Abstract
Objectives
The biomarkers of an immunological dysregulation due to a chronic HBV infection are indeed understudied. If untreated, this condition may evolve into liver impairment co-occurring with extrahepatic involvements. Here, we aim to identify a new panel of biomarkers [including immunoglobulin G (IgG) subclasses, RF, and Free Light Chains (FLCs)] that may be useful and reliable for clinical evaluation of HBV-related cryoglobulinemia.
Methods
We retrospectively analysed clinical data from 44 HBV-positive patients. The patients were stratified (according to the presence/absence of mixed cryoglobulinemia) into two groups: 22 with cryoglobulins (CGs) and 22 without CGs. Samples from 20 healthy blood donors (HDs) were used as negative controls. Serum samples were tested for IgG subclasses, RF (-IgM, -IgG, and -IgA type), and FLCs.
Results
We detected a strikingly different distribution of serum IgG subclasses between HDs and HBV-positive patients, together with different RF isotypes; in addition, FLCs were significantly increased in HBV-positive patients compared with HDs, while no significant difference was shown between HBV-positive patients with/without mixed cryoglobulinemia.
Conclusion
The immune-inflammatory response triggered by HBV may be monitored by a peculiar profile of biomarkers. Our results open a new perspective in the precision medicine era; in these challenging times, they could also be employed to monitor the clinical course of those COVID-19 patients who are at high risk of HBV reactivation due to liver impairment and/or immunosuppressive therapies.
Keywords: HBV, mixed cryoglobulinemia, vasculitis, free light chains, IgG subclasses, rheumatoid factor
Rheumatology key messages
A putative role for HBV infection in the pathogenesis of mixed cryoglobulinemia has been suggested here.
As a direct marker of B cell activity, FLC assessment may improve the diagnostics of HBV-related immunological dysfunction in the precision medicine era.
The setting of a biomarker panel for HBV-related immune disorders represents an important new diagnostic tool for those COVID-19 patients at high risk of HBV reactivation.
Introduction
HBV, an enveloped, partially double-stranded DNA virus, chronically infects 350–400 million of the world’s population, with a higher prevalence in the Indian subcontinent, subSaharan Africa, and Central Asia, although the vaccination program has reduced the global prevalence [1].
Untreated chronic HBV infection can progress to end-stage liver disease, such as cirrhosis and hepatocellular carcinoma (HCC). Liver damage caused by HBV infection is mediated by the immune responses towards the active viral replication [2]. Together with a progressive liver impairment, HBV infection causes a variety of extra-hepatic manifestations. In some patients, HBV can trigger a systemic necrotizing vasculitis, e.g. PAN, with the presence of HBsAg and anti-HBsAg antibody immune complexes in the vascular lesions [3, 4]; moreover, a systemic cryoglobulinemic vasculitis (CV) has been described in ∼3% of HBV patients, mostly affecting the skin, peripheral nervous system, and kidney [5–7].
CV is the symptomatic manifestation of a condition called mixed cryoglobulinemia (MC), characterized by the presence of circulating cryoglobulins (CGs) consisting of immunoglobulins (Igs) that precipitate at cold temperature, and are usually classified in three subsets. In types II and III, the CGs are immune complexes composed generally of polyclonal IgGs, the antigen(s), and monoclonal or polyclonal IgMs, respectively, while type I consists of only monoclonal Ig and it is therefore known as cryoglobulinemia [8]. The IgM commonly display RF activity against polyclonal IgG [9]. Types II and III CGs, associated with chronic viral infections (HBV and HCV), can cause a clinically evident CV that may evolve towards a frank B cell lymphoma [10]. Although the association of CV with HCV-chronic infection is stronger, the ex adiuvantibus criterion, showing regression of cryoglobulinemic vasculitis after successful antiviral treatment in HBV patients, clearly confirms the pathogenetic relationship with this virus [5, 11–13].
Also, different meta-analyses showed that HBV-infected patients displayed a 2–3-fold higher risk of developing B cell non-Hodgkin lymphomas. Although the HBV pathogenetic contribution to lymphomagenesis is still unknown [14], pathways common to those described for HCV-induced CV may be involved; in particular, the role of a B cell population producing polyclonal cryoglobulins and the subsequent positive selection of B-cells releasing monoclonal cryoglobulins has been postulated [15].
The natural history of pandemic coronavirus disease 19 (COVID-19) may impact onHBV infection. IL-6 represents the major pleiotropic cytokine that sustains, through a trans-signalling mechanism, chronic inflammation of any injured tissue [16, 17]. It has been reported that IL-6 receptor antagonists employed in COVID patients to control the cytokine storm syndrome unfortunately increase the risk for patients with active or past HBV infection [18]. In consideration of this event occurring in COVID patients, together with the wide spectrum of worsening outcomes related to HBV infection, it is apparent why reliable circulating immunological biomarkers of viral reactivation and for monitoring therapy and follow-up are greatly sought after.
It has been reported that chronic HBV patients display high levels of RF and low levels of C3 [19], suggesting a possible role of the HBsAg–antibody complex in the production of RF [20]. Taking into account the fact that the RF is higher in patients with persistent HBsAg positivity than in patients who have undergone seroconversion, it was suggested that abnormal level of RF [21] may be considered as a marker of an impaired immune response; moreover, HBV load is paralleled by RF titre that may decrease following to HBV vaccination [21].
Light chains of Igs are produced in excess of heavy chains during the synthesis of intact Ig and may enter the bloodstream as free light chains (FLCs); increased circulating levels of FLCs reflect polyclonal B cell activation, and determination of serum FLCs has become a useful diagnostic tool in immunopathological conditions, because they indicate systemic and organ-specific autoimmune disease [22, 23]; in the case of HCV-related cryoglobulinemic vasculitis, they represent a surrogate marker for measuring disease and monitoring the possible evolution in B cell lymphoma [24, 25].
Most of the auto-abs are class G Igs (IgG), which includes four subclasses (IgG1–4). Only recently, the serum IgG profile has been described, with its distinct patterns in different autoimmune disorders, suggesting that different subclasses could be specific for the underlying driving autoantigens [26, 27].
Here, we aim to validate a new panel of biomarkers for HBV infection, including RF, FLCs and IgG subclasses profile, which may improve the monitoring of infection-worsening outcomes towards HBV-related cryoglobulinemia.
Patients and methods
Patients and laboratory testing
This study retrospectively analysed sera and clinical data from 44 HBV-positive patients (age range 47–80 years, mean 63) of two major Italian groups: the centre for Systemic Manifestations of Hepatitis Viruses, the Department of Experimental and Clinical Medicine, the University of Florence, and the regional referral centre for Mixed Cryoglobulinemia, Policlinico Umberto I, in Rome. Patients were stratified (on the presence/absence of MCs) into two group: 22 with CGs and 22 without CGs. Demographics and clinical correlates of patients are described in Table 1. All serological determinations were performed in our institution’s laboratories (Fondazione Policlinico Universitario ‘A. Gemelli’ I.R.C.C.S., in Rome). We also collected and tested serum samples from 20 healthy blood donors (HDs) age- and sex-matched as negative controls (age range 35–60 years, mean 52).
Table 1.
Demographics and clinical correlates of patients
| HBV patients | Patients with CGs | Patients without CGs | |
|---|---|---|---|
| n° | 44 (25 M, 19 F) | 22 | 22 |
| Age (years) | 59 (29–88) | 61(45–88) | 56 (29–81) |
| ANA | 3 | 1 | 2 |
| ENA | 1 | 1 | – |
| AMA | – | – | – |
| anti-LKM-1 | – | – | – |
| ASMA | – | – | – |
| aCL-IgM | 1 | 1 | – |
| anti-MPO | – | – | – |
| Meltzer’s triad | 4 | 3 | 1 |
Anti-LKM-1: liver kidney microsomal type 1 antibodies; CG: cryoglobulin.
Patients were included in the study according to the following criteria: presence of HBV-DNA in serum; absence of HIV co-infection; absence of antiviral treatment and/or immunosuppressive therapy, presence/absence of CGs, presence of MC symptoms in patients with CGs positivity, according to the classification criteria for MC as proposed by the Italian Group for the Study of Cryoglobulinemias in 1989 and later revised in 2002 [5, 28].
Serum samples were tested for RF (IgM, IgG and IgA type), IgG subclasses (IgG 1–4) and free k and λ chains. RF level was determined by means of ELISA kits for IgG, IgA and IgM (Menarini, Italy); according to the manufacturer the cut-off is <20 U/ml. According to the Clinical and Laboratory Standards Institute (CLSI) guidelines, we tested 20 HDs from the local population to verify adherence to CLSI EP 28A3C [29, 30].
IgG subclasses were assessed by an Optilite analyser (Human IgG subclasses kit, The Binding Site, UK; normal range: IgG1: 3.824–9.286 g/l; IgG2: 2.418–7.003 g/l; IgG3: 0.2182–1.7606 g/l; IgG4: 0.0392–0.864 g/l).
FLCs were measured by means of the Optilite analyser (Freelite™ Human Kappa and Lambda Free Kits, The Binding Site, UK; normal range: 3.3–19.4 mg/l for free k and 5.7–26.3 mg/l for free λ). A ratio of k/λ < 0.26 or of >1.65 was considered abnormal.
Samples were thawed only once and immediately assayed in a single batch, following the manufacturer’s instructions. All the determinations were performed by an operator without knowledge of the clinical information of the handled sample. Each sample was tested twice to minimize eventual discrepancies, and all tests were performed in the same laboratory with the same instruments.
Ethical considerations
The ethics committee of our institution (Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario ‘A. Gemelli’ I.R.C.C.S.) approved the study (ID: 2080). All patients gave written informed consent to the use of their clinical and serological data in this study. The whole study was conducted according to the Declaration of Helsinki, as revised in 2013.
Statistical analysis
Statistical analyses were performed using the software package R (3.5.2 release) [31]. Biomarkers were tested for normality by means of a visual inspection of the QQ-Plot, followed by a Shapiro–Wilk test. It was found that some parameters deviate significantly from normality. Patients were divided in two groups based on the presence (or absence) of CGs. Comparisons between groups was performed with the Wilcoxon Unpaired Two-Sample test. The diagnostic performances of the investigated serum markers in distinguishing HBV patients with and without CGs was assessed by logistic regression and receiver operating characteristic (ROC) curves. Logistic regression is executed using the function glm from the R package stats to extract probabilities from the fitted models, either with a single biomarker or with several biomarkers used in combination. ROC curves and area under the curve (AUC) values were calculated as described in references [32] and [33], using the R package pROC [34]. A forward–backward stepwise logistic regression was used to select the best subset of markers according to the Aikake Information Criterion. The optimal cut-off was calculated by maximization of Youden’s statistics J = sensitivity + specificity – 1 [35]. The logistic classifier was validated with the leave-one-out approach using the R function cv.glm. Correlations between variables were evaluated using Spearman’s correlation coefficients. Strength of correlation was judged using correlation coefficients of >0.70 as strong correlation, 0.30–0.70 as moderate correlation and <0.3 as weak correlation. Correlation heat maps were calculated with the package corrplot implemented in the software R [36].
Results
Analysis of RF, FLC, and IgG subclasses in HBV-patients with or without cryoglobulins
A total of 64 subjects were recruited for the study: 44 HBV-positive patients (age range 47–80 years, mean 63) and 20 HDs (age range 35–60 years, mean 52) as negative controls; 22 out of 44 HBV patients were diagnosed with MC (Table 1).
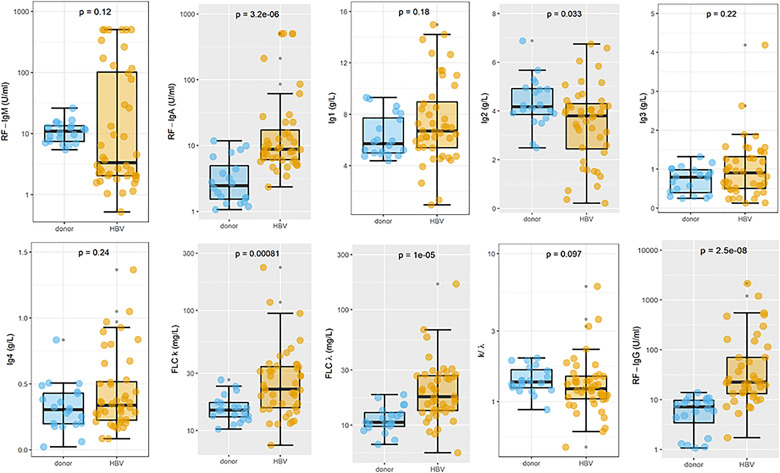
In Fig. 1, a box-plot analysis of the biomarkers serum levels is shown for HDs (cyan) and HBV patients with and without CGs (gold). Comparison between two groups is carried out with the Wilcoxon Unpaired Two-Sample test. P-values are reported in each plot. Significantly raised marker levels were found in HBV patients for RF-IgA (P = 3.2e-6), free k (P = 0.00081), free λ (P = 1e-5) and RF-IgG (P = 2.5e-8). A significant reduction in IgG2 levels was measured in HBV patients (P = 0.033).
Fig. 1.
Box-plot analysis of the biomarkers serum levels for healthy donors (cyan) and HBV patients with and without cryoglobulins (gold)
The presence of statistically significant differences is graphically shown using a grey plot background.
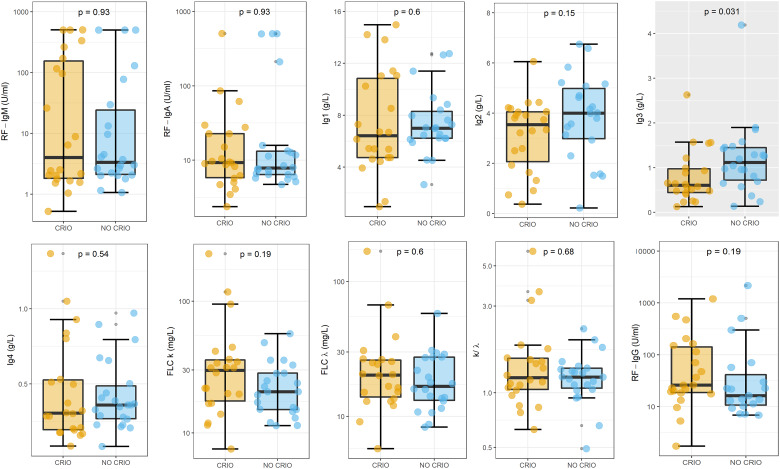
In Fig. 2, a box-plot analysis of the biomarkers serum levels is shown for HBV patients with CGs (cyan) compared with those without CGs (gold), with similar results being obtained in the two groups. A statistically significant difference could be found only for IgG3 levels. The direct comparison of these two groups with the Wilcoxon Unpaired Two-Sample Test showed a significant reduction (P = 0.031) in the IgG3 levels of patients with CGs.
Fig. 2.
Box-plot analysis of the biomarkers serum levels for HBV patients with (cyan) and without cryoglobulins (gold)
A grey plot background graphically indicates the presence of statistically significant differences.
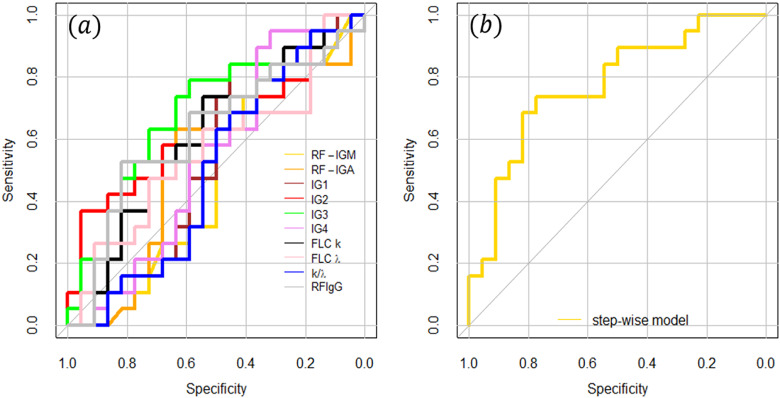
This analysis suggested that the presence of CGs cannot be inferred starting from the knowledge of one single biomarker, and a more complex model is needed. This conclusion is confirmed in Fig. 3a, in which a ROC curve analysis is shown for all the investigated parameters separately. Small AUCs are measured, with the 95% CI often containing the value 0.5, which corresponds to a random classifier. The following AUCs were measured: 0.49 (CI: 0.31, 0.67) for RF-IgM, 0.53 (CI: 0.35, 0.72) for RF-IgA, 0.53 (CI: 0.35, 0.72) for IgG1, 0.62 (CI: 0.44, 0.80) for IgG2, 0.68 (CI: 0.508, 0.85) for IgG3, 0.54 (CI: 0.35, 0.72) for IgG4, 0.61 (CI: 0.44, 0.79) for FLCk, 0.57 (CI: 0.38, 0.75) for FLC λ, 0.5 (CI: 0.33, 0.68) for k/λ, and 0.62 (CI: 0.44, 0.80) for RF-IgG.
Fig. 3.
ROC analysis of selected biomarkers, separately (a) and combining different biomarkers (b) according to a stepwise logistic regression in both directions
RF-IgM, RF-IgA, IgG3 and λ FLCs as selected biomarkers predictive for CG in HBV patients
To find a predictive model able to distinguish HBV patients with and without CGs, a stepwise logistic regression in both directions was performed taking into account all the investigated biomarkers. This analysis endeavoured to select the most suitable subset of biomarkers, which minimize the Aikake Information Criterion.
The procedure selected four out of ten parameters, namely RF-IgM, RF-IgA, IgG3 and FLC λ. In Fig. 3b, we show the ROC curve computed for the combination of the four selected biomarkers. A large (AUC = 0.77) and significant (95% CI: 0.6255, 0.9199) AUC value was obtained for the stepwise model. The following values of specificity, sensitivity, positive predictive value, and negative predictive value were calculated, respectively: 0.77, 0.73, 0.74 and 0.77. The step-wise logistic regression model allowed us to write the following functions for estimating the probability P that a patient has CG based on her/his RF-IgM, RF-IgA, IgG3, and FLC λ levels:
A leave-one-out-cross-validation (LOOCV) approach with the R function cv.glm was used to validate our logistic classifier. LOOCV provided an error rate of ∼0.30, which gives us an accuracy of ∼0.7.
This analysis indicates the selected parameters as promising immunological biomarkers for the clinical evaluation of HBV-related cryoglobulinemia. As such, it suggests the need for a further research effort based on larger sample sizes, aimed at confirming these results and, eventually, refining the coefficients in the probability function.
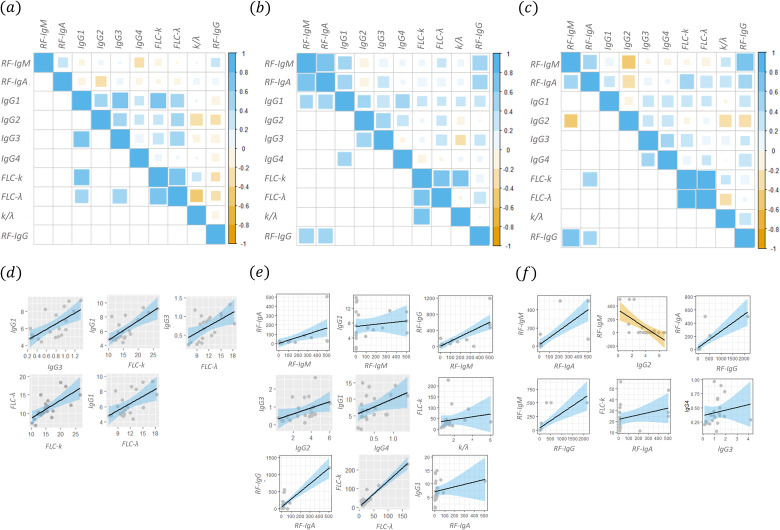
For the sake of completeness, a correlational analysis among different biomarkers was performed in the three groups (healthy donors, HBV patients with and without CGs), separately. In Fig. 4, three Spearman’s correlation maps are reported for healthy subjects: (a) HBV patients with (b), and without (c) cryoglobulinemia. The upper-right corner of the maps shows all the correlation coefficients, while the lower-left corner of the map shows only the significant ones (at the 0.05 significance level). In panels d, e and f of Fig. 4, the scatter plots corresponding to the couples of variables with a significant Spearman’s correlation are shown. The best linear fit lines of the data together with the corresponding confidence bands were also added. A qualitative analysis of these scatter-plots shows that not all the couples of variables matched the assumption of a simple linear regression model, further adding stress to our choice to use a non-parametric correlation coefficient (Fig. 4a–c). Nevertheless, a subset of these couples of variables displays sufficient regularity for taking into consideration a regression line to establish a mathematical relationship between the two variables. These scatter plots are highlighted using a grey background. The data are summarized in Table 2.
Fig. 4.
Spearman’s correlation coefficients among different biomarkers in the three groups separately, namely healthy donors (a), patients with (b) and without (c) cryoglobulins
The corresponding scatter plots for significant correlations are shown in panels d, e and f.
Table 2.
Comparative analysis of biomarkers among different groups of subjects
| Group | Biomarkers | Spearman’s correlation (P-value) | Linear regression coefficients |
|---|---|---|---|
| With CGs | RF-IgA vs RF-IgM | 0.79 (4.3e-5) | RF-IgA = (1.35 ± 23) + (0.33 ± 0.11)*RF-IgM |
| IgG1 vs RF-IgM | 0.44 (0.04) | RF-IgM = (69.4 ± 84.6) + (6.25 ± 10.1)*IgG1 | |
| RF-IgM vs RF-IgG | 0.59 (0.004) | RF-IgG = (7.4 ± 84.6) + (1.16 ± 0.21)*RF-IgM | |
| IgG2 vs IgG3 | 0.55 (0.004) | IgG3 = (0.27 ± 0.29) + (0.168 ± 0.08)*IgG2 | |
| IgG4 vs IgG1 | 0.50 (0.017) | IgG1 = (5.18 ± 1.35) + (5.00 ± 2.34)*IgG4 | |
| RF-IgA vs IgG1 | 0.53 (0.011) | IgG1 = (7.05 ± 0.90) + (0.0091 ± 0.008)*RF-IgA | |
| Freeλ vs Free k | 0.065 (0.0011) | Free k = (2.58 ± 4.83) + (1.38 ± 0.011)*Free λ | |
| k/λ vs Free k | 0.065 (0.0011) | Free k = (31.16 ± 18.0) + (6.44 ± 8.94)*k/λ | |
| RF-IgG vs RF-IgA | 0.55 (0.0077) | RF-IgG = (57.3 ± 33.1) + (2.24 ± 0.30)*RF-IgA | |
| Without CGs | RF-IgA vs RF-IgM | 0.47 (0.02) | RF-IgM = (16.3 ± 26.3) + (0.77 ± 0.14)*RF-IgA |
| IgG2 vs RF-IgM | −0.45 (0.03) | RF-IgM = (336 ± 73) − (66.3 ± 17.4)*IgG2 | |
| RF-IgG vs RF-IgM | 0.72 (0.00054) | RF-IgM = (43.4 ± 31.9) − (0.269 ± 0.062)*RF-IgG | |
| RF-IgA vs Free k | 0.53 (0.011) | Free k = (0.36 ± 0.09) − (0.048 ± 0.060)*RF-IgA | |
| RF-IgG vs RF-IgA | 0.45 (0.04) | RF-IgA = (27.3 ± 248) − (0.25 ± 0.05)*RF-IgG | |
| Healthy donors | IgG3 vs IgG1 | 0.65 (0.002) | IgG1 = (3.96 ± 0.068) + (3.22 ± 0.86)*IgG3 |
| Free k vs IgG1 | 0.62 (0.003) | IgG1 = (3.82 ± 2.03) + (0.49 ± 0.13)*Free k | |
| Free λ vs IgG1 | 0.48 (0.0034) | IgG1 = (2.74 ± 1.21) + (0.31 ± 0.10)*Free λ | |
| Free λ vs IgG3 | 0.53 (0.017) | IgG3 = (0.026 ± 0.25) + (0.06 ± 0.01)*Free λ | |
| Free λ vs Free k | 0.70 (0.005) | Free k = (0.026 ± 0.25) + (0.06 ± 0.01)*Free λ |
Correlation between different biomarkers (2nd column) among the three different groups of subjects.
Discussion
The major pathogenetic mechanism for determining cryoglobulinemia involves the production of aberrant autoantibodies by dysregulated B-cells; a chronic inflammatory stimulus could promote this worsening evolution, interfering with normal B cell function. CGs are associated with systemic autoimmune diseases, lymphoproliferative disorders, and chronic infections, and above all, HCV infection. An association of cryoglobulinaemia with chronic HBV infection has been suggested in only ∼2% of cases of cryoglobulinemic vasculitis [37]. A worsening evolution towards cryoglobulinemic complications depends on viral-triggered chronic immune stimulation, resulting in an immune dysfunction with the production of polyclonal, oligoclonal, or monoclonal auto-immune complexes, probably due to the molecular mimicry of virus with the target auto-antigens [38].
Genetic and/or environmental cofactors can have a role in the formation of specific IgG subclasses as a trigger of the cryoglobulinemic mechanism, independent of infection. In HCV-related MC, it is still debated whether viral infection represents a simple triggering factor or whether it also contributes to the self-perpetuating mechanism of multifactorial and multistep disease process [39].
A possible role of HBV infection in the pathogenesis of MC has been postulated, also confirmed by the evidence of a relationship between undetectable HBV DNA and regression of MC after antiviral therapy [5, 11, 12]. A broad spectrum of serum biomarkers has been well analysed in relation to the progression of extra-hepatic disorders in patients with HCV-related MC [9, 40–43]; in our opinion, the biomarkers of HBV vasculitis have not been as well investigated.
Our population of HBV MC patients show high CGs in the serum, low levels of complement components (data not shown), and clinical evidence of purpura, peripheral neuropathy, renal involvement, and lymphoproliferative disorders. Our results show strikingly different serum IgG subclasses distributions between HDs and HBV-positive patients. This profile might help in the understanding of hyperstimulation of the immune system by infectious agents, such as viruses, and the chronic inflammatory response that can lead to the development of some disorders with loss of control of B and T cells. To sustain a complete and effective immune response, B and T lymphocytes cooperate in a loop in which they affect each other: the disruption of this crosstalk profoundly impairs immunological responses and regulation [44]. The specific IgG subclasses profile depends on the type of antigen and on the duration of antigen exposure, reflecting a probable specific driving viral surface antigen. Serum levels of IgG subclasses could correlate with the amount of circulating antibody, suggesting a direct relationship between the elevated IgG subclass and the disease process [26]. In an earlier paper, we reported that the early identification of IgG3 RF in sera from HCV-positive patients could represent a biomarker predictor of the development of MC [38]. Here, however, we did not find a statistically significant difference between HDs and HBV patients (P = 0.22, Fig. 1), confirming that IgG subclasses display a different distribution in different clinical settings [23, 26, 40].
Comparing the different RF isotypes (IgM, IgA and IgG) that we found between HDs and HBV-positive patients, higher levels of RF IgG and RF IgA emerged in HBV patients, as expected. This data represents a possible physiological response induced by antibody–antigen binding.
RF secretion by B lymphocytes requires simultaneous stimulation of B cell receptors by an IgG linked to an antigen, and Toll-like receptor stimulation by an epitope, which is sustained by their interaction with immune complexes, attributed, at least in part, to non-specific liver damage [45].
RF IgA is present in the SF of patients with RA, and its clinical importance is in predicting the response to a biologic drug [46]. We hypothesize that RF IgA in the setting of HBV could represent a link between autoimmune responses and production of autoantibodies, despite the fact that so far interplay between RF and HBV has not been demonstrated.
In Fig. 2, we show a trend in the levels of RF, IgG, IgA, and IgM to be higher in HBV-related MC patients then in patients without CGs. These differences were not statistically significant, reflecting the fact that, probably, the same pathogenetic mechanism determines both conditions. The ROC analysis and the Spearman correlation’s coefficient, among different biomarkers, are shown in Figs 3 and 4, respectively. Nevertheless, it can be qualitatively observed that data of patients with CGs have the tendency to be more dispersed than the data of patients without CGs. This tendency is interesting and deserves more in-depth study.
Increased FLC levels can be detected in patients suffering from different immunological disorders, suggesting that serum FLCs could be a useful biomarker in immunopathological conditions, because they reflect polyclonal B cell activation [22, 47]. The biology of FLCs provides evidence for their role in several inflammatory and immune diseases [47]. The assessment of total and antigen-specific FLCs might be of primary interest and indicate a causal role in several diseases. Their measurement may be useful as a diagnostic marker of co-morbidities and as a potential prognostic marker [48]. Our results show high levels of FLCs in HBV-positive patients, with a significant difference in comparison with HDs, whereas no significant difference was shown between HBV with vs without MC.
Biochemical structural differences of pathological FLCs could be involved, and probably the production of different FLC isotypes by B lymphocytes could be connected to any still unknown pathways. So, increasing FLC levels could exist in both HBV groups but with different immunochemical features. We could hypothesize that, in patients with HBV-related MC, the FLCs could participate in CGs formation phenomena by stimulating specific B cells.
Reactivity against specific virus antigens would identify the presence of circulating CGs, representing the first step of activation of B lymphocyte clones, which show a subclinical self-reactivity that is not yet symptomatic. The antigenic stimulus leads to formation of Ig autoantibodies that in some cases also exhibit RF activity towards virus-related Ig. The next step leads to the formation of symptomatic CGs, with the presence of RF IgM and possible clinical symptoms. HBV viruses are well-recognized causes of chronic hepatitis, cirrhosis, and even hepatocellular carcinoma.
The definition of a reliable biomarkers panel represents a very important goal in precision medicine era, to detect any disease at an early stage when it is still curable; in HBV setting, the employment of a biomarker panel including RF, FLC, IgG subclasses could be useful to better stratify immune complex disease in chronic HBV patients improving early diagnosis of HBV-related vasculitis, and ameliorate prognosis paving the way for a tailored-patient better therapy. Last, but not the least, a thin red line joins the worldwide breakdown due to the novel coronavirus disease and the urgent need of reliable biomarkers of immunological state in chronic HBV patients. In COVID-19 patients, an increased risk of HBV reactivation may be correlated to the immune dysfunction associated with liver impairment and/or immunosuppressive therapies employed to control hyper-inflammatory conditions [49]. In the setting of different studies that are ongoing in HBV-positive patients affected by COVID-19 to validate Diacerein as new active metabolite for the inhibition of inflammosome pathways but maintaining anti-HBV properties, immunological reliable biomarkers of HBV activity could play a central role [50].
Overall, in the spectrum of HBV-related extrahepatic manifestations sometimes with significant morbidity and even mortality in limited cases, for the management of HBC-chronic infected patients with different co-morbidities, serological biomarkers may play a strategical role, but in our opinion still understudied. Our major goal was to conduct an analytic study on serum levels of RF, FLCs and IgG subclasses in a population of HBV-patients. It is worth pointing out that, due to the relatively small number of patients recruited for this study, equation 1 has to be considered a preliminary estimation and the validity of the model and the robustness of its logistic weights have to be confirmed in a further study carried out on a larger sample size.
Acknowledgements
K.P. and C.N. substantially contributed to the acquisition and interpretation of data and to drafting the article; L.G. and A.L.Z. revised the article critically for important intellectual content; G.C., S.C., S.M., L.V., F.G., S.L., A.B., A.S., L.M. and M.C. substantially contributed to the acquisition, analysis and interpretation of data; G.L.R. and M.V. critically revised and approved the final version of the manuscript; M.M. critically revised, edited, approved, and submitted the final version; U.B. substantially contributed to the study conception and design, and revised and approved the final version of the manuscript.
Funding: This work was supported by Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario ‘A. Gemelli’ I.R.C.C.S., which we gratefully acknowledge, as a part of its programs on promotion and dissemination of scientific research (Linea D.1 and Linea Premio pubblicazioni di alta qualità to M.M.).
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
References
- 1. Tripathi N, Mousa OY. Hepatitis B. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2020. https://www.ncbi.nlm.nih.gov/books/NBK555945/ (18 June 2020, date last accessed) [Google Scholar]
- 2. Tseng TC, Huang LR. Immunopathogenesis of Hepatitis B Virus. J Infect Dis 2017;216:S765–70. [DOI] [PubMed] [Google Scholar]
- 3. De Virgilio A, Greco A, Magliulo G et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev 2016;15:564–70. [DOI] [PubMed] [Google Scholar]
- 4. Michalak T. Immune complexes of hepatitis B surface antigen in the pathogenesis of periarteritis nodosa. Am J Pathol 1978;90:619–32. [PMC free article] [PubMed] [Google Scholar]
- 5. Mazzaro C, Dal Maso L, Urraro T et al. Hepatitis B virus related cryoglobulinemic vasculitis: a multicentre open label study from the Gruppo Italiano di Studio delle Crioglobulinemie – GISC. Dig Liver Dis 2016;48:780–4. [DOI] [PubMed] [Google Scholar]
- 6. Cacoub P, Saadoun D, Bourlière M et al. Hepatitis B virus genotypes and extra-hepatic manifestations. J Hepatol 2005;43:764–70. [DOI] [PubMed] [Google Scholar]
- 7. Cacoub P, Terrier B. Hepatitis B-related autoimmune manifestations. Rheum Dis Clin North Am 2009;35:125–37. [DOI] [PubMed] [Google Scholar]
- 8. Brouet J-C, Clauvel J-P, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med 1974;57:775–88. [DOI] [PubMed] [Google Scholar]
- 9. Basile U, Gulli F, Gragnani L et al. Different biochemical patterns in type II and type III mixed cryoglobulinemia in HCV positive patients. Dig Liver Dis 2018;50:938–43. [DOI] [PubMed] [Google Scholar]
- 10. Basile U, Napodano C, Marino M et al. Cryoglobulins: putative effectors of adaptive immune response. Clin Exp Rheumatol 2020; Advance Access published 29 October 2020, PMID: 33124568 [DOI] [PubMed] [Google Scholar]
- 11. Boglione L, D’Avolio A, Cariti G, Di Perri G. Telbivudine in treatment of hepatitis B-associated cryoglobulinemia. J Clin Virol 2013;56:167–9. [DOI] [PubMed] [Google Scholar]
- 12. Viganò M, Martin P, Cappelletti M, Fabrizi F. HBV-associated cryoglobulinemic vasculitis: remission after antiviral therapy with Entecavir. Kidney Blood Press Res 2014;39:65–73. [DOI] [PubMed] [Google Scholar]
- 13. Enomoto M, Makanishi T, Ishii M, Tamori A, Kawada N. Entecavir to treat hepatitis B-associate cryoglobulinemic vasculitis. Ann Intern Med 2008;149:912–3. [DOI] [PubMed] [Google Scholar]
- 14. Dalia S, Chavez J, Castillo JJ, Sokol L. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res 2013;37:1107–15. [DOI] [PubMed] [Google Scholar]
- 15. Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Jt Bone Spine 2019;86:707–13. [DOI] [PubMed] [Google Scholar]
- 16. Bartoccioni E, Scuderi F, Marino M, Provenzano C. IL-6, monocyte infiltration and parenchymal cells. Trends Immunol 2003;24:299–300. [DOI] [PubMed] [Google Scholar]
- 17. Marino M, Scuderi F, Ponte E et al. Novel path to IL-6 trans-signaling through thrombin-induced soluble IL-6 receptor release by platelets. J Biol Regul Homeost Agents 2013;27:841–52. [PubMed] [Google Scholar]
- 18. Rodríguez-Tajes S, Miralpeix A, Costa J et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat 2021;28:89–94. 10.1111/jvh.13410. doi: 10.1111/jvh.13410. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arai J, Takayoshi I, Miyashita M et al. High level of rheumatoid factor is associated with hepatitis B viremia in patients with chronic hepatitis B. Showa Univ J Med Sci 2014;26:75–83. [Google Scholar]
- 20. Watanabe K, Ohkubo Y, Funahashi Y et al. An investigation on rheumatoid factor of different immunoglobulin classes in hepatitis B virus carriers. Clin Rheumatol 1991;10:31–7. [DOI] [PubMed] [Google Scholar]
- 21. Choi ST, Lee HW, Song JS, Lee SK, Park YB. Analysis of rheumatoid factor according to various hepatitis B virus infectious statuses. Clin Exp Rheumatol 2014;32:168–73. [PubMed] [Google Scholar]
- 22. Napodano C, Pocino K, Rigante D et al. Free light chains and autoimmunity. Autoimmun Rev 2019;18:484–92. [DOI] [PubMed] [Google Scholar]
- 23. Basile U, Marino M, Napodano C et al. Serological immunoglobulin-free light chain profile in myasthenia gravis patients. J Immunol Res 2018;2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basile U, Gulli F, Napodano C et al. Biomarkers of minimal residual disease in rituximab-treated patients with mixed cryoglobulinemia. Biotechnol Appl Biochem 2020; Advance Access published 25 April 2020, doi: 10.1002/bab.1929 [DOI] [PubMed] [Google Scholar]
- 25. Gulli F, Marino M, Napodano C et al. Biomarkers in HCV-related mixed cryoglobulinemia patients with non-Hodgkin lymphoma. Eur Rev Med Pharmacol Sci 2020;24:8067–74. [DOI] [PubMed] [Google Scholar]
- 26. Napodano C, Marino M, Stefanile A et al. Immunological role of IgG subclasses. Immunol Invest 2020;11:1–18. [DOI] [PubMed] [Google Scholar]
- 27. Marino M, Basile U, Spagni G et al. Long-lasting rituximab-induced reduction of specific—but not total—IgG4 in MuSK-positive myasthenia gravis. Front Immunol 2020;11:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002;55:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McEnroe RJ, Durham AP, Goldford MD et al. EP05-A3: Evaluation of precision of quantitative measurement procedure: approved guideline. 3rd edn. Wayne, PA: Clinical and Laboratory Standards Institute, 2014. [Google Scholar]
- 30. CLSI. EP28: Defining, establishing, and verifying reference intervals in the clinical laboratory: approved guideline—3rd edn. Volume 28, Number 30. CLSI document EP28-A3c. Wayne, PA: Clinical Laboratory Standards Institute, 2008. Corrected 2010.
- 31. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing, 2018. Available online at https://www.R-project.org/ [Google Scholar]
- 32. Minelli E, Ciasca G, Sassun TE et al. A fully-automated neural network analysis of AFM force–distance curves for cancer tissue diagnosis. Appl Phys Lett 2017;111:143701.doi:10.1063/1.4996300. [Google Scholar]
- 33. Ciasca G, Sassun TE, Minelli E et al. Nano-mechanical signature of brain tumours. Nanoscale 2016;8:19629–43. [DOI] [PubMed] [Google Scholar]
- 34. Robin X, Turck N, Hainard A et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 36. Wei T, Simko V, Levy M et al. corrplot: visualization of a correlation matrix. R Package v 084. 2017. https://github.com/taiyun/corrplot (4 July 2020, date last accessed)
- 37. Ferri C, Sebastiani M, Giuggioli D et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum 2004;33:355–74. [DOI] [PubMed] [Google Scholar]
- 38. Basile U, Gulli F, Gragnani L et al. IgG3 subclass: a possible trigger of mixed cryoglobulin cascade in hepatitis C virus chronic infection. Dig Liver Dis 2017;49:1233–9. [DOI] [PubMed] [Google Scholar]
- 39. Roccatello D, Saadoun D, Ramos-Casals M et al. Cryoglobulinaemia. Nat Rev Dis Primers 2018;4:11. [DOI] [PubMed] [Google Scholar]
- 40. Basile U, Marino M, Gragnani L et al. Sentinel biomarkers in HCV positive patients with mixed cryoglobulinemia. J Immunol Methods 2020;476:112687. [DOI] [PubMed] [Google Scholar]
- 41. Basile U, Napodano C, Pocino K et al. Serological profile of asymptomatic HCV positive patients with low level of cryoglobulins. Biofactors 2019;45:318–25. [DOI] [PubMed] [Google Scholar]
- 42. Gulli F, Basile U, Gragnani L et al. Autoimmunity and lymphoproliferation markers in naïve HCV-RNA positive patients without clinical evidences of autoimmune/lymphoproliferative disorders. Dig Liver Dis 2016;48:927–33. [DOI] [PubMed] [Google Scholar]
- 43. Basile U, Gragnani L, Piluso A et al. Assessment of free light chains in HCV-positive patients with mixed cryoglobulinaemia vasculitis undergoing rituximab treatment. Liver Int 2015;35:2100–7. [DOI] [PubMed] [Google Scholar]
- 44. Marino M, Bartoccioni E, Alboini PE, Evoli A. Rituximab in myasthenia gravis: a “to be or not to be” inhibitor of T cell function. Ann N Y Acad Sci 2018;1413:41–8. [DOI] [PubMed] [Google Scholar]
- 45. Moll J, Isailovic N, De Santis M, Selmi C. Rheumatoid factors in hepatitis B and C infections: connecting viruses, autoimmunity, and cancer. Isr Med Assoc J 2019;21:480–6. [PubMed] [Google Scholar]
- 46. Sakthiswary R, Shaharir SS, Mohd Said MS, Asrul AW, Shahril NS. IgA rheumatoid factor as a serological predictor of poor response to tumour necrosis factor α inhibitors in rheumatoid arthritis. Int J Rheum Dis 2014;17:872–7. [DOI] [PubMed] [Google Scholar]
- 47. Gulli F, Napodano C, Marino M et al. Serum immunoglobulin free light chain levels in systemic autoimmune rheumatic diseases. Clin Exp Immunol 2020;199:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Basile U, Gulli F, Gragnani L et al. Free light chains: eclectic multipurpose biomarker. J Immunol Methods 2017;451:11–9. [DOI] [PubMed] [Google Scholar]
- 49. Napodano C, Pocino K, Stefanile A et al. COVID-19 and hepatic involvement: the liver as a main actor of the pandemic novel. Scand J Immunol 2020;93:e12977. [DOI] [PubMed] [Google Scholar]
- 50. Gonçalves de Oliveira P, Termini L, Durigon EL et al. Diacerein: a potential multi-target therapeutic drug for COVID-19. Med Hypotheses 2020;144:109920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.