Abstract
We evaluated 100 postacute coronavirus disease 2019 (COVID-19) patients a median (interquartile range) of 60 (48–67) days after discharge from the Careggi University Hospital, Italy. Eighty-four (84%) had at least 1 persistent symptom, irrespective of COVID-19 severity. A considerable number of hospital readmissions (10%) and/or infectious diseases (14%) during the postdischarge period were reported.
Keywords: COVID-19, follow-up, sequelae, SARS-CoV-2, long-term
On December 31, 2019, the world received the first notice of a cluster of atypical pneumonia cases due to a novel coronavirus, later named severe acute respiratory syndrome 2 (SARS-CoV-2) [1]. Twelve months later, nearly 90 million cases of coronavirus disease 2019 (COVID-19) have been reported worldwide, with almost 2 million deaths [2].
Clinical presentation can be variable, ranging from SARS-CoV-2 asymptomatic carriers to life-threatening and fatal disease. The most common symptoms include fever, cough, and shortness of breath. Musculoskeletal symptoms, such as myalgia, joint pain, headache, and fatigue have also been reported, as well as enteric symptoms (abdominal pain, vomiting, and diarrhea), anosmia, and dysgeusia [3, 4]. Critically ill patients often require prolonged hospital stay, mechanical ventilation, and an intensive level of treatment, being at higher risk of severe complications, such as septic shock, thromboembolic events, and acute kidney injury [5].
Initial reports are emerging about the persistence of a significant symptom burden in the aftermath of recovery from acute COVID-19; this phenomenon has been called “long COVID” [6–8]. More insight about the short- and long-term consequences of SARS-CoV-2 infection is essential to properly inform follow-up programs for patients who experienced symptoms of COVID-19 [9]. An earlier clinical review within 4–8 weeks postdischarge has been recommended, at least in patients who experienced more severe symptoms [10]. In Tuscany, Italy, a comprehensive 12-month follow-up, including multidisciplinary evaluations according to disease severity and patient characteristics, is guaranteed to all individuals diagnosed with SARS-CoV-2 infection [11].
In this paper, we report the results of the first step of the follow-up program for postacute COVID-19 patients discharged from Careggi University Hospital, Florence, Italy, consisting of a clinical and biochemical assessment 8 weeks after hospital discharge.
METHODS
Since May 20, 2020—soon after the end of the epidemic phase—an outpatient service dedicated to the follow-up of postdischarge COVID-19 patients has been active at the Careggi University Hospital, Florence, Italy, in accordance with the program of the Tuscany Region. Several specialists from different disciplines are involved with this program, including infectious diseases specialists, pulmonologists, cardiologists, immunologists, and physiotherapists.
All patients discharged from the hospital were offered a clinical visit. Exclusion criteria were (i) patients discharged for more than 10 weeks; (ii) patients unable to attend the visit because of hospitalization or residents in care facilities; (iii) patient refusal. Data on subjects’ death after discharge were collected.
Data on previous hospital admissions were retrieved from electronic medical records. Disease severity was classified as mild, moderate, severe, and critical, according to World Health Organization (WHO) definitions [12]. A detailed postdischarge clinical history was collected through a standardized questionnaire focused on persistence of symptoms potentially related to recent SARS-CoV-2 infection. Symptom count included any self-reported symptom persisting at the time of the follow-up visit. Postdischarge symptoms resolved before the visit were not considered in the count. The symptom inventory questionnaire used for the study is shown in the Supplementary Data. Moreover, hospital readmissions and postdischarge infections were ascertained by medical record review, or, alternatively, they were self-reported and supported by all available medical documentation. A full physical examination was performed.
Laboratory tests included complete blood count, coagulation profile, serum biochemical tests and serum inflammatory markers, and arterial blood gas test.
Descriptive analysis was used to illustrate population characteristics. Categorical variables were evaluated with the Pearson chi-square/Fisher exact test, as appropriate. Continuous variables were evaluated with the Mann-Whitney test. A multivariate logistic regression was performed, aiming to investigate the association between symptom persistence (categorical) and demographic factors (age, gender), comorbidity burden (by Charlson comorbidity index), and clinical severity of COVID-19 (as per WHO classification).
Patient Consent Statement
Data collection was approved by the local ethics committee (17104_oss). Patient consent was obtained. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and with the International Conference on Harmonization Good Clinical Practice guidelines.
RESULTS
Between May 20 and August 26, 2020, 178 patients were potentially eligible for the 8-week follow-up review, of whom 71 presented at least 1 exclusion criterion (41 residents of health care facilities, 7 patients readmitted to the hospital, 3 already followed by other outpatient services, 20 refused or did not answer). In addition, 7 patients died after discharge. One hundred patients (41% female; median age [interquartile range {IQR}], 67.5 [56–78.5] years) attended the postdischarge follow-up visit. Baseline characteristics and data on the COVID-19-related hospitalization of the 100 patients are reported in detail in Table 1. In brief, 12/100 (12%) and 47/100 (47%) experienced severe and critical COVID-19, respectively; the median hospital length of stay (IQR) was 16 (8–27) days, with 31/100 (31%) admitted to the intensive care unit (ICU). Most of the evaluated subjects received antiretrovirals (76/100, 76%) and/or hydroxychloroquine (88/100, 88%). Immune-modulator drugs (such as tocilizumab and ruxolitinib) and high-dose steroids (methylprednisolone equivalent ≥1 mg/kg/d) were used in 48/100 (48%) and 26/100 (26%) cases, respectively; 90/100 (90%) required oxygen supplementation, including high-flow nasal cannulae (7/100, 7%), noninvasive ventilation (26/100, 26%), and mechanical ventilation (21/100, 21%).
Table 1.
Baseline Characteristics and Data on COVID-19-Related Hospitalization of the Studied Population (n = 100 Patients)
| Baseline Characteristics | Total (n = 100) |
|---|---|
| Gender female, No. (%) | 41 (41) |
| Age, y | |
| Median (IQR) | 67.5 (56–78.5) |
| Range | 24–90 |
| Charlson comorbidity index, median (IQR) | 3 (1–4) |
| Hypertension, No. (%) | 50 (50) |
| Diabetes, No. (%) | 21 (21) |
| COPD, No. (%) | 12 (12) |
| CHD, No. (%) | 12 (12) |
| CKD, No. (%) | 7 (7) |
| Obesity, No. (%) | 25 (25) |
| Length of hospital stay, median (IQR), d | 16 (8–27) |
| Time to microbiological cure, median (IQR), da | 27 (14–44) |
| Treatments, No. (%) | |
| Antiretrovirals (LPV/r, DRV/c) | 76 (76) |
| Hydroxychloroquine | 88 (88) |
| Remdesivir | 9 (9) |
| Immune-modulators | 48 (48) |
| High-dose steroid (≥1 mg/kg 6-MP) | 26 (26) |
| Antibiotics | 49 (49) |
| ICU admission, No. (%) | 31 (31) |
| Highest oxygen supplementation, No. (%) | |
| No support | 13 (13) |
| Standard oxygen therapy | 33 (33) |
| High flow nasal cannulae | 7 (7) |
| Noninvasive ventilation | 26 (26) |
| Mechanical ventilation | 21 (21) |
| COVID-19 severity (WHO), No. (%) | |
| Mild | 9 (9) |
| Moderate | 32 (32) |
| Severe | 12 (12) |
| Critical | 47 (47) |
| Follow-up timing, median (IQR) | |
| Days since symptoms onset | 82 (70–101) |
| Days since hospital discharge | 60 (48–67) |
| Days since microbiological cure | 50 (36–66) |
Abbreviations: CHD, coronary heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; LPV/r, lopinavir/ritonavir; DRV/c, darunavir/cobicistat; ICU, intensive care unit; IQR, interquartile range; WHO, World Health Organization.
aTime from the first positive to the first negative nasopharyngeal swab.
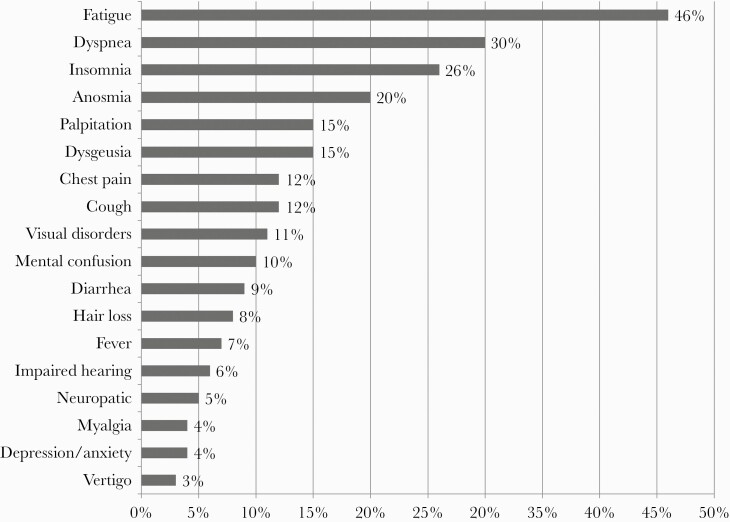
At the time of the follow-up visit, a median (IQR) of 60 (48–67) days after hospital discharge, 84/100 (84%) had at least 1 persistent symptom, and 36/100 (36%) reported >2 symptoms. The more frequent symptoms were fatigue (46%), dyspnea (30%), insomnia (26%), anosmia (20%), and dysgeusia and palpitation (15%). Unusual symptoms, such as visual disorders (11%), hair loss (8%), and impaired hearing (6%), were also reported. Other neurological disorders included mental confusion (10%), peripheral neuropathies (5%), and vertigo (3%). Furthermore, 4% of patients had psychological symptoms, such as anxiety and depression (Figure 1).
Figure 1.
Persistent symptoms reported among 100 postacute coronavirus disease 2019 patients a median of 60 days after hospital discharge.
The persistence of symptoms was not associated with COVID-19 severity (83% vs 85% in patients with mild/moderate vs severe/critical disease, respectively; P = .807) or with ICU admission (84% vs 84% in ICU- vs non-ICU-admitted patients, respectively; P = .981) or with length of hospital stay (16 vs 13.5 days in patients with and without persistent symptoms, respectively; P = .559) (Table 2).
Table 2.
Clinical and Laboratory Findings at Follow-up in a Postacute COVID-19 Population (n = 100 Patients)
| Persistent Symptoms | Total (n = 100), No. (%) | Mild to Moderate (n = 41), No. (%) | Severe to Critical (n = 59), No. (%) | P Value | Non-ICU (n = 69), No. (%) | ICU (n = 31), No. (%) | P Value |
|---|---|---|---|---|---|---|---|
| No | 16 (16) | 7 (17) | 9 (15) | .807 | 11 (16) | 5 (16) | .981 |
| Yes | 84 (84) | 34 (83) | 50 (85) | .342 | 58 (84) | 26 (84) | |
| No. of symptoms | |||||||
| 0 | 17 (17) | 7 (17) | 10 (17) | 11 (16) | 5 (17) | ||
| 1–2 | 47 (47) | 16 (39) | 31 (53) | 30 (43) | 17 (59) | ||
| >2 | 36 (36) | 18 (44) | 18 (30) | 28 (41) | 7 (24) | .362 | |
| Postdischarge infectious diseases | 14 (14) | 5 (12) | 9 (15) | .665 | 9 (13) | 5 (16) | .681 |
| Rectal colonization | 19 (19) | 3 (7) | 16 (27) | .018 | 8 (12) | 11 (35) | .005 |
| Blood Test at Follow-up | Reference Values | Median Values (IQR) | |||||
| White blood cell, 109 cells per L | 4.0–10.0 | 6.65 (5.38–7.72) | |||||
| Neutrophil count, 109 cells per L | 1.5–7.5 | 3.98 (3.13–5.84) | |||||
| Lymphocyte count, 109 cells per L | 0.5–5.0 | 1.84 (1.47–2.22) | |||||
| Platelet count, 109 cells per L | 140–440 | 239 (194–290) | |||||
| ALT, U/L | 10–50 | 15 (12–21) | |||||
| Creatinine mg/dL | 0.7–1.2 | 0.9 (0.8–1.1) | |||||
| D-dimer, mg/L | <500 | 444 (302–816) | |||||
| C-reactive protein, mg/L | 0–5 | 4 (4–5) | |||||
| Serum ferritin, μg/L | 30–400 | 152 (69–276) | |||||
| Lactate dehydrogenase, U/L | 135–225 | 194 (174–219) |
Abbreviations: ALT, alanine aminotransferase; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range.
Likewise, no statistical difference was observed between patients with and without symptom persistence by gender, frequency of comorbidities (including hypertension, diabetes, chronic obstructive pulmonary disease, coronary heart disease, chronic kidney disease, and obesity), or median Charlson comorbidity index. By multivariate analysis, only age was associated with an increased risk of symptom persistence (odds ratio, 1.09 for each 1-year increase; 95% CI, 1.02–1.16) (data not shown).
Some patients required hospital readmission (10/100, 10%). Causes for hospital readmission included cardiac disease, such as heart failure and myocardial infarction (n = 5), infectious diseases (n = 2), respiratory symptoms (n = 1), and neurologic disorders (n = 2). Overall, 14 patients (14%) experienced an infection during the postdischarge period, including urinary tract infections, skin and soft tissue infections, and Clostridioides difficile colitis. Nineteen (19%) presented rectal colonization with multidrug-resistant bacteria (vancomycin-resistant Enterococcus spp. and/or carbapenem-resistant Enterobacterales) during the hospital stay, with a higher risk in more severe patients (16/59, 27%) in comparison with milder cases (3/41, 7%; P = .010), and ICU-admitted patients (11/31, 5%) in comparison with non-ICU patients (8/69, 12%; P = .016) (Table 2).
No significant alteration was observed in the median values of the blood test (Table 2). In our population, 22% (22/100) and 14% (14/100) showed persistence of elevated C-reactive protein and ferritin, respectively. Thirteen (13%) patients presented high D-dimer values (>1000 ng/mL). None presented respiratory failure (pO2 < 60 mmHg). Fifteen patients (15/100, 15%) were discharged with long-term oxygen therapy (LTOT), 5 of whom (5/100, 5%) were still on LTOT at the time of the follow-up visit (2 were already on LTOT before SARS-CoV-2 infection).
DISCUSSION
We analyzed clinical and laboratory results from follow-up reviews of 100 COVID-19 patients a median of 60 days after hospital discharge. At the time of the visit, a high percentage of patients (84%) complained of 1 or more persistent symptoms. Similar findings have been observed in recent studies, based on face-to-face reviews or telephone/web surveys, on both COVID-19 inpatient and outpatient populations [6–8, 13–14]. In our study, persistence of symptoms was not related to COVID-19 severity, ICU admission, or length of hospital stay. Among demographic and clinical characteristics, only increasing age was independently associated with higher risk of SARS-CoV-2 infection sequelae. Moreover, in most cases, symptoms were not accompanied by blood test abnormalities, as median values of lymphocyte count, D-dimer, and inflammation markers resulted in range and abnormal results occurred in a minority of patients. Fatigue was the most frequent self-reported symptom (46%). Persistent fatigue has been already reported as a common sequela following SARS-CoV-2 infection, raising concern that SARS-CoV-2 has the potential to trigger postviral chronic fatigue syndrome, similarly to other infectious diseases [15]. In the same study, postviral fatigue was associated with female gender and a preexisting diagnosis of depression/anxiety, while no correlation was observed with the severity of initial SARS-CoV-2 infection, nor with inflammatory biomarker abnormalities.
The available data suggest that chronic sequelae are not limited to more severe COVID-19 cases. The results of a multistate telephone survey in the United States confirmed that return to baseline health after COVID-19 can take a long time, even in young adults with milder diseases and no chronic conditions [13]. Age, female gender, obesity, and burden of comorbidities have been variously identified as predictors of long-term symptom persistence during follow-up assessment performed from a few weeks to 6 months after acute illness [7, 16–17].
Hospital readmission after an initial COVID-19 hospitalization was experienced in 10% of the patients in our population. A large nationwide US study, including more than 100 000 electronical records of COVID-19 patients’ hospitalizations, found a 9% rate of readmission to the same hospital within 2 months of discharge [18]. The odds of hospital readmission increased with age and in the presence of chronic conditions, such as chronic obstructive pulmonary disease, heart failure, diabetes, chronic kidney disease, and obesity. In our study, heart failure and other cardiac conditions accounted for half of hospital readmissions within the 8-week postdischarge period, including an 82-year-old man diagnosed with heart failure and referred to the emergency room during the follow-up visit. Moreover, at least 1 case of sudden death due to heart attack was recorded close to the follow-up visit in a 70-year-old man with a previous history of coronary heart disease. Although it is hard to definitively establish whether these events are due to direct or indirect effects of COVID-19, rather than concurrent complications of underlying conditions, more information about burden and risk factors for COVID-19 patients’ readmission is needed to inform both clinical practice and public health decisions [19–20]. However, postdischarge cardiopulmonary manifestations, such as dyspnea, palpitations, and chest pain, require careful consideration, especially in elderly patients with multiple comorbidities. The wide range of reported symptoms reflects the multi-organ involvement of COVID-19, mediated by direct tissue damage, hyperinflammation, and COVID-19-related coagulopathy. A number of symptoms reported in our study, like chemosensory dysfunction, insomnia, mental confusion, and vertigo, belong to the neuropsychiatric sphere. SARS-CoV-2 tropism of the central nervous system (CNS), likely due to widespread angiotensin-converting enzyme 2 (ACE2) expression in the brain tissue, has been documented [21]. Long-term neurological sequelae in patients with previous SARS-CoV-2 infection will be fully understood only in the coming months, when longitudinal assessments will be performed.
In addition, several patients complained of unusual symptoms, like visual disorders, impaired hearing, and hair loss. Vision and hearing impairment may be part of peripheral nervous system manifestations [22]. A high frequency of male pattern hair loss among patients hospitalized for COVID-19 has been observed in Spain, suggesting that androgen expression might be a clue to COVID-19 severity [23]. Although the aforementioned symptoms are not life-threatening conditions, they can affect patients’ overall well-being and functional status.
Finally, 14% of patients had an infectious event after discharge. Immune system damage induced by SARS-CoV-2 infection, treatment with steroids and other immune-suppressing drugs, and longstanding hospitalization exposed patients to a high risk of infectious complications, which continued after hospital discharge. As a further element of concern, a significant rate of rectal colonization by multidrug-resistant (MDR) bacteria was detected in our population (19%), especially those admitted to the ICU. Considering that in 2019 the overall rate of rectal colonization by MDR bacteria in our hospital amounted to 14.4% (personal communication with Elisabetta Mantengoli, MD, Infectious and Tropical Diseases Unit, Careggi University and Hospital, Florence, Italy), it may be speculated that the COVID-19 pandemic has negatively influenced infection control practices.
Our study has some limitations. Only hospitalized patients were candidates for follow-up in our outpatient clinic, and no data were collected from COVID-19 outpatients. Moreover, due to the cross-sectional nature of the analysis, information about postdischarge symptoms that had resolved before the visit was missed. Finally, the study reflects an early follow-up review, limited to clinical and laboratory assessment. Further information, which is needed to better characterize the burden and pathogenesis of possible chronic sequelae, will be obtained from future radiological and functional testing, including chest radiograph, spirometry, exercise testing, and echocardiography, in addition to other specialist evaluations, to be selected case-by-case. Future studies may also confirm the real frequency of unusual symptoms reported in our study and may provide new insights into their pathogenesis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Careggi Post-acute COVID-19 Study Group. Carlo Fumagalli, Maria Vittoria Silverii, Luca Ciani, Chiara Zocchi, Luigi Tassetti, Rossella Marcucci, Betti Giusti, Luca Livi, Lorenzo Giovannoni, Paola Parronchi, Fabio Almerigogna, Francesco Annunziato, Alessio Mazzoni, Laura Maggi, Francesco Liotta, Lorenzo Cosmi, Alessandra Vultaggio, Andrea Matucci, Silvia Sticci, Martina Donati, Cecilia Defraia, Fabrizio Giansanti, Daniela Bacherini.
Financial support. This work was supported by funds from the Ministry of Education, University and Research (Italy) Excellence Departments 2018–2022 (Project for the Department of Experimental and Clinical Medicine).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Careggi Post-acute COVID-19 Study Group:
Carlo Fumagalli, Maria Vittoria Silverii, Luca Ciani, Chiara Zocchi, Luigi Tassetti, Rossella Marcucci, Betti Giusti, Luca Livi, Lorenzo Giovannoni, Paola Parronchi, Fabio Almerigogna, Francesco Annunziato, Alessio Mazzoni, Laura Maggi, Francesco Liotta, Lorenzo Cosmi, Alessandra Vultaggio, Andrea Matucci, Silvia Sticci, Martina Donati, Cecilia Defraia, Fabrizio Giansanti, and Daniela Bacherini
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Weekly update on COVID-19—05 January 2021. 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update---5-january-2021. Accessed 12 February 2021. [Google Scholar]
- 3. Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C investigators . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lagi F, Piccica M, Graziani L, et al. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill 2020; 25:2000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandal S, Barnett J, Brill SE, et al. “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19 [published online ahead of print November 10, 2020]. Thorax 2020. doi: 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chopra V, Flanders SA, O’Malley M, et al. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med 2020. doi: 10.7326/M20-5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19 [published online ahead of print October 5, 2020]. JAMA 2020. doi: 10.1001/jama.2020.19719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.British Thoracic Society. British Thoracic Society guidance on respiratory follow-up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia. 2020. Available at: https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/resp-follow-up-guidance-post-covid-pneumonia/. Accessed 12 February 2021.
- 11. Regione Toscana. Follow-up dei pazienti clinicamente guariti da COVID-19. Percorsi diagnostici multidisciplinari: primi indirizzi. Delibera n.938 del 20/07/20. 2020. Available at: http://www301.regione.toscana.it/bancadati/atti/Contenuto.xml?id=5259310&nomeFile=Delibera_n.938_del_20-07-2020. Accessed 12 February 2021.
- 12. World Health Organization. Clinical Management of COVID-19 Interim Guidance. Geneva: World Health Organization; 2020. [Google Scholar]
- 13. Tenforde MW, Kim SS, Lindsell CJ, et al. ; IVY Network Investigators; CDC COVID-19 Response Team; IVY Network Investigators . Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort [published online ahead December 3, 2020]. Thorax. 2020; doi: 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One 2020; 15:e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stavem K, Ghanima W, Olsen MK, et al. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study [published online ahead December 3, 2020]. Thorax. 2020; doi: 10.1136/thoraxjnl-2020-216377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of 1 Long-COVID: analysis of COVID cases and their symptoms collected by the Covid symptoms study app. medRxiv 10.1101/2020.10.19.20214494 [Preprint]. 21 October 2020. Available at: 10.1101/2020.10.19.20214494. Accessed 12 February 2021. [DOI] [Google Scholar]
- 18. Lavery AM, Preston LE, Ko JY, et al. Characteristics of hospitalized COVID-19 patients discharged and experiencing same-hospital readmission—United States, March-August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeon WH, Seon JY, Park SY, Oh IH. Analysis of risk factors on readmission cases of COVID-19 in the Republic of Korea: using nationwide health claims data. Int J Environ Res Public Health 2020; 17:5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atalla E, Kalligeros M, Giampaolo G, et al. Readmissions among patients with COVID-19. Int J Clin Pract 2020; 00:e13700. [DOI] [PubMed] [Google Scholar]
- 21. Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 2020; 77:1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goren A, Vaño-Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain—a potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol 2020; 19:1545–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.