Abstract
The stark racial disparities related to the coronavirus disease 2019 (COVID-19) pandemic in the United States, wherein minority populations are disproportionately getting infected and succumbing to the disease, is of grave concern. It is critical to understand and address the underlying causes of these disparities that are complex and driven by interacting environmental, social and biological factors. In this article we focus on the African American community and examine how social and environmental determinants of health intersect with biological factors (comorbidities, underlying genetics, host immunity, vitamin D levels, epigenetics) to exacerbate risk for morbidity and mortality.
Keywords: African Americans, comorbidities, COVID-19, epigenetics, health disparities
The stark racial disparities already observed in coronavirus disease 2019 (COVID-19) in the United States utterly decry the view that the coronavirus is a great leveler. Put simply, Indigenous Americans, African Americans (AAs), Hispanic Americans, and Pacific Islander Americans are disproportionately getting infected and succumbing to the disease, causing grave concerns [1, 2]. There is a clear urgency for understanding and addressing the underlying causes of these disparities as the pandemic rages on. Although more information is needed and substantive research has yet to be conducted to truly understand their causes, it is already evident that these disparities are complex and likely fueled by interacting biological, environmental, and social factors.
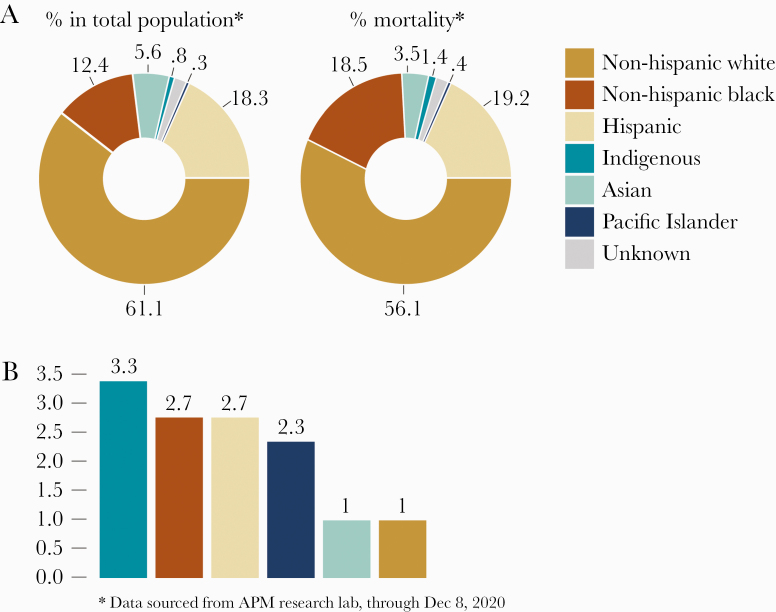
In principle, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus affects the young and the elderly as well as the rich and the poor. When it comes to eliminating its human host, however, the biases come to the fore; a higher mortality rate has been observed in older (>65 years), immunocompromised patients and those with underlying disorders [3]. In the United States, AAs have the second highest overall COVID-19 mortality rates, which is 1.6 times higher than that of whites and 2.4 times than that of Asians [2]. Currently (December 2020), AAs account for 18.5% of COVID-19-related deaths despite representing only 12.4% of the US population (Figure 1A). After adjusting for age, the data expose an even larger gap in mortality rates, with AAs experiencing a 2.7 times higher mortality rate than whites [2] (Figure 1B). It is clear that disparities are observed in all age brackets, and, alarmingly, more young AAs are now succumbing to the disease.
Figure 1.
(A) Disproportionate overall actual coronavirus disease 2019 mortality rates in different racial groups in the United States. (B) Age-adjusted mortality rates by which racial groups are more likely to have died when compared to non-Hispanic whites.
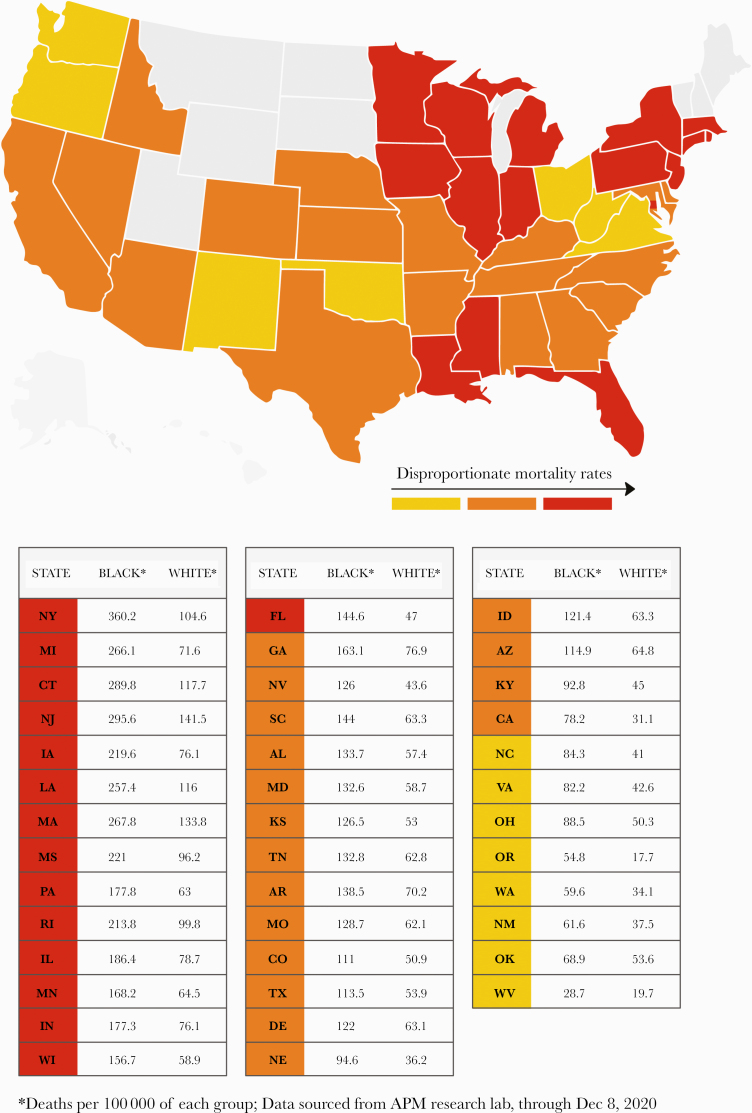
In New York City—one of the hardest hit, COVID-19 hot spots—the death rate in AAs is 360.2 per 100 000 versus 104.6 per 100 000 in whites [2]. Similar disproportionate mortality rates are now being reported from other US states (Figure 2). The racial health disparities in the COVID-19 pandemic mirror those of its predecessor, the H1N1 [4] pandemic, and inadvertently lays bare the fault lines of inequity that run deep in the American healthcare and social system. Vulnerabilities are underscored by disparities in exposure to the virus, differences in susceptibility to illness upon exposure, inequalities in access to treatment once disease develops, and pre-existing conditions that augment pathogenicity. These factors create a vicious cycle of disadvantage that leads to disparate levels of disease and death [5]. Within the framework of these factors, we will examine how biological, environmental, and social factors may exacerbate these health inequities in the rapidly evolving COVID-19 pandemic. Given the unprecedented surge in published research and clinical studies, and that information on this novel disease is rapidly evolving, some reports lack scientific rigor. In our review, we caution the readers that we cite a few preliminary reports (preprints) that have not yet undergone peer-review with a note that some findings may change with time.
Figure 2.
United States states that are experiencing race-related, age-adjusted coronavirus disease 2019 disparity in mortality rates are marked on the map, and corresponding data are displayed in the table (in decreasing order of absolute disparity).
The history of racial health disparities in the United States is unfortunately long-standing and complex. The reasons for inequity are multifactorial and have been a subject of extensive debate and research. There is already a call for a National Commission on COVID-19 Racial and Ethnic Disparities [6] and efforts by scientists to debunk the myth of black immunity to the virus. In this report, we outline the factors noted thus far that seem most closely related to the health disparities within COVID-19. We begin by discussing the social and environmental factors that play a sizeable role in poorer disease outcomes in AAs. Although these pertain to most minority groups in the United States, our article is exclusively devoted to discussing factors responsible for health disparities in the AA community. The subsequent section spotlights possible biological factors that, in conjunction with the social and environmental factors, may contribute in heightening the disparities in AAs. We also briefly discuss possible strategies to address these inequities.
ENVIRONMENTAL AND SOCIAL FACTORS: THE SOCIAL DETERMINANTS OF HEALTH
The environmental and social factors that drive health disparities in the United States are well documented and rooted in the current socioeconomic, environmental, and cultural conditions. These are often described as the social determinants of health. In fact, the social determinants of health framework is very helpful and highly relevant in terms of understanding and providing context to health disparities including those observed with COVID-19 [7]. Typically, the framework outlines 5 specific but intersecting domains: health and healthcare; economic stability; education; neighborhood and the built environment; and social and community context. In the case of COVID-19, these social determinants of health may intersect with biological factors to exacerbate the risk of morbidity and mortality [8].
In brief, there are several social determinants of health that may help to provide context to the COVID-19 disparities. Low socioeconomic status or lack of economic stability is often identified as a predominant factor underlying a range of health disparities. For example, 21% of AAs compared with 9.1% of whites live below the poverty line [9]. Lack of health insurance (11% of AAs vs 6% of whites were uninsured in 2017 [10]) or adequate health coverage, inequalities in access to healthcare, lack of primary care doctor as a point of contact, and lack of trust in the healthcare system are all major impediments [11]. These factors have an immediate and substantial bearing on the racial disparities in COVID-19, because timely access to health resources, such as the emergency department and intensive care, is critical. However, there is also a disparity in preventative care being provided to AA populations, which significantly contributes to disparities in chronic health conditions found to increase risk of poor COVID-19 outcomes.
A recent review stated that the fundamental cause of the pandemic inequity in the United States is racial capitalism [12]. It is indisputable that numerous well documented environmental characteristics pertaining to the neighborhood and built environment as well as the social and community context, such as residential segregation, high-density residential areas, crowded living conditions, distance from grocery stores and medical facilities, neighborhood disadvantage and violence, and air pollution, exacerbate health inequities in the AA community [11].
Air pollution is likely a critical environmental factor that increases susceptibility to COVID-19. A recent, unpublished report from Harvard University suggests that long-term exposure to PM2.5 can lead to an increase in the COVID-19 death rate in the United States [13]. The impact of long-term air pollution, particularly on lung health, is well known. A strikingly large proportion of AAs live in the proximity of oil and gas refineries and other settings with high levels of air pollution. As such, their overall levels of exposure are generally higher, likely impacting their health more adversely than whites [14, 15].
Disadvantaged neighborhoods also create a whirlpool of stress, which is linked to a higher incidence of chronic diseases and inflammation, as well as an increased risk of mortality [11]. Intriguingly, a study reported that AA youths who were subjected to racial discrimination expressed a higher prevalence of low-grade inflammation (indicated by elevated cytokine levels). Those who identified positively with their racial identities did not evince such an outcome [16]. Many of the neighborhood disadvantages or environmental factors are also considered social in nature and are often directly related to racism and discrimination, which clearly falls under the rubric of social determinants of health [17].
Finally, at this moment in time, in the midst of the COVID-19 pandemic, it is very clear how systematic racism and disadvantage serve as underpinnings of both short- and long-term health inequities. Of particular concern with respect to minority health is the limited access to preventative care, which may be the key driver for many of the chronic diseases that in turn exacerbate risk for COVID-19. In fact, there is a medical term for this known as “acute on chronic”, which refers to a longer term illness or medical condition that is aggravated by an acute illness, and that the acute illness has worse health outcomes than it would have in the absence of the chronic illness or condition [18]. This is exactly what we have seen with the COVID-19 pandemic in minority communities, which are already heavily burdened by chronic diseases such as cardiovascular diseases, cancers, diabetes, and metabolic syndrome, among others [18]. Obesity as a health condition is a key risk factor for COVID-19 and future research will likely provide a more specific determination of the risk levels. However, in a recent study that simulated a hypothetical scenario using large national databases, researchers found that eliminating both obesity and tobacco use in the US population would reduce disparities and result in greater health equity, demonstrating the need for prevention and access to preventative care [19].
STRATEGIES FOR ADDRESSING THE HEALTH DISPARITIES IN THE CONTEXT OF CORONAVIRUS DISEASE 2019
Since the Department of Health and Human Services published their “Action Plan to Reduce Racial and Ethnic Health Disparities: Implementation Progress Report 2011–2014” in 2015 [20], an ongoing focus on the multifaceted approach required was outlined to recognize the need for the following: (1) expanding access to quality healthcare; (2) addressing diversity and cultural competence in the healthcare workforce; (3) supporting population health; and (4) enhancing data collection and research. Although there remains grave disparities that urgently need to be addressed, we also need to factor in that the infrastructure to address these disparities was put in place or elevated relatively recently. Within the past decade, tremendous progress was made such as establishing Offices of Minority Health across several US health agencies, elevating the National Center on Minority Health and Health Disparities as an Institute within the National Institutes of Health, among other structural changes designed to specifically address minority health inequities [20]. However, the weaknesses and lack of integration across systems and health agencies have severely worsened the impact of COVID-19 in minority communities and underscored the need for additional strategies and action.
Because a multifaceted approach is required to address disparities in general, so too is the engagement across sectors and an understanding of the factors that drive providers and payers in their decision-making process for addressing health disparities [21]. For example, The American Medical Association (AMA) has made available a range of resources and training materials for physicians such as the “Health Disparities Toolkit” and the “AMA’s Code of Medical Ethics” on their website [22]. It specifically states that they work to increase the number of minority physicians to ensure that they reflect the US population, that they have made the elimination of racial and ethnic health disparities a top priority, and that they are working with physicians to recognize and manage low health literacy among their patients [22]. Likewise, the American Hospital Association is addressing health disparities specifically through innovative partnerships and the implementation of community health workers program as outlined on their website [23]. Despite these initiatives, concerns about inequity in treatments for COVID-19 have been made and reported in the news. As an example, an AA female physician, internist, recently and tragically died of COVID-19 several weeks after having raised concerns regarding the inadequate treatment received, which she determined was attributable only to her race [24]. However disturbing, social psychologists have outlined the disparities present in healthcare delivery, specifically regarding physician’s implicit biases that may affect their decision-making process regarding treatments and also implicit biases that may impact the physician-patient relationship, which in turn may adversely impact communication [25]. The most practical strategy for addressing these biases is to accelerate the process of diversifying the work force and to enhance the cultural competency of healthcare workers [26].
Priorities for reducing racial health disparities from academic scholars have also suggested that we should focus on “what we already know.” In their powerful review, Williams and Cooper [27] provide recommendations for developing communities of opportunities to mitigate the adverse impact of systematic racism, enhancing access to high-quality care for all, particularly focusing on preventative care, and finally conducting research to identify the optimal strategies for building both political will and also support to address the social inequities in health, all of which have direct relevance for the COVID-19 pandemic in the United States.
However, perhaps it is that last point that was raised by Williams and Cooper [27], about ensuring the political will and support for addressing the social inequities in health, where we need to start. It seems that in many settings, the message around health disparities and social inequities in health have not been fully embraced or endorsed. As such, the message from the former Secretary of Health and Human Services, Kathleen G. Sebelius, remains a critically important communication strategy for the work ahead: “It is time to refocus, reinforce, and repeat the message that health disparities exist and that health equity benefits everyone” (see https://www.minorityhealth.hhs.gov/assets/pdf/hhs/HHS_Plan_complete.pdf, page 1).
In addition, there are a few key strategies that may prove helpful in the short term to stem the pandemic and its impact in minority communities. In particular, strategies that support COVID-19 testing, contact tracing, and mitigating measures for community spread remain critically important factors to reduce the COVID-19 disparities.
Education and improved health literacy can play a fundamental role in tackling the already noted COVID-19 health disparities. The ability to obtain, process, and understand basic health information is key to engaging in protective measures. It has already been noted that health literacy is an underestimated problem related to COVID-19 [28] because it is of tremendous importance in supporting the public in taking precautions to limit exposure, assessing the value of information, making well informed decisions, weighing the benefits and disadvantages of situations, comprehending the statistics that news articles may include, and knowing the specific strategies recommended for social distancing, frequent handwashing, and wearing a mask and other key elements required to fight the pandemic. In addition, the distribution of myths and misinformation have likely been a barrier in the response and uptake of necessary precautions. In particular, the early myths regarding AAs immunity to the virus [6] may have been particularly devastating in communities already experiencing stigma and mistrust of medicine and government [29].
At the start of the pandemic, dangerous and baseless rumors about how AAs were impervious to the disease were circulating in some communities. Outreach community programs for minorities can help inform and rectify such misinformation, but, in this regard, educational interventions seem to be falling short. Education and health literacy initiatives can also be a great complement to awareness in determining a successful strategy to fight the disease. It is unfortunate that only 22% of AAs compared with 36% of non-Hispanic whites complete 4 years of college [30], a factor that needs to be considered when designing new strategies that are also recognized by the AMA in the training and support of physicians (https://www.ama-assn.org/).
Social distancing is an effective way to stymie the spread of the highly contagious coronavirus. However, a high proportion of AAs are employed in essential services and industries (eg, healthcare, transportation, grocery stores/supermarkets, public transit, burial services) where exposure to the virus is high, social distancing strategies are difficult to implement, and options for working remotely are absent. Likewise, because of the living conditions and lower socioeconomic status, minorities are more heavily reliant on public transportation for commuting to and from work [31], which further exacerbates the risk of exposure to the virus. Similarly, wearing protective gear has been highly recommended and is required in some states. However, because of the racial profiling and racism, some minorities, in particular AA men, are reluctant to wear face masks, fearing for their safety, particularly when visiting retail stores [32]. These issues underscore the underlying causes of health disparities and the need for broader, cross-cutting prevention strategies that reflect cultural context, racism, and structural inequities.
BIOLOGICAL FACTORS
Comorbidities: Achilles’ Heel
Although SARS-CoV-2 is a close relative of the SARS-CoV virus, they are markedly different in their biology and epidemiological dynamics [33], with the COVID-19 disease pathology appearing to be distinct and challenging in several ways. One of the commonalities is that people above the age of 60 or those with underlying conditions are at higher risk of developing severe complications after infection with either virus [34–36]. Data from COVID-19 patients show poorer clinical outcomes (admitted to ICU, invasive ventilation, or death) in patients having at least 1 of the following comorbidities: hypertension, diabetes, obesity, chronic metabolic disease, chronic obstructive pulmonary disease (COPD), cardiovascular or cerebrovascular diseases, hepatitis B infections, chronic kidney disorders, malignancy, and immunodeficiency [36–40]. According to the Centers for Disease Control and Prevention (CDC), cardiovascular diseases, diabetes, chronic lung disease, and obesity are the most frequently reported underlying medical conditions in hospitalized COVID-19 patients. This may largely explain why the AA community is so badly hit by the pandemic, because AAs exhibit a higher incidence of comorbidities, including cardiovascular diseases and diabetes [41–43]. A compromised innate immunity and proinflammatory state in patients with diabetes can increase susceptibility to cytokine storms and thrombotic events that are characteristic severe COVID-19 outcomes [44]. In addition, COVID-19 itself can worsen glucose control in diabetics thus whipping up a vicious cycle [44].
Patients with asthma are also considered high risk [45]. The AA population bears a greater burden of asthma compared with white Americans [46]. The high pollen season, which coincided with the COVID-19 pandemic in the United States, can cause allergy flare-ups and trigger or aggravate asthma [47].
Obesity is associated with several comorbidities including asthma, cardiovascular disorders, and type II diabetes [48, 49]. Among different racial groups, the highest prevalence of obesity has been observed in non-Hispanic blacks and could be one of the factors resulting in a higher incidence of comorbidities in this population [50].
The cancer burden in AAs is also disproportionately high compared with other racial and ethnic groups in the United States. After cardiovascular disorders, cancer is the second cause of death in AAs [51]. More specifically, prostate, breast, lung, and colorectal cancer are the most commonly diagnosed malignancies in this group [51]. The rates of lung cancer in AA males is 15% higher than in white males (although 14% lower in AA women vs white women) [51]. This sex difference is largely attributed to differential smoking patterns. Cigarette smoking is linked to chronic inflammation, which can lead to COPD, lung cancer, and other lung diseases [52]. Although overall tobacco use is not higher among AAs (compared with whites), studies suggest that AAs are more vulnerable to the damaging effects of smoking [53–55].
Microbes: The Showrunners?
The genetic composition of humans has been greatly influenced by coexisting microbes. Some of the medical conditions that AAs are predisposed to have a genetic basis. For example, malaria-causing Plasmodium species are an evolutionary driving force selecting for advantageous genetic traits that proffer protection against the dreaded disease. Certain polymorphisms, such as those causing sickle cell disease (SCD), Glu-6-phosphatase deficiency, and beta-thalassemia, are prevalent in African populations (and other malaria-endemic regions) and have been selected as they protect against severe forms of malaria [56]. In the United States, SCD occurs in approximately 1 of 365 black/AAs, and 1 in 16 300 Hispanic-Americans [57]. The sickle cell trait ([SCT] carrying 1 allele) occurs in approximately 1 in 13 black/AAs [58]. The majority of SCD patients display lung function abnormalities, including asthma and hypoxemia [58]. It has been observed that people with SCD who were infected with seasonal influenza or H1N1 developed more severe complications (acute chest syndrome, mechanical ventilation requirement) [59, 60]. It is too early to gauge the impact of SCD on COVID-19 prognosis, but those with SCD could potentially be at higher risk. These observations may have prompted the Harvard Medical School’s Coronavirus Resource Center to add SCD in the updated list of conditions that could lead to severe COVID-19 symptoms [61]. Although individuals with SCT typically show no symptoms, they can experience SCD complications under certain circumstances such as under conditions of increased pressure or low oxygen in the atmosphere (as in altitude sickness) [62]. These conditions are simulated in the lungs of patients with COVID-19, whose clinical manifestations are very heterogeneous, ranging from pneumonia to acute respiratory distress syndrome (ARDS), hypoxemia, and atypical symptoms resembling those observed at high altitudes [63, 64]. Therefore, individuals with SCT may experience severe COVID-19 symptoms. This nascent connection is worth exploring when sufficient clinical data are available. It is also of interest to determine whether the preponderance of SCD and SCT in AAs may explain the observed racial disparity in COVID-19 outcomes.
Another interesting feature of malaria is that it may also be exerting a selective pressure in AAs. The hormone angiotensin II (Ang II) is a major regulator of blood pressure, and its concentration is determined by angiotensin-converting enzyme (ACE) and ACE2. Gene polymorphisms in ACE (eg, I/D polymorphism in intron 16) [65] and ACE2 [66] lead to higher levels of Ang II and hypertension, and they are suggested to confer protection against severe malaria [67]. These polymorphisms have been linked to populations in malaria-endemic regions, including Africa [67, 68]. The incidence of hypertension in AAs (>45%) [69] is higher than other groups, and its genetic basis can, in part, be attributed to these polymorphisms [70]. Despite conferring a protective role against malaria, these polymorphisms increase the risk of other illnesses and possibly severe COVID-19-related complications. It is interesting to note that the ACE (I/D) polymorphism has also been associated with type 2 diabetes [71] and obesity [72, 73] and is also considered a risk factor for ARDS mortality [74]. A comprehensive large-scale study of the prevalence of these polymorphisms in AAs is required to elucidate their contribution to hypertension and other comorbidities that enhance susceptibility to COVID-19.
Instigating the Host Immunity: A Cytokine Storm Brews
Microbial pathogens can also shape differential ancestry-related immune response to infections [75]. Compared with whites, AAs are significantly more likely to carry genetic variants of proinflammatory cytokines [76, 77]. Compounding this effect is the observation that they also carry genotypes known to down-regulate the anti-inflammatory response, including variants of interleukin (IL)-1, IL-6, IL-10, and tumor necrosis factor (TNF)-α [76]. This may explain why a higher proportion of inflammatory diseases strike the AA community [78]. Chronic inflammation is also the underlying cause of several diseases (eg, cardiovascular disorders, diabetes, obesity, kidney ailments, and cancer) that are more frequent in AAs than in whites. In addition to inflammatory responses, marked differences in antiviral responses have also been observed among populations [75] and are of considerable significance, because a subset of COVID-19 patients with severe symptoms develop a “cytokine storm,” wherein the body mounts an “overenthusiastic” response to the invading virus. Cytokine storm is a type of systemic inflammatory response caused by proinflammatory cytokines that attack the host tissues (primarily the lungs), thereby leading to pneumonia and hypoxemia [79].
Clogging the Vasculature: Hypercoagulopathy
Mounting evidence shows that COVID-19 is also associated with distinct coagulation abnormalities (eg, thrombotic events and elevated levels of D-dimer, von Willebrand Factor [vWF], and Factor VIII), which may cause complications in severe cases [80]. The COVID-19 autopsies have revealed abnormal clotting in multiple organs and the presence of megakaryocytes and platelet-rich thrombi in the lungs, heart, kidneys, and liver [81]. These findings are particularly alarming because a prothrombotic state may start early on in the disease course; it has been observed even among younger patients, and it can occur despite anticoagulation therapy. In COVID-19 survivors, coagulation abnormalities may cause long-term complications of the heart, brain, and lungs. Abnormal blood clotting can cause multiorgan failure, strokes, various neurological conditions (eg, dizziness, headaches, seizures, brain fog, loss of smell, and taste), hypoxia, pulmonary embolism, and arterial thromboembolism, deep vein thrombosis, and mortality [80]. It has been established that uncontrolled or hyperactive host immune responses triggered by the virus contribute to disease severity in COVID-19 patients. A recent study reported that in a subset of patients, sustained and systemic activation of the complement pathways, a key component of the innate immunity, may be resulting in microvascular injury and thrombosis [82]. The risk factors for abnormal coagulation include older age, being male, diabetes, and obesity.
Abnormal coagulopathy, reflected by elevated levels of D-dimer, vWF, and Factor VIII, is fast emerging as a crucial marker of disease severity and mortality in COVID-19 patients and calls for an investigation into racial differences, if any, in the molecular and genetic nature of blood clotting. D-dimer is a degradation product of fibrin, and elevated D-dimer levels are indicative of presence or risk of thrombotic events [83]. von Willebrand Factor is an essential component of the blood clotting system and is produced in endothelial cells and megakaryocytes. Elevated levels of vWF may indicate endothelial injury and an increased risk of thrombosis. It has been suggested that hypercoagulopathy and respiratory distress in COVID-19 patients may, in part, be due to a vWF-dependent mechanism [84]. Likewise, a high level of Factor VIII, an essential blood coagulation factor, is a marker of increased risk of deep vein thrombosis and pulmonary embolism [85]. A comparison of platelet function and transcriptome between healthy AAs and whites has shown that the molecular route of blood clotting differs between the 2 racial groups [86]. This may also partly explain the differences in heart disease incidence and outcomes between AAs and whites. Studies have also shown that AAs have higher levels of D-dimer, vWF, and Factor VIII compared with whites and Hispanics. The higher levels of these markers in AAs are due to both genetic and environmental factors [87–89] and may contribute to severe COVID-19 outcomes. Plasma D-dimer levels are higher in AAs than whites even among hypertensive patients [90]. Thus, it is probable that even within high-risk groups with underlying heart conditions, ethnic disparities in COVID-19 outcomes may be observed.
Dwindling D Levels to Blame?
Chronic D hypovitaminosis is associated with increased risk of respiratory tract infections, including pneumonia and ARDS, apart from several comorbidities (eg, obesity, diabetes, hypertension) [91–94].Vitamin D deficiency may increase the risk of severe COVID-19. There is strong evidence supporting the link between vitamin D deficiency and mortality in AAs and European populations [91, 92, 95, 96]. Vitamin D deficiency is disproportionately higher in AAs (estimated at 76%) and may contribute to the higher rates of COVID-19 infection and mortality in the AA population [96]. Furthermore, individuals aged over 50 often have lower vitamin D levels, which may, in part, explain the high numbers of elderly succumbing to the infection [97]. Vitamin D is thought to reduce the risk of infection by attenuating inflammatory responses (eg, cytokine storm in COVID-19 patients) and lowering viral replication rates [91, 92]. The impact of vitamin D supplementation on the COVID-19 disease course is currently being investigated in a multicenter, randomized, and controlled clinical trial (CoVitTrial [98]).
Epigenetics: Threading Biology and the Environment
Continuous social and environmental assaults lead to stress, inducing chronic inflammation, and ultimately the development of various diseases (eg, obesity, diabetes mellitus, cancer, and cardiovascular diseases). Stress activates the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis that release chemical mediators of inflammation (C-reactive protein, IL-6, nuclear factor-κB, TNF-α) [11]. Environmental factors, including diet, smoking, pollution, and neighborhood characteristics, affect epigenetic modifications, thereby increasing or decreasing the susceptibility to various diseases. Deoxyribonucleic acid (DNA) methylation, histone modifications, and alterations in the expression of noncoding ribonucleic acids form the molecular basis of the epigenetic regulation of gene expression. Each environmental factor can establish a unique epigenomic imprint with potentially long-lasting or even transgenerational impact [99].
A growing body of research has also outlined the transgenerational effects specifically of racism, described as the psychophysiological inheritance across generations in response to white dominance. Alternatively, it has also been described as inheriting racist disparities in health. There are new studies demonstrating the biological underpinnings of these health disparities, many of which can be directly attributed to racism. For example, a recent study using national longitudinal data demonstrate that upwardly mobile AAs were more likely to experience both acute and chronic discrimination compared with those not upwardly mobile, raising important questions about social context and environment as well as for epigenetics [100]. Likewise, although research is emerging across health topics, researchers have questioned whether epigenetics can serve to explain how differential environmental exposure may manifest in observed racial differences in chronic pain [101]. Similarly, research indicates that altered DNA methylation patterns are linked to age-associated health disparities for AAs. Epigenetics have been used to examine a range of health conditions among AAs including renal function, metabolic syndrome, and cardiovascular health [102–106],
Unspooling the Biological Underpinnings of Coronavirus Disease 2019 Disparities
Both biological and nonbiological factors seem to contribute to racial disparities in COVID-19. Large, multicenter, comprehensive clinical studies are warranted to elucidate the biological impact of each of these factors. Biobanking COVID-19 patient samples is foundational and crucial to enable researchers to conduct such studies. It is abundantly clear that COVID-19 cannot be treated with a “one size fits all” approach. The identification of immunological and metabolomic differences among genetically diverse groups throughout the disease course will provide further insight into the biological underpinnings of COVID-19 disparities. For instance, susceptibility to the virus and disease severity could be affected by variations in the human leukocyte antigen (HLA) complex. Proof-of-concept studies are required to determine the role of HLA variants in infection, susceptibility, and disease outcomes, because HLA subtyping could aid in early patient stratification. Although an HLA susceptibility map for SARS-CoV-2 has already been developed using in silico analyses [107], these results need to be verified in clinical samples.
The SARS-CoV-2 enters the host cells upon binding to cellular receptors (eg, ACE2) via its spike (S) proteins and S protein priming by host serine proteases including transmembrane protease serine 2 (TMPRSS2) [108]. Evaluation of the genetic variants of these host factors within and across populations can be invaluable in identifying individuals and populations more susceptible to the virus and likely to have worse outcomes. A few studies have begun to determine genetic variants of ACE2 that can impact its expression and its enhanced or disruptive interactions with the virus along with effects on tissue specific expression [109–111]. Studies are also underway to identify polymorphisms that alter TMPRSS2 expression and its protease activity [109–112]. Thus far, there appears to be significant variability in genetic determinants of these host factors’ expression among individuals and populations. One study found that African populations are genetically predisposed to lower expression of ACE2 and TMPRSS2 and may therefore be less susceptible to the coronavirus [111]. In contrast, the findings of another study suggest that AAs are likely more susceptible to the virus due to a commonly observed polymorphism in the androgen receptor (AR) gene [112]. African Americans often carry a lower number of CAG repeats in the AR gene [113–115], which leads to higher AR levels [116]. The TMPRSS2 promoter contains an androgen response element, which induces TMPRSS2 transcription upon AR activation [117]. In addition, androgens have been shown to increase ACE2 activity in males [118], which may explain the racial and gender disparities in COVID-19 infection and outcomes. It must be noted, however, that these contradictory reports lack clinical validation. As more clinical samples from COVID-19 patients become available, the relevance of ACE2 and TMPRSS2 polymorphism in racial disparities in COVID-19 warrants further study.
Several promising drugs (both repurposed and new) and vaccine trials are underway. However, AAs and other minorities remain underrepresented in clinical trials [119]. Considering that differences in the immune landscape among racial/ethnic groups can impact drug and vaccine potency/antigenicity, dosage, and side effects, it is crucial to ensure a balanced cohort composition in clinical studies. In addition, the identification of the differences in the lung microbiome among different demographic subgroups is critical, because the human microbiome can profoundly affect host immune response, secondary bacterial infections, and drug efficacy. Several public opinion surveys make it plain that AAs are reluctant to get the COVID-19 vaccine, reflecting their continued mistrust in the medical system and the pharmaceutical industry, that finds its origins in the Tuskegee study and the Hela cell line research in the past. This pattern is in line with the CDC’s analysis of flu coverage data, which show that AAs are less likely than whites and Asians to get vaccinated [120].
To address these barriers, largely stemming from lack of data and integrated research that examine the intersection of the socioecological context, human behavior, and biological factors, new rigorous mixed-methods studies need to be conducted. Because the pandemic evolved so rapidly, the need for new data created a surge of research that either examined clinic samples or that involved community surveys. These studies have helped us greatly by providing a wealth of data. Many strategic projects also capitalized on existing infrastructure to expand projects already underway in clinical settings or communities. However, to drive the science forward so as to better understand these disparities, studies that link clinical data with survey data will be of critical importance.
As mentioned earlier, the social determinants of health play a significant role in the transmission of COVID-19, its manifestation, as well as treatment outcomes. As such, multiple data sources reflecting both biological samples and patient data and clinical records will be key. Previous studies have been well executed with similar goals and can serve as examples for future research such as the Dallas Heart Study for cardiovascular disease [121].
CONCLUSIONS
Coronavirus disease 2019 is a novel, rapidly evolving disease, with unprecedented scale and ferocity, and for which the unknowns far exceed the knowns. The pandemic is nothing short of a siege, because, in a very short span of time, it has exacted a heavy human and economic toll. In the United States, which is in the throes of the ravaging pandemic, a searing image of racial disparity has emerged—the pandemic is a rattling wake-up call for the nation. Addressing health disparities will require sweeping reforms in the healthcare system and public health infrastructure as well as addressing structural inequities. Moreover, we need to provide better education to our students and substantially improve health literacy across all population groups.
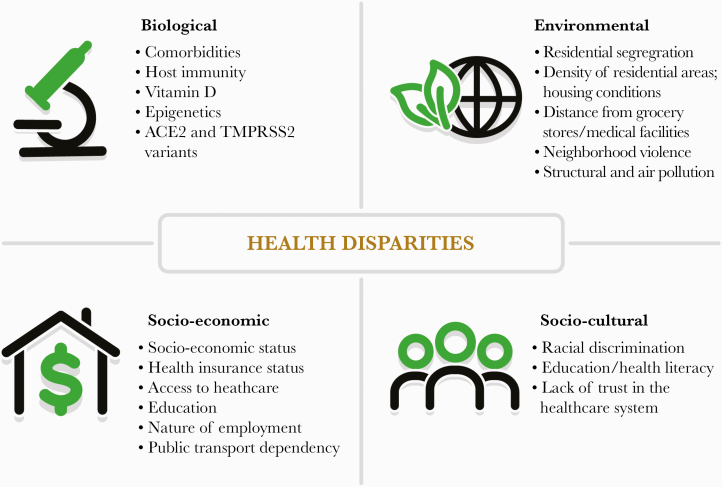
In this review, we have presented a list of key biological factors that, individually or in combination with environmental and social factors, may elucidate the underlying pathways responsible for the grave disparities noted so far (Figure 3). Meanwhile, there is an urgent need for our communities to come together and confront these alarming disparities.
Figure 3.
Health disparities lie at the crossroads of various biological, environmental, socioeconomic, and cultural factors, as outlined in the graphic.
Acknowledgments
Financial support. This study was funded by the National Cancer Institute at the National Institute of Health (Grant R01 CA169127; to R. A.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Available at: https://www.cdc.gov/covid-data-tracker/index.html#demographics. Accessed 05 January 2021.
- 2.Available at: https://www.apmresearchlab.org/covid/deaths-by-race. Accessed 05 January 2021.
- 3.Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed 05 January 2021.
- 4. Quinn SC, Kumar S, Freimuth VS, et al. Racial disparities in exposure, susceptibility, and access to health care in the US H1N1 influenza pandemic. Am J Public Health 2011; 101:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blumenshine P, Reingold A, Egerter S, et al. Pandemic influenza planning in the United States from a health disparities perspective. Emerg Infect Dis 2008; 14:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laurencin CT, , McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities 2020; 7:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNeely CL, Schintler LA, Stabile B. Social determinants and COVID-19 disparities: differential pandemic effects and dynamics. World Med Health Policy 2020; 12:206–17. [Google Scholar]
- 8. Singu S, Acharya A, Challagundla K, Byrareddy SN. Impact of social determinants of health on the emerging COVID-19 pandemic in the United States. Front Public Health 2020; 8:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fontenot K, Semega J, Kollar M; US Census Bureau. Income and Poverty in the United States: 2017 . Current Population Reports P60-263. Washington, DC: US Government Printing Office; 2018. [Google Scholar]
- 10. Pan HY, Walker GV, Grant SR, et al. Insurance status and racial disparities in cancer-specific mortality in the United States: a population-based analysis. Cancer Epidemiol Biomarkers Prev 2017; 26:869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saini G, Ogden A, McCullough LE, et al. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control 2019; 30:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laster Pirtle WN. Racial capitalism: a fundamental cause of novel coronavirus (COVID-19) pandemic inequities in the United States. Health Educ Behav 2020; 47:504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. Sci Advances 2020; 6:eabd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Available at: https://naacp.org/latest/naacp-clean-air-task-force-national-medical-association-release-landmark-study-impact-oil-gas-pollution-african-american-communities/. Accessed 10 July 2020.
- 15.Available at: https://insideclimatenews.org/news/01032018/air-pollution-data-african-american-race-health-epa-research. Accessed 10 July 2020.
- 16. Brody GH, Yu T, Miller GE, Chen E. Discrimination, racial identity, and cytokine levels among African-American adolescents. J Adolesc Health 2015; 56:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castle B, Wendel M, Kerr J, et al. Public health’s approach to systemic racism: a systematic literature review. J Racial Ethn Health Disparities 2019; 6:27–36. [DOI] [PubMed] [Google Scholar]
- 18.Available at: https://www.healthaffairs.org/do/10.1377/hblog20200716.620294/full/. Accessed 02 January 2021.
- 19. Frisco ML, Van Hook J, Hummer RA. Would the elimination of obesity and smoking reduce U.S. racial/ethnic/nativity disparities in total and healthy life expectancy? SSM Popul Health 2019; 7:100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Available at: https://www.minorityhealth.hhs.gov/assets/pdf/hhs/HHS_Plan_complete.pdf. Accessed 02 January 2021.
- 21. Johnson M, McPheron H, Dolin R, et al. Making the case for addressing health disparities: what drives providers and payers? Health Equity 2018; 2:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Available at: https://www.ama-assn.org/delivering-care/patient-support-advocacy/reducing-disparities-health-care. Accessed 02 January 2021.
- 23.Available at: https://www.aha.org/news/blog/2018-11-08-how-hospitals-are-addressing-health-disparities-through-community-health. Accessed 02 January 2021.
- 24.Available at: https://www.cnn.com/2020/12/24/us/black-doctor-susan-moore-covid-19/index.html. Accessed 02 January 2021.
- 25. Penner LA, Blair IV, Albrecht TL, Dovidio JF. Reducing racial health care disparities: a social psychological analysis. Policy Insights Behav Brain Sci 2014; 1:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson CS, Gracia JN. Addressing health and health-care disparities: the role of a diverse workforce and the social determinants of health. Public Health Rep 2014; 129 Suppl 2:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams DR, Cooper LA. Reducing racial inequities in health: using what we already know to take action. Int J Environ Res Public Health 2019; 16(4): 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paakkari L, Okan O. COVID-19: health literacy is an underestimated problem. Lancet Public Health 2020; 5:e249–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koch JW. Racial minorities’ trust in government and government decision makers. Soc Sci Q 2019; 100:19–37. [Google Scholar]
- 30. US Census Bureau. Educational Attainment in the United States: 2017. Table 1. Educational Attainment of the Population 18 Years and Over, by Age, Sex, Race, and Hispanic Origin: 2017. Suitland, MD: US Census Bureau; 2017. [Google Scholar]
- 31.Available at: https://www.pewresearch.org/fact-tank/2016/04/07/who-relies-on-public-transit-in-the-u-s/. Accessed 20 August 2020.
- 32.Available at: https://www.nytimes.com/2020/04/14/us/coronavirus-masks-racism-african-americans.html. Accessed 20 August 2020.
- 33. Zhang YZ, Holmes EC. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell 2020; 181:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang P, Wang X. COVID-19: a new challenge for human beings. Cell Mol Immunol 2020; 17:555–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chan KS, Zheng JP, Mok YW, et al. SARS: prognosis, outcome and sequelae. Respirology 2003; 8 Suppl:S36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J 2020; 55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lazzerini M, Putoto G. COVID-19 in Italy: momentous decisions and many uncertainties. Lancet Glob Health 2020; 8:e641–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carnethon MR, Pu J, Howard G, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular Health in African Americans: a scientific statement from the American Heart Association. Circulation 2017; 136:e393–423. [DOI] [PubMed] [Google Scholar]
- 42. Goff LM, Ladwa M, Hakim O, Bello O. Ethnic distinctions in the pathophysiology of type 2 diabetes: a focus on black African-Caribbean populations. Proc Nutr Soc 2020; 79:184–93. [DOI] [PubMed] [Google Scholar]
- 43. Marshall MC Jr. Diabetes in African Americans. Postgrad Med J 2005; 81:734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pal R, Bhadada SK. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr 2020; 14:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html. Accessed 10 July 2020.
- 46. Nyenhuis SM, Krishnan JA, Berry A, et al. Race is associated with differences in airway inflammation in patients with asthma. J Allergy Clin Immunol 2017; 140:257–265.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Available at: https://www.aafa.org/asthma-triggers-causes/. Accessed 10 July 2020.
- 48. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haslam DW, James WP. Obesity. Lancet 2005; 366:1197–209. [DOI] [PubMed] [Google Scholar]
- 50.Available at: www.cdc.gov/obesity/data/adult.html. Accessed 10 July 2020.
- 51. DeSantis CE, Miller KD, Goding Sauer A, et al. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019; 69:211–33. [DOI] [PubMed] [Google Scholar]
- 52. Potton E, McCaughan F, Janes S. Chronic obstructive pulmonary disease and lung cancer. Respir Med 2009; 5:34–7. [Google Scholar]
- 53.Available at: https://www.cdc.gov/tobacco/disparities/african-americans/index.htm. Accessed 10 July 2020.
- 54. Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med 2006; 100:1110–6. [DOI] [PubMed] [Google Scholar]
- 55. Chatila WM, Wynkoop WA, Vance G, Criner GJ. Smoking patterns in African Americans and whites with advanced COPD. Chest 2004; 125:15–21. [DOI] [PubMed] [Google Scholar]
- 56. Luzzatto L. Sickle cell anaemia and malaria. Mediterr J Hematol Infect Dis 2012; 4:e2012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Available at: https://www.cdc.gov/ncbddd/sicklecell/data.html. Accessed 10 July 2020.
- 58. Dei-Adomakoh YA, Afriyie-Mensah JS, Forson A, et al. Lung function abnormalities in sickle cell anaemia. Adv Hematol 2019; 2019:1783240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Available at: https://www.bmc.org/healthcity/population-health/covid-19-sickle-cell-disease-and-critical-need. Accessed 10 July 2020.
- 60. Strouse JJ, Reller ME, Bundy DG, et al. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood 2010; 116:3431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Available at: https://www.health.harvard.edu/diseases-and-conditions/if-you-are-at-higher-risk. Accessed 10 July 2020.
- 62.Available at: https://www.cdc.gov/ncbddd/sicklecell/traits.html. Accessed 10 July 2020.
- 63. Gattinoni L, Chiumello D, Caironi P, et al. COVID -19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46:1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gattinoni L, Coppola S, Cressoni M, et al. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990; 86:1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fan X, Wang Y, Sun K, et al. ; Study Group for Pharmacogenomic Based Antihypertensive Drugs Selection, Effects and Side Effects, in Rural Area Chinese. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of Captopril in women. Clin Pharmacol Ther 2007; 82:187–96. [DOI] [PubMed] [Google Scholar]
- 67. Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Gene polymorphisms in angiotensin I converting enzyme (ACE I/D) and angiotensin II converting enzyme (ACE2 C–>T) protect against cerebral malaria in Indian adults. Infect Genet Evol 2010; 10:337–41. [DOI] [PubMed] [Google Scholar]
- 68. Mengesha HG, Petrucka P, Spence C, Tafesse TB. Effects of angiotensin converting enzyme gene polymorphism on hypertension in Africa: a meta-analysis and systematic review. PLoS One 2019; 14:e0211054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020; 141:e139–596. [DOI] [PubMed] [Google Scholar]
- 70. Gallego-Delgado J, Walther T, Rodriguez A. The high blood pressure-malaria protection hypothesis. Circ Res 2016; 119:1071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zarouk WA, Hussein IR, Esmaeil NN, et al. Association of angiotensin converting enzyme gene (I/D) polymorphism with hypertension and type 2 diabetes. Bratisl Lek Listy 2012; 113:14–8. [DOI] [PubMed] [Google Scholar]
- 72. Mao S, Huang S. A meta-analysis of the association between angiotensin-converting enzyme insertion/ deletion gene polymorphism and the risk of overweight/obesity. J Renin Angiotensin Aldosterone Syst 2015; 16:687–94. [DOI] [PubMed] [Google Scholar]
- 73. Mehri S, Mahjoub S, Hammami S, et al. Renin-angiotensin system polymorphisms in relation to hypertension status and obesity in a Tunisian population. Mol Biol Rep 2012; 39:4059–65. [DOI] [PubMed] [Google Scholar]
- 74. Adamzik M, Frey U, Sixt S, et al. ACE I/D but not AGT (-6)A/G polymorphism is a risk factor for mortality in ARDS. Eur Respir J 2007; 29:482–8. [DOI] [PubMed] [Google Scholar]
- 75. Quach H, Rotival M, Pothlichet J, et al. Genetic adaptation and Neandertal admixture shaped the immune system of human populations. Cell 2016; 167:643–56.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and white Americans. Am J Epidemiol 2004; 160:1033–8. [DOI] [PubMed] [Google Scholar]
- 77. Nédélec Y, Sanz J, Baharian G, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell 2016; 167:657–69.e21. [DOI] [PubMed] [Google Scholar]
- 78. Brinkworth JF, Barreiro LB. The contribution of natural selection to present-day susceptibility to chronic inflammatory and autoimmune disease. Curr Opin Immunol 2014; 31:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Agbuduwe C, Basu S. Haematological manifestations of COVID-19: from cytopenia to coagulopathy. Eur J Haematol 2020; 105:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 2020; 24:100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cushman M, Folsom AR, Wang L, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood 2003; 101:1243–8. [DOI] [PubMed] [Google Scholar]
- 84. Ladikou EE, Sivaloganathan H, Milne KM, et al. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med (Lond) 2020; 20:e178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jenkins PV, Rawley O, Smith OP, O’Donnell JS. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol 2012; 157:653–63. [DOI] [PubMed] [Google Scholar]
- 86. Edelstein LC, Simon LM, Montoya RT, et al. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med 2013; 19:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lutsey PL, Cushman M, Steffen LM, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost 2006; 4:2629–35. [DOI] [PubMed] [Google Scholar]
- 88. Swystun LL, Lillicrap D. Genetic regulation of plasma von Willebrand factor levels in health and disease. J Thromb Haemost 2018; 16:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aksenova AY. Von Willebrand factor and endothelial damage: a possible association with COVID-19. Ecol Genet 2020; 18:135–8. [Google Scholar]
- 90. Khaleghi M, Saleem U, McBane RD, et al. African-American ethnicity is associated with higher plasma levels of D-dimer in adults with hypertension. J Thromb Haemost 2009; 7:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020; 12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McCartney DM, Byrne DG. Optimisation of vitamin D status for enhanced immuno-protection against Covid-19. Ir Med J 2020; 113:58. [PubMed] [Google Scholar]
- 93. Dancer RC, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015; 70:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Papandreou D, Hamid ZT. The role of vitamin D in diabetes and cardiovascular disease: an updated review of the literature. Dis Markers 2015; 2015:580474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J 2020; 287:3693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 2020; 32:1195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Boucher BJ. The problems of vitamin d insufficiency in older people. Aging Dis 2012; 3:313–29. [PMC free article] [PubMed] [Google Scholar]
- 98.Available at: https://clinicaltrials.gov/ct2/show/NCT04344041. Accessed 10 July 2020.
- 99. Gonzalez-Jaramillo V, Portilla-Fernandez E, Glisic M, et al. Epigenetics and inflammatory markers: a systematic review of the current evidence. Int J Inflam 2019; 2019:6273680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Colen CG, Ramey DM, Cooksey EC, Williams DR. Racial disparities in health among nonpoor African Americans and Hispanics: the role of acute and chronic discrimination. Soc Sci Med 2018; 199:167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Aroke EN, Joseph PV, Roy A, et al. Could epigenetics help explain racial disparities in chronic pain? J Pain Res 2019; 12:701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bomotti SM, Smith JA, Zagel AL, et al. Epigenetic markers of renal function in African Americans. Nurs Res Pract 2013; 2013:687519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Akinyemiju T, Do AN, Patki A, et al. Epigenome-wide association study of metabolic syndrome in African-American adults. Clin Epigenetics 2018; 10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol 2009; 21:2–15. [DOI] [PubMed] [Google Scholar]
- 105. Shannon Sullivan. The physiology of sexist and racist oppression - The Epigenome: On the transgenerational effects of racism. 2015: Chapter 4, Oxford Scholarship Online. doi: 10.1093/acprof:oso/9780190250607.003.0004. [DOI] [Google Scholar]
- 106. Shannon S. Inheriting racist disparities in health: epigenetics and the transgenerational effects of white racism. Crit Philos Race 2013; 1:190–218. [Google Scholar]
- 107. Nguyen A, David JK, Maden SK, et al. Human leukocyte antigen susceptibility map for SARS-CoV-2. J Virol 2020; 94:e00510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 2020; 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020; 12:10087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ortiz-Fernández L, Sawalha AH. Genetic variability in the expression of the SARS-CoV-2 host cell entry factors across populations. Genes Immun 2020; 21:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McCoy J, Wambier CG, Vano-Galvan S, et al. Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19? J Cosmet Dermatol 2020; 19:1542–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Giovannucci E, Stampfer MJ, Krithivas K, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A 1997; 94:3320–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bennett CL, Price DK, Kim S, et al. Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. J Clin Oncol 2002; 20:3599–604. [DOI] [PubMed] [Google Scholar]
- 115. Sartor O, Zheng Q, Eastham JA. Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology 1999; 53:378–80. [DOI] [PubMed] [Google Scholar]
- 116. Irvine RA, Yu MC, Ross RK, Coetzee GA. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res 1995; 55:1937–40. [PubMed] [Google Scholar]
- 117. Clinckemalie L, Spans L, Dubois V, et al. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol Endocrinol 2013; 27:2028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dalpiaz PL, Lamas AZ, Caliman IF, et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 2015; 10:e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nazha B, Mishra M, Pentz R, Owonikoko TK. Enrollment of racial minorities in clinical trials: old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book 2019; 39:3–10. [DOI] [PubMed] [Google Scholar]
- 120.Available at: https://www.cdc.gov/flu/fluvaxview/reportshtml/trends/index.html. Accessed 2 January 2021.
- 121. Victor RG, Haley RW, Willett DL, et al. ; Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93:1473–80. [DOI] [PubMed] [Google Scholar]