Abstract
The role of obesity in the pathophysiology of respiratory virus infections has become particularly apparent during the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, where obese patients are twice as likely to suffer from severe coronavirus disease 2019 (COVID-19) than healthy weight individuals. Obesity results in disruption of systemic lipid metabolism promoting a state of chronic low-grade inflammation. However, it remains unclear how these underlying metabolic and cellular processes promote severe SARS-CoV-2 infection. Emerging data in SARS-CoV-2 and Influenza A virus (IAV) infections show that viruses can further subvert the host’s altered lipid metabolism and exploit obesity-induced alterations in immune cell metabolism and function to promote chronic inflammation and viral propagation. In this review, we outline the systemic metabolic and immune alterations underlying obesity and discuss how these baseline alterations impact the immune response and disease pathophysiology. A better understanding of the immunometabolic landscape of obese patients may aid better therapies and future vaccine design.
Keywords: obesity, SARS-CoV-2, influenza, virus, metabolism, immune response, COVID-19, inflammation
INTRODUCTION
Obesity has emerged as an unexpected feature of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) [1]. The SARS-CoV-2 pandemic has highlighted the global health crisis that is obesity [2] representing a risk factor independent of other co-morbidities [3]. Evidence of a link between obesity and infectious respiratory disease first became apparent after the 2009 influenza A pandemic (H1N1pdm). Obesity accounted for 12% of total deaths from H1N1pdm [4–7]. Early COVID-19 data emerging from China did not report body mass index (BMI), however, a study in Seattle showed that for critically ill patients, 85% were obese requiring mechanical ventilation and 62% of them died [8]. Two retrospective studies in New York City at the epicentre of the outbreak found COVID-19 patients aged <60 years with a BMI of 30–34 were 2.0 and 1.8 times more likely to suffer severe disease requiring critical care and a BMI ≥ 35 were 2.2 times more likely [9]. A BMI > 40 is a strong predictor of hospitalization (OR 6.2, 95% CI 4.2–9.3) [10]. In a French centre, 47.6% of severe COVID-19 patients were obese (BMI > 30) and 28.2% were severely obese (BMI > 35) with a total of 68.6% of patients requiring invasive mechanical ventilation, particularly males [3]. In the UK, several prospective studies based on UK biobank data sets revealed detrimental effects of obesity on COVID-19 susceptibility and disease severity, even after adjustment for other co-morbidities such as age, lifestyle and chronic diseases [11, 12]. Interestingly, Ho et al. demonstrated that increased inflammation which is associated with increased adiposity may increase susceptibility for severe COVID-19 in obese individuals [12]. Data from 265 patients hospitalized with severe COVID-19 disease, revealed a significant inverse correlation between age and BMI. Younger individuals (<50 years) who were admitted to intensive care units for COVID-19 were more likely to be obese compared with older individuals [13]. These studies highlight the risk of obesity in this generally considered low-risk age group [9, 13].
In this review, we will discuss how obesity impacts viral shedding, propagation, host metabolism, inflammation and immunity. Furthermore, we highlight ongoing therapeutic approaches to improve COVID-19 patient survival in the obese.
Viral shedding
Obesity may play a role in prolonged viral shedding of infected individuals, potentially increasing transmission within the population. Viral shedding of IAV in obese adults can last 42% longer than lean individuals and in asymptomatic cases 104% longer [14], data confirmed in diet-induced obesity (DIO) mouse models [15]. Similarly, obese patients with SARS-CoV-2 required longer hospitalization stays and time to test negatively by pharyngeal swab compared to non-obese patients [16]. This indicates that obese individuals may require longer time to overcome viral infections and may promote viral spread in the population.
Viral receptors
Obesity may contribute to viral propagation by promoting the expression of the SARS-CoV-2 cell entry receptor angiotensin-converting enzyme 2 (ACE2) on a variety of cell types including bronchial epithelial cells [17, 18]. ACE2 is also expressed on adipocytes [19, 20]. Importantly, obesity may upregulate ACE2 [21], possibly under the control of sterol regulatory element-binding protein 1 (SREBP1), a transcription factor which has an important role in lipogenesis, adipogenesis and cholesterol homeostasis to prevent lipotoxicity. Mouse studies show that ACE2 mRNA expression remained elevated in high-fat-fed mice compared with low-fat-fed mice demonstrating that adipocytes express ACE2 and is nutritionally regulated by high-fat feeding [22]. These findings suggest that obese people should be considered an at-risk population as ACE2 expression might be higher than in non-obese people. Indeed, ACE inhibitors (ACEi) represent good candidates for COVID-19 treatment [23–27] with telmisartan (NCT04355936) completing Phase IV and losartan (NCT04311177; NCT04312009) and captopril (NCT04355429) in Phase II Clinical Trials. All of these drug candidates are effective therapies for high blood pressure and heart failure, which are comorbidities more common in obese individuals. Telmisartan and losartan are angiotensin II receptor type 1 (AT1) antagonists and thus selectively block the binding of angiotensin II to the AT1 receptor [28]. This competitive replacement of endogenous angiotensin II will lower the pro-inflammatory and vascular effects by receptor activation and therefore may prevent hyperinflammation in COVID-19 patients. In contrast, captopril directly inhibits the enzymatic function of ACE preventing the conversion of angiotensin I to angiotensin II thus also increasing activation of the renin–angiotensin–aldosterone system (RAAS) [28]. The usage of ACEi has sparked debate about its safety since blocking RAAS can increase expression of ACE2 on the cell surface and thus could enhance viral uptake. On the contrary, the receptor is associated with tissue-protective, anti-inflammatory pathways and vasodilation [29] and could therefore reduce inflammation and cardiovascular insults caused by COVID-19. Early prospective cohort studies from more than 8 million participants in the UK suggest a reduced risk of COVID-19, when individuals were taking ACEi [30]. However, only completion of clinical trials will reveal whether ACEi can reduce COVID-19 risk and mortality associated with cardiovascular events.
Viral impact on cellular lipid metabolism
Upon viral infection, SARS-CoV-2 hijacks cellular metabolism for replication and production of new virions. SARS-CoV-2 infection in monocytes leads to an accumulation of intracellular lipids and an increase of proteins involved in lipid metabolism such as the fatty acid transporter CD36 and lipogenic transcription factors such as the peroxisome proliferator-activated receptor γ (PPARγ) and SREBP-1 [31]. Furthermore, SARS-CoV-2 may use lipid droplets as replication sites. Pharmacological inhibition of diacylglycerol O-acyltransferase 1 (DGAT1), a key enzyme for triacylglyceride synthesis and lipid droplet formation, prevents viral replication [31]. Similarly, IAV infection increases the extracellular uptake of palmitic acid promoting viral replication, whereas blocking CD36-dependent palmitate uptake using sulfo-N-succinimidyl oleate prevents enhanced viral replication [32]. Circulating levels of palmitic acid are increased in obese individuals and can promote inflammation through binding to the pattern recognition receptors Toll-like receptor 2 and 4 [33]. In cells, palmitoylation of viral membrane proteins facilitate membrane fusion by SARS-CoV and some IAV strains [34, 35]. Although the mechanism has not been elucidated, an increase of palmitoylation of some proteins in obese mice suggests an alteration in this pathway [36, 37].
Besides palmitoylation, enveloped viruses up-regulate cholesterol metabolism [38]. Cholesterol is required for the formation of lipid rafts and is thought to assist viral spreading by serving as an assembly point at the plasma membrane and to promote efficient viral cell entry [39]. Due to the central role of cholesterol metabolism in viral replication, the lipid-lowering therapy statins (HMG-CoA reductase inhibitors), have been investigated as a potential antiviral therapy. Two recent retrospective studies on the use of statins in SARS-CoV-2 survival revealed variable outcomes with one showing a 50% improved overall survival [40] whereas the other could not identify any survival advantage among the statin cohort [41].
Drugs targeting lipid metabolism present novel therapeutic tools for combating obesity-related viral infection through their central role in the viral infection cycle. Thus, statins and other lipid-lowering drugs represent a promising candidate for COVID-19 treatment in obese patients [42].
In the following section, we discuss how obesity changes the host’s metabolic and immunological state during viral infections and how this may provide a potent environment for respiratory viral infections such as SARS-CoV-2.
THE OBESOGENIC HOST
Systemic chronic inflammation
Enhanced adipose tissue deposition in the obese (particularly in the abdomen) not only creates alterations in abdominal pressure with inadequate lung function and reduced oxygen saturation [43], but also represents the ‘soil’ for chronic low-grade systemic inflammation by activated immune cells in response to over-nutrition [44–46]. Activation of the innate immune response in adipose tissues creates altered cytokine and adipokine profiles. Dysfunctional hypertrophic adipocytes promote a chronic inflammatory state by producing pro-inflammatory cytokines and accumulating pro-inflammatory ‘M1-like’ macrophages [47–49] thereby outnumbering anti-inflammatory ‘M2-type’ macrophages that maintain normal tissue homeostasis [50]. This underlying chronic inflammation impairs innate and adaptive immune responses to pathogens [51] therefore leaving the obesogenic host vulnerable to viral persistence, secondary infection and vaccine failure [52].
Obese individuals infected for example with IAV show delayed and attenuated innate and adaptive immune response with reduced numbers of activated and functional memory CD4+ and CD8+ T cells, leading to increased viral spread with prolonged infection, lung damage and susceptibility to secondary bacterial infections [53]. Titers of influenza virus-specific antibodies increasingly decline at 1-year post-vaccination in obese vaccinees, increasing their 2-fold risk of severe/critical influenza infection and increased hospital admissions compared to their healthy weight counterparts [4].
Impaired B and T cell responses are a hallmark feature of severe COVID-19 disease [54]. Low naïve CD4+ and CD8+ T cells (lymphopenia) and CD4+ T cells with Th17 and Th22 phenotypes represent impaired adaptive immunity and a pro-inflammatory phenotype [55, 56]. Other studies show increased numbers of CD4+ T cells, reduced cytotoxic CD8+ T cells [57] combined with reduced regulatory T cells [58]. Activated T cells infiltrate adipose tissue possibly in response to antigens developed through high-fat feeding [59], thus contributing to local tissue inflammation. Coupled with T helper cells producing inflammatory cytokines, including IL-6, IL-10 and TNF-α in both obesity and COVID-19 patients, these factors influence disease progression [3]. Obesity promotes increased effector and memory T cell populations but reduced T cell receptor diversity compared with lean normal chow-fed mice [60]. Thus, obesity may impact the ability of circulating T cells to respond to a disparate pool of antigens, leaving the obese individual susceptible to infection. Adipose tissues represent an important site for many adaptive immune cells, in particular, to provide a metabolic niche for memory T cell development and maintenance supporting a protective response to infection [61]. During reinfection, antigen-specific memory T cells from adipose tissue show stronger activation and increased lipid uptake compared to memory T cells from lymphoid organs. Interestingly, obesity increases memory T cell numbers in white adipose tissue in a DIO mouse model. While able to clear lymphocytic choriomeningitis virus (LCMV) during primary infection, restimulation of cross-reactive memory T cells during a heterologous challenge promotes a pathological, lethal effect in obese mice. Thus, pathogenesis occurs when increased numbers of memory T cells kill virus-infected white adipose tissue and release IFNγ and TNF cytokines [62].
In contrast, little is known about the effects of obesity on B cell function during infection. Adipose tissue-derived B cells from lean animals protect against peritoneal antigens via T cell-dependent class switching and hypermutation [63]. Obesity increases the number of B cells in visceral adipose tissue and produces autoreactive immunoglobulins [64]. Two subpopulations of B cells (BRegs and B-1a cells) regulate glucose metabolism through secretion of the anti-inflammatory cytokine IL-10. The protective effects of B-1a cells through IL-10 secretion support normal insulin regulation. In diabetic patients and obese mice, the function of B-1a cells is impaired, promoting chronic, low-grade inflammation and insulin resistance [65]. Furthermore, B-1 cells have been shown to promote protection to influenza infections by producing cross-reactive natural IgM [66]. However, it remains unclear if antibody-secreting cells accumulate in adipose tissues upon primary infection or vaccination and whether they contribute to the secondary response during reinfection. The formation of an adaptive immune memory to SARS-CoV-2 may be crucial to identify the best vaccination strategy for the obese population that is so much more at risk of severe disease course.
Dyslipidaemia
Lipid homeostasis is predominantly regulated by adipose tissues and the liver in order to provide cells with essential lipids for biosynthesis and energy metabolism. Many lipids, including cholesterol, are transported in lipoprotein complexes in blood to avoid lipotoxicity. These are crucial for immune and stromal cell function. For instance, macrophages rely on high-density lipoproteins (HDLs) to control cholesterol efflux [67]. Obese individuals have lower levels of high-density lipoprotein cholesterol (HDL-C) that leads to an accumulation of cholesterol in cells which can promote inflammation and viral replication while decreased total circulating cholesterol levels limit T cell expansion [68]. A common feature of viral infections is an imbalance in systemic lipid metabolism often leading to a significant reduction in circulating HDL-C levels. In line with this, severe to critical SARS-CoV-2 patients exhibit reduced HDL-C levels which are inversely correlating with inflammation markers such as C-reactive protein [69, 70]. Knowing that dysregulation of HDL-C levels occurs frequently in critical viral infections, treatment of this imbalance by administration of an Apo-A1 mimetic, the main lipoprotein in HDL-C complexes, significantly reduced influenza-induced lung damage in a murine model [71]. Taken together, this would suggest that decreased circulating HDL-C due to obesity may raise the risk of increased disease severity in viral infections.
Besides cholesterol, other lipid species can directly impact on immune function such as derivates of arachidonic acid. This poly-unsaturated fatty acid is transformed in innate immune cells to lipid mediators known to promote inflammation such as prostaglandins. COVID-19 patients show a gradual decrease in the precursor arachidonate with increasing disease severity [72]. This may indicate that SARS-CoV-2 can propagate more efficiently. In line with this, arachidonic acid supplementation can limit Middle East Respiratory Syndrome (MERS) virus production [73]. Other lipid mediators derived from eicosanoid acid and docosanoic acid are also dysregulated in SARS-CoV-2 patients and show an overall increase with disease severity, with some pro-resolving lipid mediators showing decreased presence in severe patients [74]. This is in line with observations of highest cyclooxygenase activity in moderate compared to severe patient groups. Furthermore, the activity of the arachidonate 5-lipoxygenase (ALOX5), a key enzyme in the production of arachidonic-derived mediators exhibits increased expression in obese individuals and severe COVID-19 patients. ALOX5 is predominantly expressed in monocytes and macrophages of SARS-CoV-2-infected patients and may contribute to inflammation through increased leukotrienes which can promote pro-inflammatory adipokine production [75–77]. Similar changes have been observed in influenza patients [78]. Based on these studies, it is likely that the underlying obesity-associated lipid imbalance is further worsened by SARS-CoV-2 promoting development of severe disease pathology, impairing resolution and hence prolonged inflammation.
Oxidative stress and mitochondrial dysfunction
Mitochondria play a key role in cellular metabolism including tricarboxylic acid cycle, decarboxylation of fatty acids or β-oxidation to generate and sustain adenosine triphosphate (ATP) levels for cellular energy demands, while also being the main producer of reactive oxygen species (ROS). Accumulating evidence links the accelerated progression of COVID-19 to increased levels of inflammation leading to increased ROS production and cellular oxidative stress which in turns can trigger mitochondrial dysfunction [79]. Indeed, oxidative stress was described as a main player in COVID-19 pathogenesis [80] and oxidative stress-associated genes were enriched in bronchoalveolar lavage fluid of severe COVID-19 patients [81]. This was demonstrated in SARS-CoV-2-infected monocytes, which play a key role in SARS-CoV-2-driven inflammation [81], displaying higher mitochondrial ROS production and reduced mitochondrial oxidative metabolism including a reduction in the spare respiratory capacity, a critical complement of mitochondrial bioenergetics that can be utilized during increased energy demands [82]. In agreement with these findings, mitochondrial antioxidant treatment leads to inhibition of SARS-CoV-2 replication and prevents upregulation of inflammatory cytokines [83]. This suggests dysregulated mitochondrial function of monocytes could contribute to SARS-CoV-2 pathogenesis.
Obesity causes profound metabolic alterations leading to mitochondrial dysfunction [84–86] which can contribute to obesity-related COVID-19 pathogenesis. Imbalance of nutrient intake, high free fatty acids concentration and hyperglycaemia result in increased ROS production and adipocyte mitochondrial dysfunction [87]. Consistent with these findings, adipocytes in obese hosts display a reduced mitochondrial oxidative capacity and biogenesis [85, 86] and a downregulation of fatty acid oxidation (FAO) and the tricarboxylic acid cycle pathways which inversely correlate with low-grade inflammation [85, 88]. These obesity-associated host factors could serve as dual roles in the metabolism of virus-infected cells. First, these factors may ‘fuel the fire’ of viral-induced metabolic reprogramming and mitochondrial alterations. Second, obesity-associated mitochondrial defects can contribute to modulating antiviral immune responses [53, 79]. For example, lipid accumulation in natural killer (NK) cells leads to impaired NK cellular metabolism, including mitochondria respiration, and trafficking of the cytotoxic machinery, causing a complete ‘paralysis’ of their cytotoxicity [89]. In addition to NK cells, accumulation of lipids in dendritic cells (DCs) results in reduced capacity in processing antigens, leading to a defect in stimulating allogeneic T cells [90]. Type-17 mucosal-associated invariant T (MAIT) cells which have been reported to increase in obese hosts [91] and linked to the pathogenesis of chronic infections [92], displayed altered mitochondrial metabolism in obesity [92]. Interestingly, these subsets have been described as the prominent IL-17-producing cells in the airways of COVID-19 patients [93].
T cell metabolism
In the context of acute viral infection, increased energy demand for effector T cell function is derived from a variety of fuel sources. Shifting from oxidative phosphorylation to glycolysis and production of ATP provides the fuel for increased metabolic demands. The conversion of effector T cells to long-lived memory T cells requires a switch back to oxidative metabolism with fatty acids as the fuel source [94, 95]. Additionally, the maintenance of tissue-resident memory CD8+ T cells depends on the uptake of exogenous free fatty acids [96]. Although T cells respond to antigens by altering their metabolic state in this way, it is not clear how systemic metabolic conditions affect T cell function. Saturated fatty acid-induced metabolic alteration leads to a preferential migration of effector T cells to inflammatory sites, contributing to low-grade systemic inflammation observed in obese individuals [97]. Treatment with palmitic acid results in increased oxidative phosphorylation in naïve T cells promoting their differentiation into pro-inflammatory effector memory phenotype, suggesting a preferential usage of FAO during T cell activation in fat-rich environment promotes pro-inflammatory effector T cell differentiation [97]. Consistent with these findings, T cells isolated from the spleen of mice with DIO display altered mitochondrial phosphorylation and a preferential utilization of fatty acids as mitochondrial fuel. In COVID-19, T cells from patients with severe disease show an increased oxidative phosphorylation compared with mild or recovered groups, suggesting altered T cell metabolism in severe infection [98]. Furthermore, T cells from patients with progressed COVID-19 displayed an altered mitochondria morphology, increased mitochondrial mass and accumulation of ROS production. Interestingly, COVID-19 patients with pre-existing metabolic disorders such as obesity showed different capacities for nutrient uptake compared to uninfected controls [99]. In addition, obesity dysregulates glucose usage and FAO and storage [100, 101] and changes in T cell metabolism are associated with impaired T cell response to influenza. Even prior to influenza infection, obese mice had altered cellular metabolism characterized by T cells with increased oxidative phosphorylation and glycolysis [102]. After influenza infection, CD4+ and CD8+ T cells from obese mice significantly increased the oxidative phosphorylation to glycolysis ratio in comparison to lean mice [102]. Weight loss in obese mice reversed systemic hyper-insulinaemia and hyperglycaemia but failed to prevent infiltration of T cells into adipose tissue and did not reverse memory T cell dysfunction. These findings suggest that obesity and metabolic disturbance creates epigenetic reprogramming on T cell function. This has not been studied in SARS-CoV-2 patients but is likely to contribute as T cell responses from critically ill patients are significantly impaired.
CONCLUSION
Obesity causes severe impairment of systemic lipid homeostasis due to calorie excess. Hypertrophic adipocytes create a state of systemic lipid imbalance and low-grade inflammation. This change in the host’s metabolic and inflammatory status renders immune cells dysfunctional by substantially altering mitochondrial structure and function. Consequently, immune cell metabolism shifts away from oxidative phosphorylation towards more pro-inflammatory glycolytic pathways, exacerbating SARS-CoV-2-induced inflammatory processes. It remains to be seen if obese patients can mount an effective memory B and T cell response to SARS-CoV-2 infection since dysfunctional adipose tissues in obese individuals may impair the necessary immunometabolic switch of memory cells towards FAO and consequently alter their reactivation potential. Previous studies examining influenza infections show that obesity impairs memory responses leaving the host more susceptible to reinfection. It remains to be seen whether obese patients are more likely to experience reinfection with SARS-CoV-2 over the coming years. Dyslipidemia may further promote viral replication through increasing ACE2 expression on adipocytes and epithelial cells.
Although global health measures to reduce obesity may represent a long-term solution to reduce obesity-driven impairments in immune response to viral infections [103], patients are in desperate need of new and effective treatments and vaccines to reduce disease severity and mortality. Drugs targeting host and or viral lipid metabolism are currently under investigation and statins have shown early promising results in promoting overall patient survival in retrospective studies [40]. However, it remains unclear whether the effects are mediated through their impact on the host’s lipid metabolism or through direct anti-inflammatory effects or both.
Future studies elucidating the metabolic and immunologic mechanisms of obesity and risk of SARS-CoV-2 infection and severe disease are warranted. Understanding these pathways at the molecular level may enable development of targeted therapeutic approaches and aid future vaccine design for the obesogenic host.
Box 1: Why does obesity matter during COVID-19?
Several studies underline the significant changes in lipid metabolism observed during viral infections, some of which are reminiscent of changes observed in obese uninfected individuals. It is clear that obese individuals are 2 times more likely to experience severe COVID-19 progression. This shows striking parallels to heightened risk of influenza-related hospitalization and disease severity. Obesity fundamentally alters the host’s metabolism and promotes obesity-associated inflammation, which has been linked in the context of influenza infections to increased viral replication and disease severity. Thus, obese individuals represent a more susceptible population group that requires particular attention in disease prevention and treatment during this pandemic.
Box 2: What is the consensus on obesity and COVID-19?
Obesity in the UK has been increasing over the past decades. Today, ∼30% of the UK population is classified as obese. Obesity increases the risk of other chronic diseases and makes individuals more susceptible to viral infections like influenza and as observed now during the SARS-CoV-2 outbreak. The UK government has recently acknowledged the necessity of tackling this issue and started promoting long-term public health measures (see https://www.gov.uk/government/news/new-obesity-strategy-unveiled-as-country-urged-to-lose-weight-to-beat-coronavirus-covid-19-and-protect-the-nhs) [103] to decrease obesity in its population. However, it will take time until these measures show effects. It remains crucial to further continue broadening our understanding of the metabolic and immunological changes in obese individuals. Improving our understanding of how obesity promotes viral infections such as SARS-CoV-2 may lead to better therapies and vaccine designs in this patient group.
ACKNOWLEDGEMENTS
We wish to express our gratitude to all members of the Oxford-Cardiff COVID-19 Literature Consortium for their relentless support and hard work during this pandemic. We also thank Professor Katja Simon and Professor Awen Gallimore for their helpful comments on this manuscript.
FUNDING
F.C.R. is funded by the Infection, Immunology and Translational Medicine Wellcome Trust Studentship. A.A. is funded by the Saudi Ministry of Education Studentship. A.T.C. is funded by Cancer Research Wales. S.H.-C. is funded by the Wellcome Trust Collaborative Award in Science. Grant codes: Sarah Hulin-Curtis from Wellcome Trust (513546), Felix Richter from Wellcome Trust (203803/Z/16/Z), Alicia Teijeira Crespo from Cancer Research Wales PhD studentship (514472).
AUTHORS’ CONTRIBUTION
All named authors have contributed by reviewing the current peer-reviewed and preprint literature and writing of the manuscript. The consortium has provided a platform for a wide-range literature assessment in the context of local and joint COVID-19 literature initiatives and organized cross-university review efforts. All authors have approved the final version.
F.C.R., S.H.-C., A.A. and A.T.C. contributed to conceptualization; F.C.R. contributed to project administration; S.H.-C. contributed to supervision; A.A. contributed to visualization; S.H.-C., F.C.R., A.A., A.T.C. contributed to writing—original draft and writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
DATA AVAILABILITY
No new data were generated or analysed in support of this research. The review was, in part, based on weekly releases by the Oxford-Cardiff COVID-19 Literature consortium—an online database on preprint digests and assessments (https://www.immunology.ox.ac.uk/covid-19/covid-19-immunology-literature-reviews/).
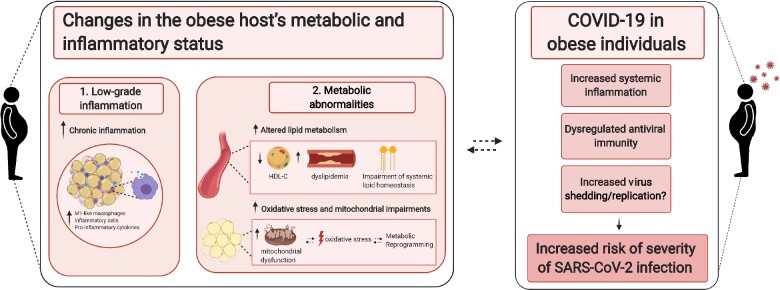
Figure 1:
Obesity alters host metabolism and immune response to promote viral infections. Obesity is linked with increased systemic inflammation [104] and metabolic abnormalities which can contribute to severe lung manifestation, exacerbation of the inflammatory process and dysregulation of innate and adaptive antiviral immunity, contributing to COVID-19 pathogenesis. The figure was created with BioRender.
REFERENCES
- 1. World Health Organisation (2020, January 12). Retrieved January 29, 2021. from https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ (28 January 2021, date last accessed)
- 2. Dai H, Alsalhe TA, Chalghaf N et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: an analysis of the Global Burden of Disease Study. PLoS Med 2020;17:e1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simonnet A, Chetboun M, Poissy J et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun Y, Wang Q, Yang G et al. Weight and prognosis for influenza A(H1N1)pdm09 infection during the pandemic period between 2009 and 2011: a systematic review of observational studies with meta-analysis. Infect Dis (Lond) 2016;48:813–22. [DOI] [PubMed] [Google Scholar]
- 5. Van Kerkhove MD, Vandemaele KA, Shinde V et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 2011;8:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaillant L, La Ruche G, Tarantola A et al. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009;14. [DOI] [PubMed] [Google Scholar]
- 7. Morgan OW, Bramley A, Fowlkes A et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One 2010;5:e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatraju PK, Ghassemieh BJ, Nichols M et al. COVID-19 in critically ill patients in the seattle region - case series. N Engl J Med 2020;382:2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lighter J, Phillips M, Hochman S et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis 2020;71:896–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrilli CM, Jones SA, Yang J et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamer M, Kivimäki M, Gale CR et al. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun 2020;87:184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ho FK, Celis-Morales CA, Gray SR et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open 2020;10:e040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020;395:1544–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maier HE, Lopez R, Sanchez N et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018;218:1378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karlsson EA, Meliopoulos VA, van de Velde NC et al. A perfect storm: increased colonization and failure of vaccination leads to severe secondary bacterial infection in influenza virus-infected obese mice. mBio 2017;8:e00889–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moriconi D, Masi S, Rebelos E et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes Res Clin Pract 2020;14:205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higham A, Singh D. Increased ACE2 expression in bronchial epithelium of COPD patients who are overweight. Obesity (Silver Spring) 2020;28:1586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamming I, Timens W, Bulthuis ML. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinheiro TA, Barcala-Jorge AS, Andrade JMO et al. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J Nutr Biochem 2017;48:74–82. [DOI] [PubMed] [Google Scholar]
- 20. Li MY, Li L, Zhang Y et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupte M, Thatcher SE, Boustany-Kari CM et al. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 2012;32:1392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupte M, Boustany-Kari CM, Bharadwaj K et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 2008;295:R781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Engin AB, Engin ED, Engin A. Two important controversial risk factors in SARS-CoV-2 infection: obesity and smoking. Environ Toxicol Pharmacol 2020;78:103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kakuma T, Gotoh K, Masaki T et al. Telmisartan reduced abdominal circumference and body weight with decreasing triglyceride level in patients with type 2 diabetes and metabolic syndrome. Obes Res Clin Pract 2010;4:e83–162. [DOI] [PubMed] [Google Scholar]
- 25. Araki K, Masaki T, Katsuragi I et al. Telmisartan prevents obesity and increases the expression of uncoupling protein 1 in diet-induced obese mice. Hypertension 2006;48:51–7. [DOI] [PubMed] [Google Scholar]
- 26. de Kloet AD, Krause EG, Kim DH et al. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology 2009;150:4114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nami B, Ghanaeian A, Ghanaeian K et al. The Effect of ACE2 Inhibitor MLN-4760 on the Interaction of SARS-CoV-2 Spike Protein with Human ACE2: A Molecular Dynamics Study. ChemRxiv. Preprint. 10.26434/chemrxiv.12159945.v1. 2020. [DOI]
- 28. Messerli FH, Bangalore S, Bavishi C et al. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 2018;71:1474–82. [DOI] [PubMed] [Google Scholar]
- 29. Kuster GM, Pfister O, Burkard T et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J 2020;41:1801–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hippisley-Cox J, Tan PS, Coupland C. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart 2020;106:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dias SSG, Soares VC, Ferreira AC, et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog 2020;16:e1009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Limsuwat N, Boonarkart C, Phakaratsakul S et al. Influence of cellular lipid content on influenza A virus replication. Arch Virol 2020;165:1151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res 2019;68:915–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gordon DE, Jang GM, Bouhaddou M et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020;583:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grantham ML, Wu WH, Lalime EN et al. Palmitoylation of the influenza A virus M2 protein is not required for virus replication in vitro but contributes to virus virulence. J Virol 2009;83:8655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ren W, Jhala US, Du K. Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte 2013;2:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spinelli M, Fusco S, Grassi C. Nutrient-dependent changes of protein palmitoylation: impact on nuclear enzymes and regulation of gene expression. Int J Mol Sci 2018;19: 3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin S, Liu N, Yang Z et al. GC/MS-based metabolomics reveals fatty acid biosynthesis and cholesterol metabolism in cell lines infected with influenza A virus. Talanta 2010;83:262–8. [DOI] [PubMed] [Google Scholar]
- 39. Sviridov D, Bukrinsky M. Interaction of pathogens with host cholesterol metabolism. Curr Opin Lipidol 2014;25:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang XJ, Qin JJ, Cheng X et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab 2020;32:176–87.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hui Jin, Junji He, Chuan Dong et al. Altered lipid profile is a risk factor for the progression and recurrence of COVID-19: from two retrospective cohorts, 19 August 2020, PREPRINT (Version 1) available at Research Square [ 10.21203/rs.3.rs-60159/v1]. [DOI] [Google Scholar]
- 42. Abu-Farha M, Thanaraj TA, Qaddoumi MG et al. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int J Mol Sci 2020;21: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shetty S, Parthasarathy S. Obesity hypoventilation syndrome. Curr Pulmonol Rep 2015;4:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnston RA, Suratt BT. Mechanisms and Manifestations of Obesity in Lung Disease. 1st Edition. Academic Press: London. 2018:353. [Google Scholar]
- 45. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177–85. [DOI] [PubMed] [Google Scholar]
- 46. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 47. Guilherme A, Virbasius JV, Puri V et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maurizi G, Della Guardia L, Maurizi A et al. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J Cell Physiol 2018;233:88–97. [DOI] [PubMed] [Google Scholar]
- 49. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wensveen FM, Valentić S, Šestan M et al. The "Big Bang" in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol 2015;45:2446–56. [DOI] [PubMed] [Google Scholar]
- 51. Nieman DC, Henson DA, Nehlsen-Cannarella SL et al. Influence of obesity on immune function. J Am Diet Assoc 1999;99:294–9. [DOI] [PubMed] [Google Scholar]
- 52. Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr 2016;7:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Honce R, Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front Immunol 2019;10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Giamarellos-Bourboulis EJ, Netea MG, Rovina N et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020;27:992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frydrych LM, Bian G, O'Lone DE et al. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol 2018;104:525–34. [DOI] [PubMed] [Google Scholar]
- 56. McLaughlin T, Ackerman SE, Shen L et al. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest 2017;127:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. O'Rourke RW, Kay T, Scholz MH et al. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes Surg 2005;15:1463–8. [DOI] [PubMed] [Google Scholar]
- 58. Feuerer M, Herrero L, Cipolletta D et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McLaughlin T, Liu LF, Lamendola C et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol 2014;34:2637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang H, Youm YH, Vandanmagsar B et al. Obesity accelerates thymic aging. Blood 2009;114:3803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han SJ, Glatman Zaretsky A, Andrade-Oliveira V et al. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity 2017;47:1154–68.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Misumi I, Starmer J, Uchimura T et al. Obesity expands a distinct population of T cells in adipose tissue and increases vulnerability to infection. Cell Rep 2019;27:514–24.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 2009;30:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Winer DA, Winer S, Shen L et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 2011;17:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shen L, Chng MH, Alonso MN et al. B-1a lymphocytes attenuate insulin resistance. Diabetes 2015;64:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baumgarth N, Herman OC, Jager GC et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med 2000;192:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tall AR, Yvan-Charvet L, Terasaka N et al. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 2008;7:365–75. [DOI] [PubMed] [Google Scholar]
- 68. Chyu KY, Lio WM, Dimayuga PC et al. Cholesterol lowering modulates T cell function in vivo and in vitro. PLoS One 2014;9:e92095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Song JW, Lam SM, Fan X et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab 2020;32:188–202.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuleshov MV, Stein DJ, Clarke DJB et al. The COVID-19 drug and gene set library. Patterns (N Y) 2020;1:100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Van Lenten BJ, Wagner AC, Anantharamaiah GM et al. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation 2002;106:1127–32. [DOI] [PubMed] [Google Scholar]
- 72. Shen B, Yi X, Sun Y et al. Proteomic and metabolomic characterization of COVID-19 Patient Sera Cell 2020;182:59–72.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yan B, Chu H, Yang D et al. Characterization of the lipidomic profile of human coronavirus-infected cells: implications for lipid metabolism remodeling upon coronavirus replication. Viruses 2019;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schwarz B, Sharma L, Roberts L et al. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators.. J Immunol 2021;206:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wilk AJ, Rustagi A, Zhao NQ et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 2020;26:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liao M, Liu Y, Yuan J et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020;26:842–4. [DOI] [PubMed] [Google Scholar]
- 77. Horrillo R, González-Périz A, Martínez-Clemente M et al. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol 2010;184:3978–87. [DOI] [PubMed] [Google Scholar]
- 78. Tam VC, Quehenberger O, Oshansky CM et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 2013;154:213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Saleh J, Peyssonnaux C, Singh KK et al. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020;54:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res 2020;51:384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bost P, Giladi A, Liu Y et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell 2020;181:1475–88.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pfleger J, He M, Abdellatif M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis 2015;6:e1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Codo AC, Davanzo GG, Monteiro LB et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis [published correction appears in Cell Metab. 2020 Sep 1;32(3):498–499]. Cell Metab 2020;32:437–446.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Putti R, Sica R, Migliaccio V et al. Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol 2015;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Heinonen S, Buzkova J, Muniandy M et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 2015;64:3135–45. [DOI] [PubMed] [Google Scholar]
- 86. Yin X, Lanza IR, Swain JM et al. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 2014;99:E209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. de Mello AH, Costa AB, Engel JDG et al. Mitochondrial dysfunction in obesity. Life Sci 2018;192:26–32. [DOI] [PubMed] [Google Scholar]
- 88. Ayari A, Rosa-Calatrava M, Lancel S et al. Influenza infection rewires energy metabolism and induces browning features in adipose cells and tissues. Commun Biol 2020;3:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Michelet X, Dyck L, Hogan A et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 2018;19:1330–40. [DOI] [PubMed] [Google Scholar]
- 90. Herber DL, Cao W, Nefedova Y et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 2010;16:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brien AO, Kedia-Mehta N, Tobin L et al. Targeting mitochondrial dysfunction in MAIT cells limits IL-17 production in obesity. Cell Mol Immunol 2020;17:1193–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Toubal A, Nel I, Lotersztajn S et al. Mucosal-associated invariant T cells and disease. Nat Rev Immunol 2019;19:643–57. [DOI] [PubMed] [Google Scholar]
- 93. Parrot T, Gorin JB, Ponzetta A et al. MAIT cell activation and dynamics associated with COVID-19 disease severity. Sci Immunol 2020;5:eabe1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pearce EL, Walsh MC, Cejas PJ et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009;460:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van der Windt GJ, Everts B, Chang CH et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012;36:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pan Y, Tian T, Park CO et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017;543:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mauro C, Smith J, Cucchi D et al. Obesity-induced metabolic stress leads to biased effector memory CD4. Cell Metab 2017;25:593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yao C, Bora SA, Parimon T et al. Cell type-specific immune dysregulation in severely ill COVID-19 patients. Cell Rep. 2021;34:108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Siska P. Metabolic stress and disease-stage specific basigin expression of peripheral blood immune cell subsets in COVID-19 patients. medRxiv 2020.
- 100. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15:6184–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: A review. World J Diabetes 2010;1:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rebeles J, Green WD, Alwarawrah Y et al. Obesity-induced changes in T-cell metabolism are associated with impaired memory T-cell response to influenza and are not reversed with weight loss. J Infect Dis 2019;219:1652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Department of Health, United Kingdom (2020). New obesity strategy unveiled as country urged to lose weight to beat coronavirus (COVID-19) and protect the NHS. Retrieved from https://www.gov.uk/government/news/new-obesity-strategy-unveiled-as-country-urged-to-lose-weight-to-beat-coronavirus-covid-19-and-protect-the-nhs (28 January 2021, date last accessed)
- 104. Afshin A, Forouzanfar MH, Reitsma MB et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research. The review was, in part, based on weekly releases by the Oxford-Cardiff COVID-19 Literature consortium—an online database on preprint digests and assessments (https://www.immunology.ox.ac.uk/covid-19/covid-19-immunology-literature-reviews/).
Figure 1:
Obesity alters host metabolism and immune response to promote viral infections. Obesity is linked with increased systemic inflammation [104] and metabolic abnormalities which can contribute to severe lung manifestation, exacerbation of the inflammatory process and dysregulation of innate and adaptive antiviral immunity, contributing to COVID-19 pathogenesis. The figure was created with BioRender.