Rheumatology key message
Clinicians should be vigilant for axial presentations of reactive arthritis secondary to COVID-19.
Dear Editor, the novel coronavirus SARS-CoV-2, known as COVID-19, is continually being observed to cause new and varied multisystem sequalae. There have been several reports of peripheral oligoarthritis attributed to COVID-19 [1, 2], but this case potentially represents the first axial presentation of COVID-19 reactive arthritis (ReA).
This 53-year-old gentleman presented in April 2020 with new onset debilitating inflammatory back pain in the lumbar, thoracic and cervical regions, associated with anterior chest wall pain. This was preceded by a short period of self-resolving constitutional symptoms: fever, night sweats, malaise, 2 kg weight loss and also a loss of sense of smell. Although no nasopharyngeal swab was obtained at the time, these symptoms were felt to be highly suggestive for COVID-19, and a subsequent SARS-CoV-2 antibody test was positive. Relevant past medical history included lumbar disc herniation secondary to a sporting injury in his mid-20s, associated with radiculopathy and foot drop, which was successfully managed with discectomy. More recently, he has been treated for hypercholesterolaemia with a statin.
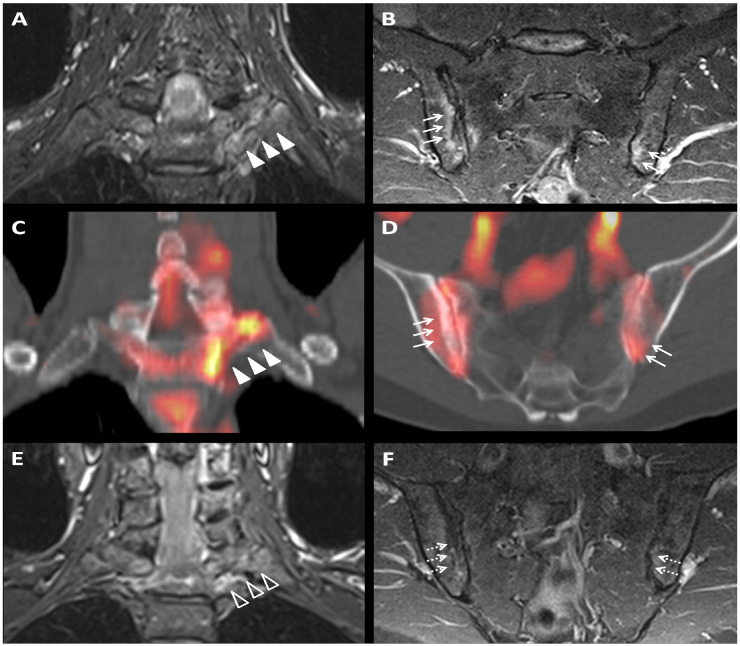
Laboratory investigations showed raised CRP 13 mg/l (reference range 0–10 mg/l) and positive HLA-B27. MRI of the sacro-iliac joints and spine [axial spondyloarthritis (axSpA) protocol] demonstrated bone marrow oedema at both sacroiliac joints and at the left first costovertebral and costotransverse joints (Fig. 1). Hybrid PET-CT demonstrated focal increased 18F-Flurodeoxyglucose (FDG) uptake at the same sites as the bone marrow oedema on MRI.
Fig. 1.
Baseline coronal STIR MRI
(A) and fused PET-CT (C) images showing left 1st costovertebritis and costotransversitis (arrowheads). Baseline coronal oblique STIR MRI (B) and fused PET-CT (D) images showing bilateral asymmetrical sacroiliitis (arrows). Follow-up MRI demonstrates partial resolution of left first costovertebritis and costotransversitis (E; arrowhead outlines) and near complete resolution of sacroiliitis (F; dashed arrows).
In early June 2020, he was treated with intra-muscular methylprednisolone 120 mg and commenced on diclofenac 75 mg once daily. At three-month review, he reported significant clinical improvement, resolution of inflammatory back and anterior chest wall pain, feeling less fatigued and had resumed all his usual activities. CRP was undetectable at <1 mg/dl. A repeat axSpA protocol MRI showed near complete resolution of the previously observed inflammatory changes (Fig. 1). He then stopped diclofenac. At review 6 weeks later, he remains asymptomatic.
ReA is traditionally defined as a sterile arthritis in response to genitourinary or gastrointestinal bacterial infection; however, viral triggers including HIV have been cited [3]. ReA usually presents as an oligoarthritis but is known to cause axial changes in some patients. In this case, there was a definite temporal relationship between COVID-19 infection and the onset of axial disease, in a timeframe that would typically be expected for ReA. HLA-B27 is positive, which is known to be an important risk factor for the development of ReA, as well as being implicated in ReA prognosis, conferring a higher probability of chronicity [4].
A potential limitation of this case is the absence of a nasopharyngeal swab at the time of his suspected COVID-19 infection; however, this was the only symptomatic illness to which the subsequent positive SARS-CoV-2 antibody test could be attributed. Other differentials for the MRI findings include a flare of pre-existing axSpA; however, there were no symptoms to suggest pre-existing disease. New onset disease is theoretically possible; however, this would be exceedingly rare in this age group [5]. A follow-up review is planned for 6 months to re-assess for this possibility, or of course sooner should the patient have a recurrence of symptoms in the interim. Given that this patient was asymptomatic before COVID-19 infection, and the rapid improvement within 6 months, we believe this case represents the first reported axial presentation of ReA secondary to COVID-19 infection. Clinicians should be vigilant regarding this potential complication of COVID-19.
Acknowledgements
There are no other contributors or collaborators for this manuscript. All three authors meet the International Committee of Medical Journal Editors (ICMJE) recommendations for authorship in that they have provided substantial contribution to the analysis of the clinical case and investigations (imaging), drafting and critical revision of the manuscript, approval of the final version submitted and agreement to be accountable for all aspects of this work.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: There are no relevant associations with commercial or non-financial entities that provided support for this work, either for the authors or their family members.
Data availability statement
All data relevant to this case report are included in the article.
References
- 1. Saricaoglu EM, Hasanoglu I, Guner R. The first reactive arthritis case associated with COVID-19. J Med Virol 2021;93:192–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ono K, Kishimoto M, Shimasaki T et al. Reactive arthritis after COVID-19 infection. RMD Open 2020;6:e001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathew AJ, Ravindran V. Infections and arthritis. Best Pract Res Clin Rheumatol 2014;28:935–59. [DOI] [PubMed] [Google Scholar]
- 4. Hannu T. Reactive arthritis. Best Pract Res Clin Rheumatol 2011;25:347–57. [DOI] [PubMed] [Google Scholar]
- 5. Rudwaleit M, van der Heijde D, Landewe R et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to this case report are included in the article.