Abstract
When solid or liquid stimuli contact the tongue tip during eating, the sensations of taste, touch and temperature are immediately evoked, and tongue function relies on these simultaneous multimodal responses. We focus on the fungiform papilla of the anterior tongue as a complex organ for taste, tactile and thermal modalities, all via chorda tympani nerve innervation from the geniculate ganglion. Rather than a review, our aim is to revise the classic archetype of the fungiform as predominantly a taste bud residence only and instead emphasize an amended concept of the papilla as a multimodal organ. Neurophysiological maps of fungiform papillae in functional receptive fields demonstrate responses to chemical, stroking and cold lingual stimuli. Roles are predicted for elaborate extragemmal nerve endings in tactile and temperature sensations, and potential functions for keratinocytes in noncanonical sensory signaling. The fungiform papilla is presented as a polymodal lingual organ, not solely a gustatory papilla.
Keywords: taste, chorda tympani, geniculate ganglion, trigeminal ganglion, chemosensory, somatosensory, receptive field
Introduction
The instantaneous and simultaneous sensations of taste, touch and temperature when solid or liquid stimuli contact the tongue tip are necessary for eating. Once in contact with the tip, potential food stimuli are manipulated by licking and tongue movements to create a bolus, relying on co-attendant saliva secretion [1,2]. The detection and recognition of oral sensory stimuli are transformed into perceptions related to flavor via central projections.
The tongue is clearly reliant on multimodal sensation to execute lingual functions. Our premise here is that the fungiform papilla (FP), and chorda tympani innervation only, is a complete multimodal organ on the anterior tongue for taste, gentle touch, and moderate temperature reception that serves principally to transmit sensations for eating. The FP has a dual innervation: the chorda tympani nerve with soma in the geniculate ganglion (GG) directed to taste buds and the immediate perigemmal surround, and the lingual nerve with soma in the trigeminal ganglion (TG) directed mainly to papilla walls.
We propose a re-conceptualization of the classic archetype of the FP as a gustatory organ that functions via chorda tympani fibers responding principally to taste stimuli and separately via lingual nerve fibers responding principally to touch and temperature stimuli. The FP and chorda tympani innervation should no longer be considered as simply a taste papilla, but rather as a taste and somatosensory organ or polymodal sensory papilla. For optimal sensitivity that colocates taste, touch and temperature responses, one organ with innervation from functional subsets of neurons in one ganglion, the GG, is adaptive for eating. On the other hand, the lingual nerve innervation in FP is especially dedicated to responding to irritant chemicals, and noxious tactile or thermal stimuli.
Our re-conceptualization of the FP is based on emerging data in the taste field and is not intended as a review. In earlier recordings from the chorda tympani nerve, innervating anterior tongue taste buds, responses to chemical, temperature and tactile lingual stimulation were shown [3,4]. Only recently, however, have responses from the intact nerve itself been experimentally separated from taste bud responses, with the potential for long term experiments and recovery [5,6]. Taste buds require Hedgehog (HH) signaling for development, homeostasis and regeneration [7,8]. When signaling from the HH pathway is repressed or blocked in the tongue epithelium, genetically [9] or pharmacologically [5], taste buds are eliminated from the tongue. There are selective effects of signaling inhibition in proliferation and differentiation in FP without widespread cell death or loss of ganglion cells [6–9]. With restoration of signaling, effects are rapidly reversible. Throughout, the nerves that innervate the taste buds are retained [5–7, 9]. Therefore, it is possible to study chorda tympani nerve responses with intact taste buds or without taste buds. Recordings from the chorda tympani after taste bud elimination demonstrate that responses to chemical stimuli are lost (as the taste buds are lost) but responses to tactile and temperature responses remain [5,6,10] (Figure 1). Thus the fungiform papilla is a complex organ that robustly subserves taste, touch and temperature modalities in the anterior tongue, all via the chorda tympani nerve innervation alone.
Figure 1.
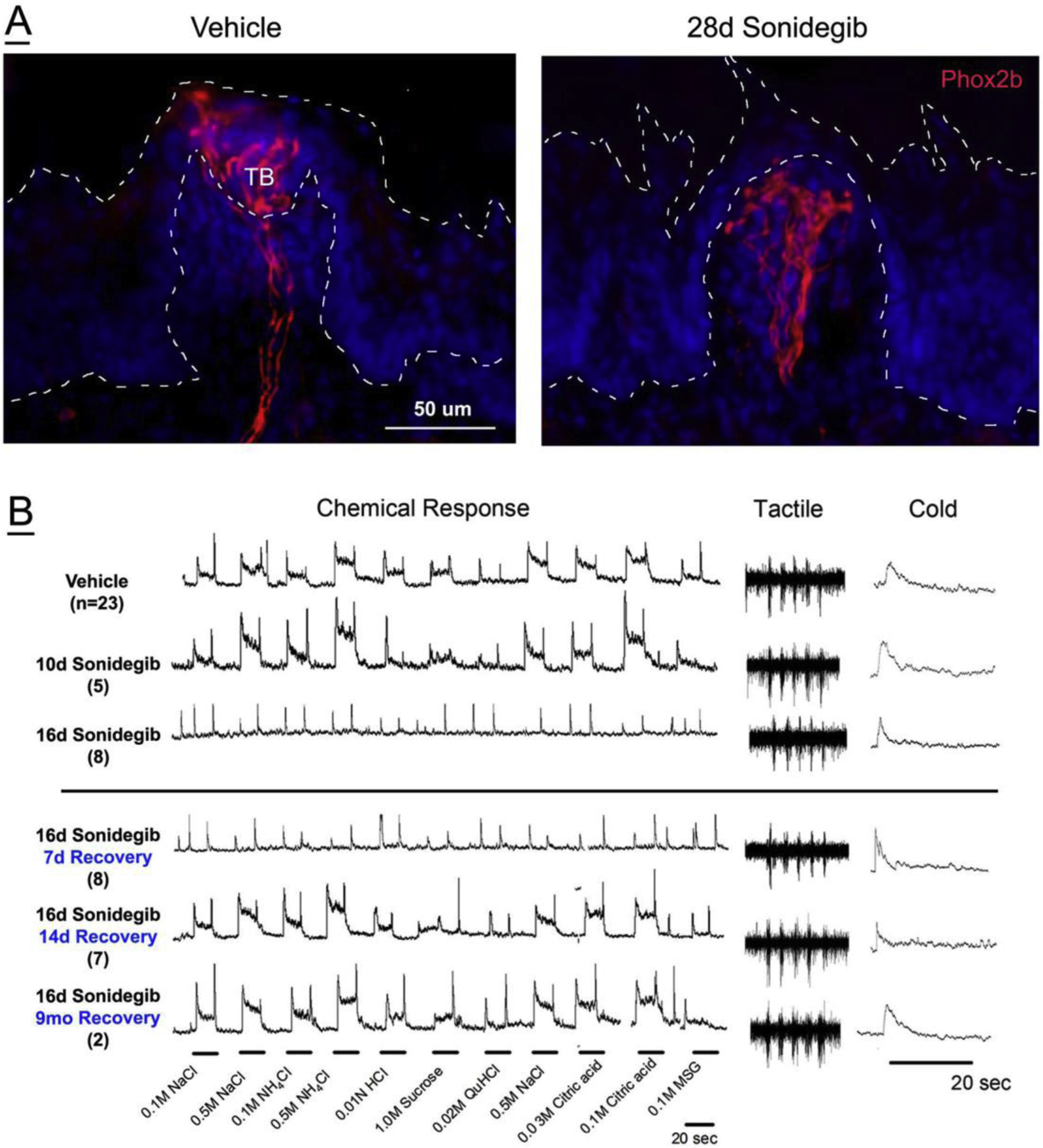
Chorda tympani nerve fibers remain and are functional after taste bud elimination in Hedgehog (HH) pathway inhibition. A. Chorda tympani fibers (red) in fungiform papillae (FP) in Phox2b-tdTomato mice after treatment with Vehicle or the HH pathway pharmacologic inhibitor Sonidegib for 28 days. Dashed lines outline the papillae. Chorda tympani fibers are in the FP connective tissue core, in the taste bud (TB, Vehicle) and extend into the epithelium outside of the taste bud. After Sonidegib treatment, and HH pathway inhibition, taste buds are eliminated in all FP. (Images are from work in progress and AChemS 2019.) B. Recordings from the chorda tympani nerve in Vehicle- (with taste buds) and Sonidegib-treated (with no taste buds) mice demonstrate loss of responses to chemical stimuli and retained robust responses to tactile and cold stimuli after treatment. At various times after drug removal, Recovery of responses to chemical stimuli is demonstrated. (Data from Figure 8, Ref. 6.)
What is the fungiform papilla and where are these papillae located?
The anterior tongue is rich in polysensory fungiform papillae (FP) that inform about taste, touch and temperature. In rat over 50% of the 187 FP are on the tongue tip with a gradual, posterior reduction in density [11] (Figure 2A). A similar FP distribution is seen in other mammals, including cat, sheep and human [12]. The high FP density at the tip, combined with mobility of the free tongue, underlies the fundamental role of the anterior tongue in initial stimulus identification [13]. Although rat, mouse, hamster and gerbil FP contain a single taste bud each, cat, sheep, primate and human FP may contain from 1 to 20 taste buds [12]. Thus, the concentrated FP distribution also indicates high taste bud density on the lingual tip, dedicated to inceptive oral stimulus processing.
Figure 2.
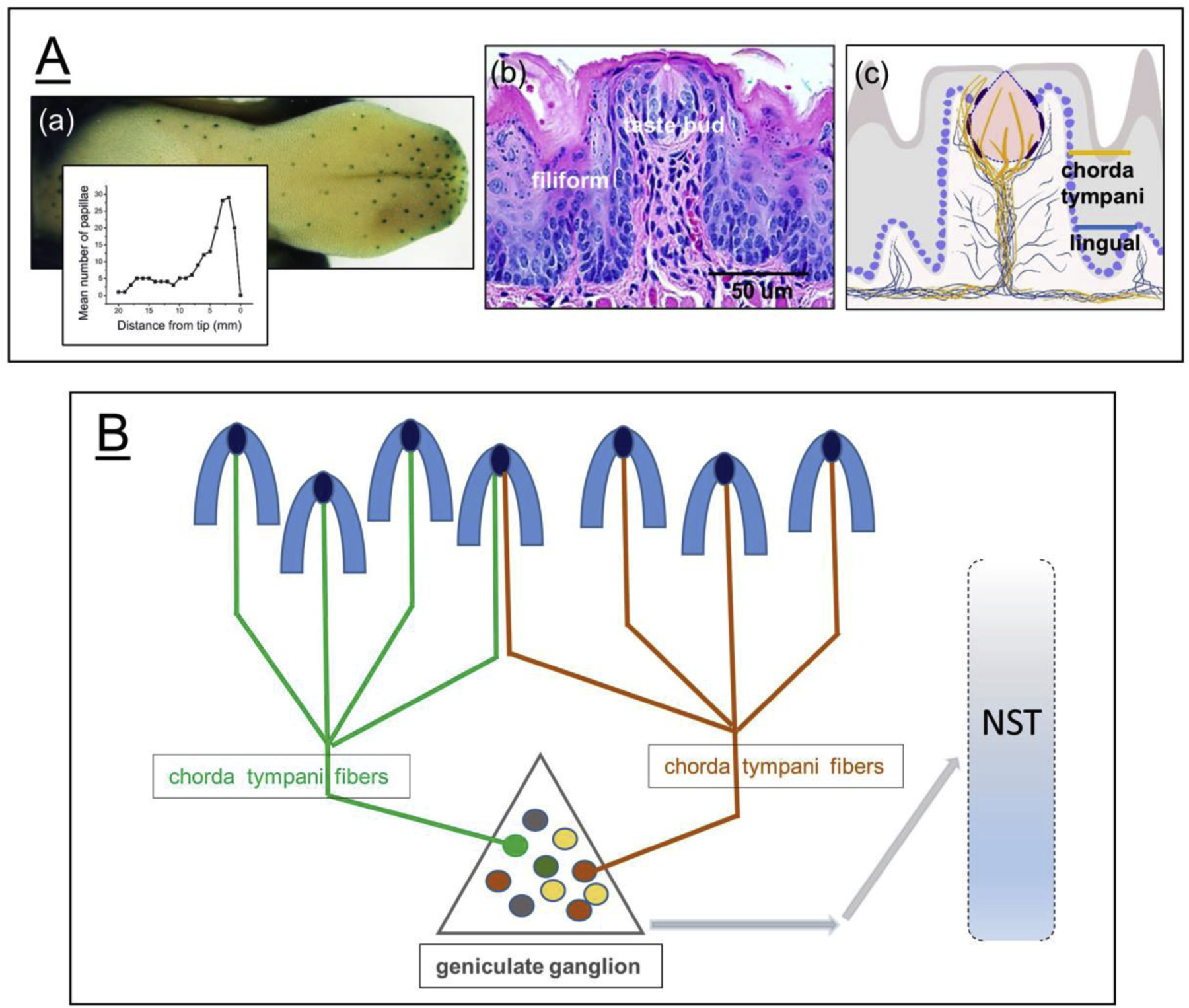
Fungiform papilla distribution, histology and innervation, and receptive fields of chorda tympani fibers and geniculate ganglion (GG) neurons. A. (a) Fungiform papillae (FP) are most dense on the anterior tongue tip, seen with blue X-Gal staining in Gli1lacZ+ mouse tongue. Data in the graph insert demonstrate lingual distribution of FP (redrawn from Ref. 11). (b) The single taste bud in rodent FP is in stratified squamous lingual epithelium encompassing a connective tissue core of cells, vessels and nerves (H&E stain). Filiform papillae, without taste buds, bracket the FP. (c) Diagram of FP innervation illustrates fibers of the chorda tympani nerve (yellow) that distribute in the central papilla core to the taste bud and to epithelium around the taste bud (and see Figure 1). Fibers of the lingual nerve (blue) project to lateral papilla walls and around, but not into, the taste bud. B. FP receptive fields of chorda tympani fibers from GG neurons vary in size, with FP converging on fibers and neurons, and potential overlap of fields. The receptive fields have functional subtypes in the GG, shown in Figure 3. GG neurons project centrally to second order cells in the nucleus of the solitary tract (NST).
The dome-shaped FP is covered with keratinocytes of a stratified squamous epithelium and incorporates taste buds (Figure 2A). The epithelium and taste buds encompass a connective tissue corpus populated with fibroblasts, inflammatory cells, blood vessels, nerves and extracellular matrix molecules. Around the fungiform are spinous, heavily keratinized, non-gustatory filiform papillae [8]. The chorda tympani nerve fibers are directed to innervate taste bud cells in the FP, and also innervate perigemmal non-taste bud or extragemmal epithelial cells, with soma in the GG. The fibers of the lingual nerve are directed to non-taste bud regions of the FP, with soma in the TG. Although the extragemmal endings in fungiform papillae had been attributed to the lingual fibers from TG neurons [14], in other papers these projections are identified as chorda tympani endings from the GG with Phox2b, P2X3 and Shh labeling [6–8,15]. Some sympathetic innervation also is proposed (15).
How are fungiform papillae connected to peripheral nerve and ganglion elements? Fungiform papilla receptive fields
Multimodal responses from chorda tympani fibers in fungiform papillae can be understood through study of all papillae in a receptive field. The sensory receptive field, or receptor elements connected to a single neuron that transmits to the central nervous system, identifies the organs and tissue area committed to a neuron [16]. Receptive field properties determine responses of projecting neurons and shape sensory circuits. For the fungiform papillae (FP) and taste buds, receptive field identifications can be made from chorda tympani nerve fibers or from GG neurons. Taste buds and FP converge via branches onto single chorda tympani fibers, and chorda fibers converge onto single GG soma (Figure 2B).
In general, several FP are innervated by branching, single chorda tympani fibers (and see Ohman and Krimm, this issue). Receptive field size ranges widely [17–21] from up to 8 papillae in rat [21] and as many as 40 papillae per field in sheep [20]. Not only do afferent fibers branch to individual FP but also a single taste bud and FP can be innervated by a number of GG neurons [22,23].
Study of GG neurons elucidates receptive field characteristics of the projecting neurons per se. In prior investigations of GG responses to tongue stimulation, the emphasis had been mainly on use of chemicals with limited attention to thermal or mechanical stimulation alone [24,25]. To address potential multimodality of GG receptive fields, individual FP were mapped during extracellular single neuron recordings with electrical stimulation of single papillae [26]. Then chemical, thermal, and tactile stimuli were applied to characterize response properties of the neuron and receptive field.
There were unique multisensory characteristics of GG neurons projecting to chorda tympani receptive fields [26]. Receptive field sizes varied from 2 to 8 FP. Subsets of neurons were observed that responded only to: chemical; chemical and thermal; thermal; or tactile stimuli (Figure 3). The largest receptive fields responded to chemicals whereas those responding specifically to thermal and tactile stimulation were smallest. Thermal responses were to cold stimuli, not warm. Responses to tactile stimuli were elicited by tongue stroking only. Pressure applied to a single fungiform papilla was ineffective.
Figure 3.
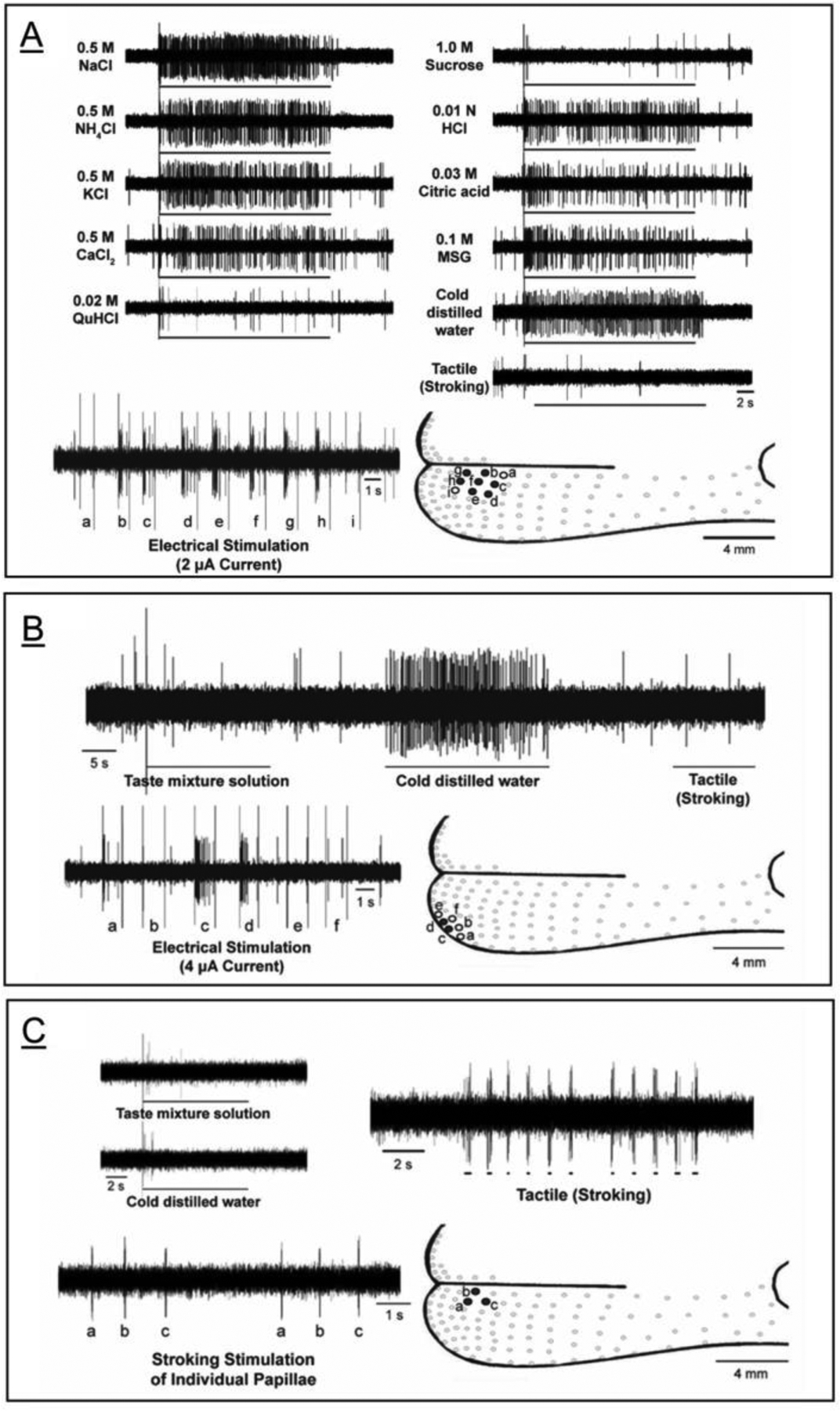
Functional groups of geniculate ganglion (GG) neuron receptive fields. Three types of GG receptive fields are shown in boxes that include extracellular recordings from single GG cells, mapping of individual FP in the receptive field by electrical stimulation, and drawing of FP in the receptive field on a tongue diagram. A. GG responses to several chemicals and to cold water (4°C) in a chemical/thermal receptive field of 7 FP. There were no responses to tactile stimuli. B. GG responses to lingual stimulation with cold water. There were no responses to a mixture of chemical or taste stimuli, or to tactile stroking. The field has 2 FP. Surrounding FP do not respond to stimulation. C. GG responses to lingual tactile stroking only, not to lingual stimulation with a mixture of chemicals or to cold water (4°C). The field has 3 FP. In general, GG receptive fields for responses to temperature and tactile stimuli are smaller than those for chemicals. (Data from Ref. 26).
We predict that the functional GG groups will relate to GG transcriptome signatures. With RNA sequencing, GG neurons were profiled broadly into gustatory (soma of chorda tympani and/or greater superficial petrosal nerves) and somatosensory (soma of ear pinna innervation) groups [27]. One cluster of gustatory neurons also expressed genes characteristic of mechanoreception [27]. Newer transcriptome data expand the earlier report and indicate that the proposed gustatory-mechanosensitive neurons innervate papillae but not taste buds, express the mechanosensitive channel Piezo2, have axons that form P2X3+ perigemmal specializations, and are hypothesized as transducing mechanical stimuli (Rosales et al. International Symposium on Olfaction and Taste, 2020). Also, in an independent study with transcriptional profiling of Phox2b-expressing, GG gustatory neurons, several ganglion clusters were defined that include a possible mechanosensitive gustatory cluster that expressed Phox2b and Piezo2 [28].
Receptive fields of fungiform papillae (FP) innervated by TG neurons.
Each FP is also innervated by lingual nerve fibers of the TG soma, although these do not project to taste buds. Conventional taste stimuli are relatively ineffectual stimuli for the lingual nerve [29,30]. Receptive field size and tactile and thermal somatosensory responses of rat TG neurons projecting through the lingual nerve were determined by mechanically stimulating individual FP (Sato H, Kumari A, Mistretta CM, Bradley RM AChemS Abstracts, 2019). Field sizes ranged from 2 to 6 FP. In contrast to GG neurons, the TG neurons for lingual nerve fibers responded to punctate pressure stimulation of a single FP and response frequency increased with increasing pressure. Responses of the FP mechanoreceptors were in two groups, of high or low sensitivity to tactile stimulation, and highest sensitivity receptors were at the tongue tip.
The TG differs from the GG in responses to oral stimuli. (1)TG neurons do not respond robustly to moderate concentrations of taste chemicals. (2) TG neurons respond to noxious, irritant chemical stimuli, not effective in eliciting in GG neuron responses. TG nociceptive neurons respond to noxious hot temperatures and temperature-receptive neurons respond with substantial overlap to capsaicin, menthol and mustard oil that elicit sensations of pungency and irritation [31,32]. Such properties are not reported in GG neurons. (3) TG neurons respond to static pressure on FP, whereas the GG neurons respond to stroking only.
What is the particular response of chorda tympani and GG neurons to fungiform papilla (FP) receptive field tactile stimulation?
Chorda tympani nerve fibers responded to tactile stimulation by stroking in the absence of taste buds [5,7,10]. GG neurons projecting via the chorda tympani responded, similarly, with a sustained discharge, only to light stroking across the lingual receptive field, not to punctate pressure application [26]. Stroking the lingual epithelium between FP did not elicit a response. Mechanosensitive responses from cat chorda tympani also were predominantly to lingual stroking [33]. What is the fiber type mediating responses to lingual stroking? In human skin Aβ fibers are a principal class for light touch sensation and Aβ field-low threshold mechanoreceptors respond to gentle skin stroking and are not responsive to hair deflection or skin indentation [34]. Mechanoreceptors that respond maximally to slow velocity stroking also have been classified as small diameter, C-fiber low-threshold mechanoreceptors [35–37]. The chorda tympani fiber types for tongue stroking have yet to be classified.
What are the transducers and receptors for fungiform papilla (FP) responses to taste, touch and temperature stimuli?
Taste bud cells and/or intragemmal fiber endings.
Responses from the FP to chemical/taste stimuli are transduced by taste bud cell types, with purported phenotypic and molecular specializations associated with chemical taste qualities [38,39]. Responses to moderate temperature and or tactile stimuli have not been directly attributable to taste bud cells.
In review of TRP channels in taste and in chemethesis (sensing irritation, pungency, pain) unequivocal attribution within taste bud cells is only ascribed to TRPM5 [40]. However, in chemethesis responses in anterior tongue, via trigeminal nerve fibers, roles for TRPV1 (capsaicin agonist) and TRPM8 (temperature sensation) are reported.
Chorda tympani fiber extragemmal endings, outside of the taste bud.
FP innervation is extensive with 196 unmyelinated and 28 myelinated nerve profiles at the papilla base, described in rat [41]. Fibers branch apically and into the taste bud, and many terminate in the epithelium around the taste bud. Outside of the taste bud, globose and varicose perigemmal nerve endings were attributed to trigeminal endings [42]. However, chorda tympani fiber endings that are extragemmal have been experimentally identified [6–8,15]. With labeling by the transcription factor Phox2B, chorda tympani fibers after Hedgehog pathway inhibition were specifically demonstrated (Kumari A, Li L, Donnelly CR, Pierchala BA, Bradley RM, CM Mistretta CM AChemS Abstracts, 2019). These fibers extend to the base of the taste bud, into the taste bud, and into complex endings around the taste bud. After taste buds are eliminated in Hedgehog signaling inhibition, the fibers remain in the FP core and in perigemmal epithelium. These remaining chorda fibers respond to tactile and cold stimuli [5,6,10] (Figure 1). Piezo2 protein, an ion channel active in touch mechanotransduction, was reported in extragemmal fiber endings around FP taste bud cells [43]. The authors noted that the fiber types were previously attributed to those with TG soma but more recently were identified as including neurons from a Phox2b lineage. We find that this Piezo2+ area is comparable to that reported for chorda tympani fiber terminations. Overall, in tongue sensation, our understanding of chorda tympani fiber endings of the GG neurons is woefully inadequate.
End organs in the fungiform papilla (FP).
The human tongue is more sensitive to tactile contact and two-point touch discrimination than the upper lip border or the fingertip [44], and there have long been attempts to identify lingual nerve endings and organs that respond to touch stimuli. Free endings, semiorganized endings, and coiled nonmyelinated fibers are in human tongue, most dense at the tip. These match well with the extragemmal chorda tympani endings that we have observed (Kumari A, Li L, Donnelly CR, Pierchala BA, Bradley RM, CM Mistretta CM, AChemS Abstracts, 2019). Single lingual nerve afferents were also recorded in human tongue [45]. Low threshold units, rapidly adapting or slowly adapting, had small receptive fields in anterior tongue. There is not, however, a known association between histological and functional classifications in the tongue, as shown for skin mechanoreceptors in the human hand [46].
Merkel cell-neurite complexes or Meissner corpuscles for skin light touch sensation [47], have not been identified in the tongue [43] but are in gingiva and hard palate [43,48]. The Meissner corpuscle is essential for gentle touch detection in glabrous skin [47], but presumably is not a principal end organ in FP. The corpuscles are innervated by Aβ neurons for gentle touch responses in skin, and two neuron subtypes, which express TrkB or Ret, have different sensitivity to tactile stimuli, [47]. Similarly there is mechanoreceptive signaling in GG neurons that include GDNF/ret [49]. Perhaps the absence of identified end organs for light touch or stroking in the FP is not that odd if one fully considers the nature of this lingual organ. In large FP with multiple taste buds and innervation (e.g., human, primate, sheep) the concept is evoked of a modified touch dome with a collection of Merkel cell-nerve complexes that have CGRP/K8/K18/K20+ paracrine/neuroendocrine cells in the epidermis innervated by Aβ, Aδ and C fibers [50,51].
Fungiform papilla keratinocytes.
Keratinocytes are prominent in the fungiform papilla (FP) epithelium and roles for these non-neuronal cells in touch reception were defined recently in a proposed paradigm shift in skin sensation [52]. Skin keratinocytes mediated innocuous and noxious touch stimuli by releasing ATP that acted via P2X4 on closely apposed neurons [52]. In taste sensation, ATP released from taste bud cells in response to lingual taste stimuli activates P2X2/P2X3 purinergic receptors on gustatory nerves [38]. Roles for lingual keratinocytes in FP multimodal sensation should be considered.
Thinking About The Multimodal Fungiform Papilla (FP) Organ
Several receptor components in the biologically powerful, multimodal, taste, temperature and tactile FP organ are dedicated to sensory detection, recognition and transmission in eating: taste bud cells; chorda tympani nerve fibers within the taste bud and extragemmal to the taste bud; specialized nerve endings or complexes; and, keratinocytes of the apical papilla epithelium (Figure 4). Lingual nerve, TG-derived elements in the fungiform organ respond to painful, noxious, irritant stimuli which participate in chemethesis [53]. Notably, however, the FP with its chorda tympani nerve, GG neuron innervation alone is a polymodal, complex taste, touch and temperature lingual organ, not solely a gustatory papilla. A recent demonstration of a multimodal epithelial organ of the octopus sucker defines receptors and cells of the octopus chemotactile sense [54]. Chemotactile receptors mediate touch-taste sensation in probing surfaces to seek prey, and these project through ganglia to a nerve cord. It is intriguing to think of this organ as an ancestor of the multimodal fungiform papillae on the tongue tip.
Figure 4.
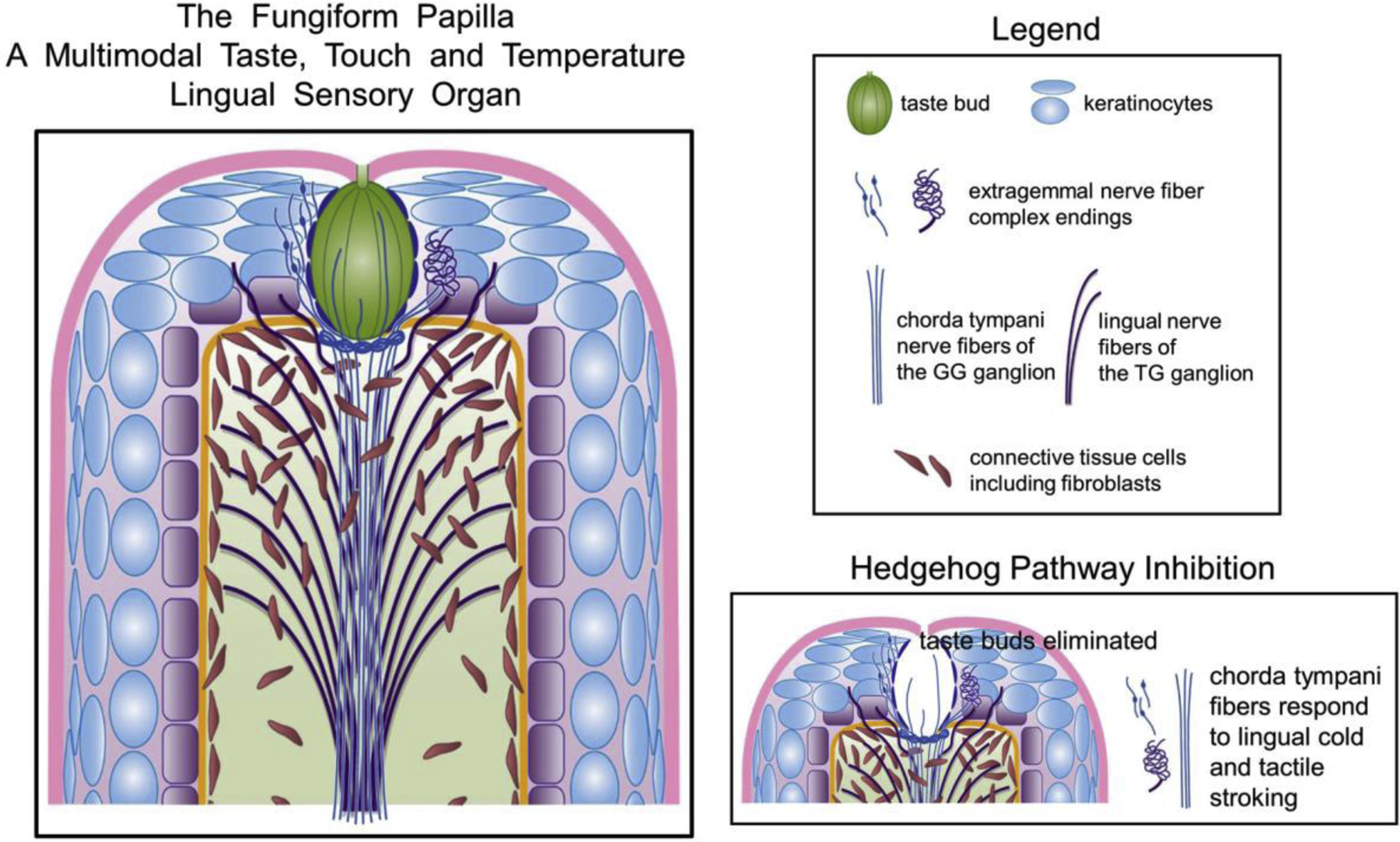
The Fungiform Papilla (FP) multimodal taste, touch and temperature organ. Taste bud cells, innervated by the chorda tympani nerve, respond to tongue stimulation with chemicals. Chorda tympani fibers (thin, blue) also project into the epithelium in extragemmal, complex nerve endings that we propose respond to tongue stroking and temperature stimuli. Lingual nerve fibers (thick, purple) project to lateral FP walls, not into the taste bud, but do project into the epithelium. The lingual nerve fibers are principally committed to responding to noxious, irritant and painful lingual stimuli, not to taste chemicals or light stroking or moderate temperatures. Keratinocytes in the FP epithelium are proposed as potential response elements for lingual stimuli through non-neuronal to neuronal pathways as shown in the skin [52]. In Hedgehog Pathway Inhibition the taste buds are eliminated and the remaining chorda tympani fibers, including extragemmal complex endings, respond to lingual cold and tactile stroking stimuli [5,6,10]. Overall, the FP with chorda tympani and GG innervation is a polymodal receptor organ to detect and transmit information about taste, touch and temperature from one organ, one nerve and one sensory ganglion to central circuits for eating and flavor perception.
Fresh perspectives in thinking about the polymodal FP and attendant chorda tympani innervation from the GG could yield a revised vision for considering how chemosensory and somatosensory modalities integrate oral taste, touch and temperature. To clarify similarities with skin somatosensation and consider noncanonical signaling, the types of chorda tympani fibers (Aβ, Aγ, Aδ, C) that respond to tactile stroking and cold sensation should be identified (e.g., see 12 functional subtypes of somatosensory neurons in the dorsal root ganglion and morphologically distinct axon endings [55]). The particular nerve endings or organs that respond to lingual stroking should be determined. Investigating potential signaling from keratinocytes to nerves would bring new ideas about sensory signaling in FP. A renewed focus on the FP and associated receptive fields as multimodal will highlight the complex lingual signaling required for eating.
ACKNOWLEDGEMENTS
Supported by National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grant R01 DC014428. We note that due to restrictions on number of references, the work from several papers associated with the topic could not be cited.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Nothing declared.
References
Papers of particular interest have been highlighted as:
** of outstanding interest
- 1.Hiiemae KM, Palmer JB: Tongue movements in feeding and speech. Crit Rev Oral Biol Med 2003, 14:413–429. [DOI] [PubMed] [Google Scholar]
- 2.Martin LE, Kay KE, Torregrossa AM: Bitter-induced salivary proteins increase detection threshold of quinine, but not sucrose. Chem Senses 2019, 44:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa H, Sato M, Yamashita S: Multiple sensitivity of chorda tympani fibers of the rat and hamster to gustatory and thermal stimuli. J Physiol 1968, 199:223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimatani Y, Grabauskiene S, Bradley RM: Long-term recording from the chorda tympani nerve in rats. Physiol Behav 2002, 76: 143–149. [DOI] [PubMed] [Google Scholar]
- 5.Kumari A, Ermilov AN, Bradley RM, Allen BL, Dlugosz AA, Mistretta CM: Hedgehog pathway blockade with cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol 2015, 113:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumari A, Ermilov A, Grachtchouk M, Allen BL, Bradley RM, and. Mistretta CM: Recovery of taste organs and sensory function after severe loss from Hedgehog/Smoothened inhibition with cancer drug sonidegib. Proc Nat Acad Sci USA 2017, 114:E10369–E10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mistretta C, Kumari A: Hedgehog signaling regulates taste organs and oral sensation: distinctive roles in the epithelium, stroma, and innervation. Int J Mol Sci 2019, 20(6) 1341; 10.3390/ijms20061341 [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Comprehensive recent review of distinctive roles for taste buds, nerves and connective tissue in taste sensory homeostasis and regeneration, with discussion of multimodal signaling.
- 8.Mistretta C, Kumari A: Tongue and taste organ biology and function: homeostasis maintained by hedgehog signaling. Ann Rev Physiol 2017, 79: 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermilov A, & Kumari A, Libo L, Joiner A, Grachtchouk M, Allen B, Dlugosz A, Mistretta C: Maintenance of taste organs is strictly dependent on epithelial hedgehog/GLI signaling. PLOS Genetics 2016. 10.1371/journal.pgen.1006442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumari A, Yokota Y, Li L, Bradley RM, Mistretta CM: Species generalization and differences in Hedgehog pathway regulation of fungiform and circumvallate papilla taste function and somatosensation demonstrated with sonidegib. Sci Rep 2018, 8, 16150. 10.1038/s41598-018-34399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller IJ Jr, Preslar AJ: Spatial distribution of rat fungiform papillae. Anat Rec 1974, 181:679–684. [DOI] [PubMed] [Google Scholar]
- 12.Mistretta CM: Developmental neurobiology of the taste system. In, Smell and Taste in Health and Disease, Getchell TV, Doty RL, Bartoshuk LM, Snow JB. (Eds) Raven Press, New York, 1991, pp35–64. [Google Scholar]
- 13.Spector AC, Glendinning JI: Linking peripheral taste processes to behavior. Curr Opin Neurobiol 2009, 19:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidi FN, Cicchini V, Kaufman D, Ko E, Ko A, Tassel H, Whitehead MC: Innervation of taste buds revealed with Brainbow-labeling in mouse. J Anat 2016, 229:778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohman-Gault L, Huang T, Krimm R: The transcription factor Phox2b distinguishes between oral and non-oral sensory neurons in the geniculate ganglion. J Comp Neurol 2017, 525:3935–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherrington CS: Observations on the scratch-reflex in the spinal dog. J Physiol. 1906, 34:1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller IJ Jr: Peripheral interactions among single papilla inputs to gustatory nerve fibers. J Gen Physiol 1971, 57:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson PP: The characteristics and regional distribution of afferent fibres in the chorda tympani of the cat. J Physiol 1988, 406:345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boudreau JC, Sivakumar L, Do LT, White TD, Oravec J, Houang NK: Neurophysiology of geniculate ganglion (facial nerve) taste systems: species comparisons. Chem Senses 1985, 10:89–127. [Google Scholar]
- 20.Nagai T, Mistretta CM, Bradley RM: Developmental decrease in size of peripheral receptive fields of single chorda tympani nerve fibers and relation to increasing NaCl taste sensitivity. J Neurosci 1988, 8:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota Y, Bradley RM: Receptive field size, chemical and thermal responses and fiber conduction velocity of rat chorda tympani geniculate ganglion neurons. J Neurophysiol 2016, 115:3062–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krimm RF, Hill DL: Innervation of single fungiform taste buds during development in rat. J Comp Neurol 1998, 398:13–24. [PubMed] [Google Scholar]
- 23.Whitehead MC, Ganchrow JR, Ganchrow D, Yao B: Organization of geniculate and trigeminal ganglion cells innervating single fungiform taste papillae: a study with tetramethylrhodamine dextran amine labeling. Neuroscience 1999, 93:931–941. [DOI] [PubMed] [Google Scholar]
- 24.Lundy RF Jr, and Contreras RJ: Gustatory neuron types in rat geniculate ganglion. J Neurophysiol 1999, 82: 2970–2988. [DOI] [PubMed] [Google Scholar]
- 25.Breza JM, Curtis KS, Contreras RJ: Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 2006, 95:674–685. [DOI] [PubMed] [Google Scholar]
- 26.Yokota Y, Bradley RM: Geniculate ganglion neurons are multimodal and variable in receptive field characteristics. Neuroscience 2017, 367:147–158. [DOI] [PubMed] [Google Scholar]; ** With fungiform papilla receptive field maps and single geniculate ganglion neuron recordings, the paper directly demonstrates multimodal taste, touch and temperature responses and shows importance of fungiform papillae and receptive fields in understanding complex lingual sensory signaling.
- 27.Dvoryanchikov G, Hernandez D, Roebber JK, Hill DL, Roper SD, Chaudhari N: Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat Comm 2017, 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson CB, Larson ED: Single cell transcriptional profiling of Phox2b-expressing geniculate ganglion neurons. bioRxiv preprint 2020. 10.1101/812578 [DOI] [Google Scholar]
- 29.Silver WI, Finger TE: The trigeminal system, In, Smell and Taste in Health and Disease, Getchell TV, Doty RL, Bartoshuk LM, Snow JB. (Eds) Raven Press, New York, 1991, 97–106. [Google Scholar]
- 30.Lundy RF Jr, and Contreras RJ: Neural responses of thermal-sensitive lingual fibers to brief menthol stimulation. Brain Res 1994, 641: 208–216. [DOI] [PubMed] [Google Scholar]
- 31.Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, Zuker CS: Coding and plasticity in the mammalian thermosensory system. Neuron 2016, 92:1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leijon SCM, Neves AF, Breza JM, Simon SA, Chaudhari N, Roper SD: Oral thermosensing by murine trigeminal neurons: modulation by capsaicin, menthol and mustard oil. J Physiol 2019, 597:2045–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biedenbach MA, Chan KY: Tongue mechanoreceptors: comparison of afferent fibers in the lingual nerve and chorda tympani. Brain Res 1971, 35:584–588. [DOI] [PubMed] [Google Scholar]
- 34.Bai L, Lehnert BP, Liu j, Neubarth NL, Dickendesher TL, New PH, Cassidy C, Woodbury CJ, Ginty DD: Genetic identification of an expansive mechanoreceptor sensitive to skin stroking. Cell 2015, 163:1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** In vivo identification of low threshold mechanoreceptors in skin as highly sensitive to gentle stroking, not responsive to hair deflections.
- 35.Ackerley R, Backlund Wasling H, Liljencrantz J, Olausson H, Johnson RD, Wessberg J: Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J Neurosci 2014, 34:2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Recordings from single human tactile afferents, with microneurography, demonstrate fibers specifically responsive to gentle stroking.
- 36.Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A: The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev 2010, 34:185–191. [DOI] [PubMed] [Google Scholar]
- 37.Le Pichon CE, Chesler AT: The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat 22 April 2014. 10.3389/fnana.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinnamon SC, Finger TE; A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci 2013: 7:264 doi: 10.3389/fncel.2013.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banik DD, Benfey ED, Martin LE, Kay KE, Loney GC, Nelson AR, Ahart GC, Kemp BT, Kemp BR, Torregrossa AM, Medler KF: A subset of broadly responsive Type III taste cells contribute to the detection of bitter, sweet and umami stimuli. Plos Genetics 2020, 16(8): e1008925. 10.1371/journal.pgen.1008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roper SD: TRPs in Taste and Chemesthesis. In: Nilius B, Flockerzi V. (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, 2014, 223: Springer, Cham. 10.1007/978-3-319-05161-1_5 [DOI] [PubMed] [Google Scholar]
- 41.Beidler LM: Innervation of rat fungiform papilla. In Olfaction and Taste III, Pfaffmann C(ed) pp 352–369. New York: Rockefeller University Press: 1969. [Google Scholar]; ** A classic paper that provides the only detailed, quantitative measures of peripheral nerve fibers throughout the fungiform papilla.
- 42.Zaidi FN, Cicchini V, Kaufman D, Ko E, Ko A, Tassel H, Whitehead MC: Innervation of taste buds revealed with Brainbow-labeling in mouse. J Anat 2016, 229:778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moayedi Y, Duenas-Bianchi LF, Lumpkin EA: Somatosensory innervation of the oral mucosa of adult and aging mice. Sci Rep 2018. 8, 9975. 10.1038/s41598-018-28195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rath EM, Essick GK: Perioral somesthetic sensibility: Do the skin of the lower face and midface exhibit comparable sensitivity? J Oral Maxillofac Surg 1990, 48: 1181–1190. [DOI] [PubMed] [Google Scholar]
- 45.Trullsson M, Essick GK: Low-threshold mechanoreceptive afferents in the human lingual nerve. J Neurophysiol 1997, 77:737–748. [DOI] [PubMed] [Google Scholar]
- 46.Johansson RS, Vallbo AB: Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci 1983, 6:27–32. [Google Scholar]
- 47.Neubarth NL, Emanuel AJ, Liu Y, Springel MW, Handler A, Zhang Q, Lehnert BP, Guo C, Orefice LL, Abdelaziz A, DeLisle MM, Iskols M, Rhyins J, Kim SJ, Cattel SJ, Regehr W, Harvey CD, Drugowitsch J, Ginty DD: Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 2020, 368:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barretta AW, Corta EM, Patela P, Berkovitz BKB: An immunohistological study of cytokeratin 20 in human and mammalian oral epithelium. Arch Oral Biol 2000, 45: 879–887. [DOI] [PubMed] [Google Scholar]
- 49.Donnelly CR, Shah AA, Mistretta CM, Bradley RM, Pierchala BA: Biphasic functions for the GDNF-Ret signaling pathway in chemosensory neuron development and diversification. Proc Nat Acad Sci USA 2018, 115: E516–E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinisch CM, Tschachler E: The touch dome in human skin is supplied by different types of nerve fibers. Ann Neurol 2005, 58:88–95. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Y, Williams JS, Brownell I: Merkel cells and touch domes: more than mechanosensory functions? Exp Neurol 2014, 23:692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL: Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. eLife 2018, 7:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Determines a role for skin keratinocytes in touch and demonstrates signaling from keratinocytes via ATP release to sensory neuron P2X4 receptors in tactile sensation. This non-neuronal to neuronal cell signaling in touch brings new thinking in cutaneous mechanotransduction.
- 53.Green BG: Chemesthesis and the chemical senses as a component of a “Chemofensor Complex”. Chem Senses 2012, 37:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Giesen L, Killian PB, Allard CAH, Bellono NW: Molecular basis of chemotactile sensation in octopus. Cell 2020, 183:594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD: The emergence of transcriptional identity in somatosensory neurons. Nature 2020, 577: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The paper reports transcriptional identities for multiple subtypes of somatosensory neurons in dorsal root ganglion. Such studies, if applied to the geniculate ganglion, can clarify lingual somatosensory classes.


