly older than that non-HH group. There was no significant difference in age and sex between the two groups. In our cohort of 51 patients, no children or adolescents were infected. The incidence of hypertension and CHD were 2 (66.67%) and 1 (33.33%), respectively, which were higher than that of 20 (41.67%) and 11 (22.92%) patients without HH. There were no DM and hepatopathy in the HH group, compared with 7/48 (14.58%) cases of DM and 1/48 (2.08%) cases of hepatopathy in the non-HH group. The most common symptoms at the onset of illness were fever (88.23%), cough (64.71%), and dyspnea (52.94%). There was no significant difference in the incidence of myalgia and COPD between the two groups. To get the outcomes of the patients, we followed up to April 5, 2020, 29 (60.42%) patients have been discharged and 19 (39.58%) died in the group of non-HH patients, while 3 (100.00%) patients have all died in the group of HH. Besides, Figure 1 also showed that the median survival time of the HH group was significantly shorter than the non-HH group (p < 0.05). Fitness for discharge was based on abatement of fever for at least 10 days, with the improvement of chest radiographic evidence and viral clearance in respiratory samples from the upper respiratory tract (Table 2).
Table 2.
Clinical characteristics of COVID-19 patients.
| Characteristics | With HH (n = 3) | Without HH (n = 48) | P value |
|---|---|---|---|
| Age (years) | 63.33 ± 7.638 | 59.94 ± 14.110 | 0.68 |
| Sex (male) | 3 (100.00) | 32 (66.67) | 0.54 |
| Current smoker (%) | 2 (66.67) | 9 (18.75) | 0.22 |
| Current drinker (%) | 1 (33.33) | 10 (20.83) | >0.999 |
| Pre-existing conditions (%) | |||
| Hypertension | 2 (66.67) | 20 (41.67) | 0.81 |
| DM | 0 | 7 (14.58) | >0.999 |
| Heart disease | 1 (33.33) | 11 (22.92) | >0.999 |
| COPD | 1 (33.33) | 1 (2.08) | 0.24 |
| Hepatopathy | 0 | 1 (2.08) | >0.999 |
| Other diseases | 3 (100.00) | 20 (41.67) | 0.09 |
| Comorbidities | 3 (100.00) | 32 (66.67) | 0.543 |
| Symptom (%) | |||
| Fever | 3 (100.00) | 42 (87.50) | >0.999 |
| Cough | 3 (100.00) | 30 (62.50) | 0.54 |
| Dyspnea | 3 (100.00) | 24 (50.00) | 0.24 |
| Myalgia | 3 (33.33) | 8 (16.67) | 0.25 |
| Chest distress | 3 (100.00) | 22 (45.83) | 0.11 |
| Other | 2 (66.67) | 17 (35.42) | 0.64 |
| Outcome (%) | |||
| Discharge | 0 | 29 (60.42) | 0.07 |
| Death | 3 (100.00) | 19 (39.58) | 0.07 |
Figure 1.
Comparison of survival probability between HH and non-HH group. Group comparisons were performed using the Kaplan-Meier and long-rank test.
Selected Laboratory Tests of COVID-19 Patients
Table 3 shows that the median values of lymphocytes and PLT were 0.26 (0.20–0.48) and 64.00 (48.25–147.25), respectively, which were significantly lower than those of patients without HH (P < 0.001). No significant difference in the level of white blood cells or hemoglobin between the two groups was observed (P > 0.05). In indexes of liver enzymes and function, patients with HH had significant degrees of ALB reduction compared with that non-HH group (P < 0.05) and the concentration of ALB has reached the level of hypoalbuminemia (ALB<30 g/L). The median levels of ALT, AST, γ-GT, LDH, and AKP in patients with HH were no significant differences between the two groups. Furthermore, ALT, AST, and γ-GT were slightly above normal, while LDH was significantly above normal. No obvious jaundice was observed in any of the patients with HH, even though the median TBIL level in the HH group was significantly higher than that non-HH group (P < 0.001). Since the coagulation proteins required for blood coagulation cascade are mainly produced by the liver, and the half-life of coagulation factors is shorter than ALB, INR is often used as the marker of liver synthesis function (13). Patients with HH had higher median INR values compared with those patients without HH (1.26 vs. 1.22), though it presented only a very slight INR elevation. Inflammatory markers among these critically ill patients, including CRP, PCT, D-dimer, and IL-6. The concentration of PCT, CRP, and IL-6 were 5.43 (1.87–20.13), 235.30 (102.80–300.50), and 356.10 (149.70–1622.25), respectively, which were significantly higher than those of patients without HH (P < 0.001). Besides, CRP as the indicator of acute inflammation, the levels of CRP in both groups was well above normal. As for the indicator of renal function, the median value of creatinine was 88.50 (71.00–124.55) and 70.00 (52.53–115.70), respectively, which were significantly higher than that non-HH group (P < 0.05), although still in the normal range. The levels of myocardial necrosis markers in patients with HH were also higher than those in patients without HH and far above normal.
Table 3.
Selected laboratory data of COVID-19 patients.
| Characteristics | With HH (n = 3) | Without HH (n = 48) | P-value |
|---|---|---|---|
| BLOOD COUNTS | |||
| WBC (× 109/L) | 9.98 (6.19–15.23) | 9.32 (6.58–14.10) | 0.89 |
| Lymphocyte (× 109/L) | 0.26 (0.20–0.48) | 0.57 (0.34–1.04) | <0.001 |
| Hgb (g/L) | 95.95 (83.90–123.70) | 111.00 (82.85–126.90) | 0.26 |
| PLT (× 109/L) | 64.00 (48.25–147.25) | 151.00 (103.00–219.50) | <0.001 |
| LIVER ENZYMES AND FUNCTION | |||
| ALB (g/L) | 28.95 (24.95–31.28) | 30.70 (27.80–34.35) | 0.02 |
| ALT (U/L) | 42.00 (21.00–54.00) | 40.00 (26.00–69.00) | 0.50 |
| AST (U/L) | 40.00 (34.00–61.00) | 38.00 (26.00–65.00) | 0.23 |
| TBIL (μmol/L) | 21.30 (14.50–32.90) | 14.20 (10.20–21.10) | <0.001 |
| γ-GT (U/L) | 70.50 (43.75–97.00) | 45.00 (24.00–111.00) | 0.07 |
| ALP (U/L) | 100.00 (87.25–120.75) | 90.50 (71.00–122.75) | 0.13 |
| LDH (U/L) | 432.00 (347.25–895.25) | 460.50 (322.25–641.75) | 0.86 |
| INR | 1.26 (1.16–1.43) | 1.22 (1.13–1.36) | 0.14 |
| ADDITIONAL MARKERS OF ORGAN DAMAGE AND INFLAMMATION | |||
| CRP (mg/L) | 235.30 (102.80–300.50) | 53.60 (23.58–112.33) | <0.001 |
| PCT (ng/ml) | 5.43(1.87–20.13) | 0.29(0.06–1.90) | <0.001 |
| D-dimer (μg/L) | 935.00 (622.50–2049.00) | 1123.00 (578.00–3254.00) | 0.61 |
| IL-6 (pg/mL) | 356.10 (149.70–1622.25) | 45.21 (17.90–103.60) | <0.001 |
| Creatinine (μmol/L) | 88.50 (71.00–124.55) | 70.00 (52.53–115.70) | 0.02 |
WBC, white blood cell;Hgb, hemoglobin; PLT, platelet count; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate transaminase; TBIL, total bilirubin; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; LDH, lactic dehydrogenase; INR, international normalized ratio; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin-6.
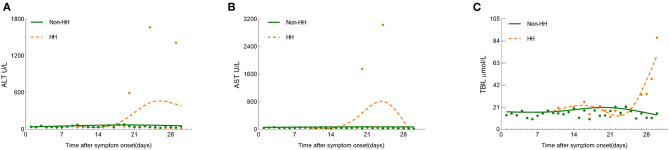
Dynamic Changes in Liver Function Indicators in COVID Patients in ICU
To determine the trajectory of liver function indicators in patients enrolled in this study, multiple liver function test results were recorded during hospitalization. LOESS models illustrated the trajectory of ALT, AST, and TBIL between the two groups (Figure 2). From the general trend, the rangeability of the non-HH group was much flatter than that of the HH group. Unlike the non-HH group, the curve of HH started on day 10. Figure 2A suggested that the curve of the non-HH group fluctuated roughly in a range of 1–2 times of ULN. ALT began to rise rapidly at the 2nd week and peaked within 3 to 4 weeks after symptom onset in the HH group. Besides, invasive mechanical ventilation was used when ALT levels reached a peak. Figure 2B illustrated that the curve of the non-HH group is close to a straight line compared with the HH group. Similar to ALT, AST also increased dramatically at the 2nd week and peaked within 3 to 4 weeks. Subsequently, the curve dropped sharply to a normal level. Figure 2C showed that the fluctuation in TBIL levels was mild and normal in the non-HH group. Moreover, TBIL levels were slightly higher than that non-HH group in the 1st week of the curve. Shortly after TBIL slowed down in the next week. However, the curve of TBIL was increased dramatically in the 3rd week. The dynamic changes in indicators suggested potential mechanisms of COVID-19 patients with HH in ICU.
Figure 2.
Smooth trajectories and scatters represent the median values (A) ALT; (B) AST; (C) TBIL in the HH and non-HH groups. The ULN in our health care system is defined as 40 U/L for ALT, 40 U/L for AST, and 21 μmol/L for TBIL, respectively.
Discussion
HH, as manifested by ALT abrupt and massive elevation, is emerging as a clinical consequence of COVID-19, and often predict a poor outcome. These severely infected patients with HH had a high rate of ARDS and a high risk of death. SARS-CoV-2 infection in humans can cause respiratory diseases, acute kidney injury, myocarditis, thrombosis, and acute liver injury (14). Most studies reported the prevalence of liver enzyme elevation has ranged from 20 to 30% and severe liver injury was uncommon (5, 15). However, extreme liver impairment (ALT >20-fold the ULN) was not rare in our study (n = 3, 5.88%) compared with previous reports (7). What's more, the significant difference of ALT elevation between HH patients and non-HH patients with liver impairment (P < 0.001) indicated that HH can be distinguished from other types of liver injury by the sharp increase of ALT level. It is also reported that liver impairment is caused by drug hepatotoxicity (such as remdesivir) (16) and immune-mediated inflammation (17). Thus, it's necessary to distinguish the causes of liver impairment.
DILI can be ruled out because hepatotoxic drugs continue to be used, and ALT also gradually decreases. Autoimmune hepatitis (AIH) was excluded because the score is not up to the diagnostic criteria(include autoantibodies, immunoglobulins, viral markers, and histological findings) proposed by the International Autoimmune Hepatitis Group (18). No imaging evidence suggested hepatic steatosis and no patients met one of the following clinical parameters, such as being overweight, having type 2 diabetes mellitus, or exhibiting metabolic dysregulation (19). Thus, metabolic associated fatty liver disease (MAFLD) was also ruled out. Henrion et al. reported that heart failure, respiratory failure, and septic toxic shock accounted for more than 90% of HH cases and the core mechanism was reduced oxygen supply to the liver (7). The hallmark of COVID-19 is respiratory failure. HH is therefore frequent in severe cases. In our study, 3 HH cases all progressed to respiratory failure, which led to hypoxia of liver tissue and abnormalities of liver function. Moreover, heart failure reduces the output, thereby decreasing the blood flow to the liver and further exacerbating hypoxia in the liver. This is consistent with previous research (7, 8). Although several hemodynamic mechanisms of liver hypoxia are involved, liver ischemia is not the only explanation for HH. On the one hand, previous studies indicated that ALT levels correlate well with markers of inflammation and are likely higher among patients with severe cytokine release syndromes (14, 20, 21). On the other hand, cytokine storm syndromes have been described in COVID-19, and are associated with severe elevations in liver enzymes (22). What's more, IL-6 is significantly increased in severe COVID-19 patients and plays a key role in the so-called “cytokine storm” (23, 24). In our study, the levels of ALT were extremely elevated and the concentrations of inflammatory markers including CRP, D-dimer, and IL-6 were significantly far above normal. Therefore, it suggested that HH may be associated with the immune-mediated inflammatory response.
Ours is the first batch of treatment centers for COVID-19 patients in the world. COVID-19 patients in ICU accounted for 7.02% (51/726) of the total number of patients diagnosed with SARA-CoV-2 infection during our observation. The prevalence of HH in patients with severe SARS-CoV-2 infection in our ICU is 5.88%. Most studies have reported an incidence of 0.9–2.4% (7). This is lower than ours, which indicated that patients with severe SARS-CoV-2 infection in ICU have a higher incidence of HH than that of patients with other causes.
At present, there has been no study on the dynamic changes of liver function in patients with COVID-19 combined with HH, and only a few studies in COVID-19-related liver injury (25, 26). The increase of ALT and AST levels in the early stage of the disease might be related to the immune-mediated inflammation in the liver. However, when ALT and AST showed a downward trend in the late stage of the disease in the HH group, the level of TBIL increased sharply. This may be a result of MOF.
The prognosis of HH is poor. Extensive analysis by Aboelsoud et al. showed that the mortality of HH was 44.1% (27). However, the mortality rate in our study was as high as 100%. MOF contributed heavily to the high mortality. We also observed an elevation in INR, significant hypoalbuminemia, and lymphopenia in patients with HH. Hypoalbuminemia is emerging as a consistent risk factor for severe disease (3, 6, 28) and is linked to poor clinical outcomes for hospitalized patients (29). Besides, increased INR in severe COVID-19 patients indicate damage of liver synthetic function. A study has also shown that lymphopenia may be a key factor related to disease severity and mortality (30). All of these factors can affect the prognosis of HH. In general, severe liver injury was not the direct cause of death. These patients died as a consequence of MOF, but the occurrence of HH had a high impact on the mortality of those critically ill patients.
In conclusion, we report a 5.88% prevalence of HH in COVID-19 patients in ICU, the first study to combine COVID-19 and HH. HH was not a rare condition in ICU, and was frequently accompanied by MOF, with high mortality. Patients with COVID-19 developed MOF accompanied by a sudden and sharp elevation of serum ALT level during hospitalization, which should be considered HH. Attention should also be paid to monitor liver function during the course of COVID-19, especially in patients with higher disease severity.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.
Ethics Statement
The analysis was approved by the Research Ethics Commission of Zhongnan Hospital of Wuhan University and the Ethics Committee of Zhejiang Provincial People's Hospital, and the need for informed consent was waived.
Author Contributions
MG and YT designed study and revised the manuscript. HH and XZ analyzed data and prepared the manuscript. HL analyzed data and performed manuscript drafting. SC performed manuscript drafting. XD arranged and filtered data. JW and JZ searched the literature and analyzed data. LS, RY, MW, and JW collect data. HH reviewed the results and made critical comments on the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We received data from the Zhongnan Hospital of Wuhan University. We thank the Zhejiang Provincial People's Hospital for their support. We also appreciate all Zhongnan Hospital of Wuhan University for their elaborate work and patient assistance.
Glossary
Abbreviations
- HH
hypoxic hepatitis
- SARA-CoV-2
severe acute respiratory syndrome coronavirus 2
- COVID-19
coronavirus disease 19
- ICU
intensive care unit
- CHD
coronary heart disease
- COPD
chronic obstructive pulmonary disease
- DM
diabetes mellitus
- ECMO
extracorporeal membrane oxygenation
- SD
standard deviation
- ULN
upper limit of normal
- BMI
body mass index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TBIL
total bilirubin
- γ-GT
γ-glutamyl transpeptidase
- ALP
alkaline phosphatase
- LDH
lactate dehydrogenase
- CRP
C-reactive protein
- IL-6
interleukin-6
- INR
international normalized ratio
- PCT
procalcitonin
- Hgb
hemoglobin
- WBC
white blood cell
- PLT
platelet count
- ALB
albumin
- AIH
autoimmune hepatitis
- MAFLD
metabolic associated fatty liver disease.
Footnotes
Funding. This study was supported by the Scientific Research Fund of the National Health Commission of China (No. WKJ-ZJ-2102), the General Scientific Research Projects of Zhejiang Education Department (No. Y202044644), the General Scientific Research Projects of Zhejiang Education Department (No. Y202044571).
References
- 1.Alexander E, Gorbalenya S, Baker C, Ralph S, Baric J, de Groot R, et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses - a statement of the Coronavirus Study Group. bioRxiv. (2020). 10.1101/2020.02.07.937862 [DOI] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020). 323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:1–11. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henrion J. Hypoxic hepatitis. Liver Int. (2012) 32:1039–52. 10.1111/j.1478-3231.2011.02655.x [DOI] [PubMed] [Google Scholar]
- 8.Dunn GD, Hayes P, Breen KJ, Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. (1973). 265:16. 10.1097/00000441-197303000-00001 [DOI] [PubMed] [Google Scholar]
- 9.Van den Broecke A, Van Coile L, Decruyenaere A, Colpaert K, Benoit D, Van Vlierberghe H, et al. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann Intensive Care. (2018) 8:15. 10.1186/s13613-018-0356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Liu J, Yu L, Zhou N, Ding W, Zheng S, et al. Prevalence and characteristics of hypoxic hepatitis in the largest single-centre cohort of avian influenza A(H7N9) virus-infected patients with severe liver impairment in the intensive care unit. Emerg Microbes Infect. (2016) 5:e1. 10.1038/emi.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kneidinger N, Funk GC, Lindner G, Drolz A, Schenk P, Fuhrmann V. Unmeasured anions are associated with short-term mortality in patients with hypoxic hepatitis. Wien Klin Wochenschr. (2013) 125:474–80. 10.1007/s00508-013-0400-9 [DOI] [PubMed] [Google Scholar]
- 12.Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine. (2003) 82:392–406. 10.1097/01.md.0000101573.54295.bd [DOI] [PubMed] [Google Scholar]
- 13.Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. (2013) 11:1064–74. 10.1016/j.cgh.2013.02.026 [DOI] [PubMed] [Google Scholar]
- 14.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large US cohort. Hepatology. (2020). 10.1002/hep.31404. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. (2020) 83:521–3. 10.1097/JCMA.0000000000000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leegwater E, Strik A, Wilms EB, Bosma LBE, Burger DM, Ottens TH, et al. Drug-induced liver injury in a COVID-19 patient: potential interaction of remdesivir with P-glycoprotein inhibitors. Clin Infect Dis. (2020). 10.1093/cid/ciaa883. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. (2020) 53:368–70. 10.1016/j.jmii.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czaja AJ. Diagnosis and management of autoimmune hepatitis: current status and future directions. Gut Liver. (2016) 10:177–203. 10.5009/gnl15352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. 10.1016/j.jhep.2020.07.045 [DOI] [PubMed] [Google Scholar]
- 20.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. (2020) 7:e438–e40. 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. (2020). 10.20944/preprints202003.0180.v1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). J Infect Dis. (2020) 221:1762–9. 10.1101/2020.02.10.2002183232227123 [DOI] [Google Scholar]
- 24.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. (2020) 55:102763. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. (2020). 10.1002/hep.31301. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, et al. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. (2020). 10.1002/hep.31326. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Aboelsoud MM, Javaid AI, Al-Qadi MO, Lewis JH. Hypoxic hepatitis - its biochemical profile, causes and risk factors of mortality in critically-ill patients: a cohort study of 565 patients. J Crit Care. (2017) 2017:7. 10.1016/j.jcrc.2017.04.040 [DOI] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT. Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg. (2017) 83:1220–7. 10.1177/000313481708301123 [DOI] [PubMed] [Google Scholar]
- 30.Chen P, Zhou B. Clinical characteristics of COVID-19 in patients with liver injury. Clin Gastroenterol Hepatol. (2020) 18:2846–7. 10.1016/j.cgh.2020.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.