Learning points for clinicians
Coronavirus disease 2019 (COVID-19) can impair male fertility with alterations in sperm morphology and DNA integrity could be a major post-COVID-19 complication in men.
All the clinicians should suggest periodic andrological assessment for COVID-19 ‘infected’ and ‘recovered’ patients. Further, all the men for ART should be tested for COVID-19 before the procedure.
Case presentation
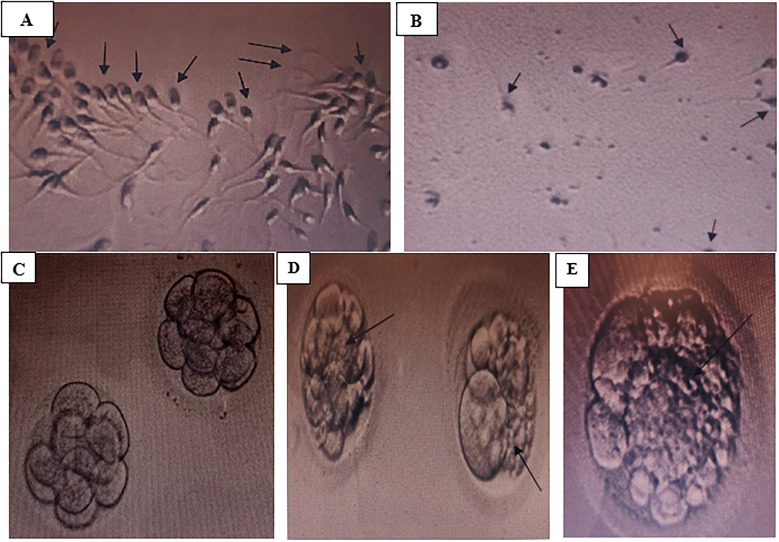
A South Indian couple with female factor infertility was planned for in vitro fertilization at our center. The man was 36 years old and the woman was 32 years old. Ovarian stimulation was achieved through an antagonist protocol. Only one metaphase-2 (M-II) oocyte was collected. The semen analysis of the man on the day of oocyte retrieval revealed ‘normozoospermia’ (Table 1, Figure 1A). On Day 3, only a single eight-cell embryo with Grade-1 quality (good quality) was formed (Figure 1C). However, in view of the limited number of embryos for transferring and the increasing age of the woman, embryo pooling was planned for the couple. The embryo was frozen at the Day-3 stage.
Table 1.
Semen analysis findings of man
| First cycle (pre-COVID-19) | Second cycle (1 month post-COVID-19) | Third cycle (4 months post- COVID-19) | Reference range (WHO 2010)6 | |
|---|---|---|---|---|
| Microscopic examination | ||||
| Sperm concentration (million/ml) | 40.4 | 10 | 22 | ≥15 |
| Total sperm count (million/ejaculate) | 172 | 30 | 67 | ≥39 |
| Motility | ||||
| Progressive motility (%) | 51 | 10 | 50 | ≥32 |
| Non-progressive motility (%) | 11 | 50 | 18 | |
| Non-motile (%) | 38 | 40 | 32 | |
| Morphology | ||||
| Normal forms (%) | 12 | <1 | 1 | ≥4 |
| Abnormal forms (%) | 88 | >99 | 99 | |
| Vitality | ||||
| Live sperms (%) | 76 | 22 | 54 | ≥58 |
| Dead sperms (%) | 24 | 8 | 46 | |
| Impression | Normozoospermia | Oligo-astheno-teratozoospermia | Teratozoopsermia | Normozoospermia |
| Method | Double density gradient method | Double density gradient method | Double density gradient method | |
WHO, World Health Organization.
Figure 1.
Sperm and embryo characteristics. (A) Normozoospermia with an excellent sperm count, motility and normal morphology in man before acquiring COVID-19. (B) Oligo-astheno-teratozoopsermia with decreased sperm count, motility and normal morphology in man after acquiring COVID-19. (C) Grade-1 quality embryo of couple showing eight cells, with no fragmentation, before the man got infected with COVID-19. (D) Grade-2 quality embryo of couple showing severe fragmentation, after 1 month of man recovery from COVID-19. (E) Grade-2 quality embryo of couple showing severe fragmentation, after 4 months of man recovery from COVID-19.
After 2 days of embryo freezing, unfortunately, the man tested positive for coronavirus disease 2019 (COVID-19). He was experiencing mild symptoms and was prescribed antipyretics, multivitamins and antioxidants. Following continuous treatment, he had tested negative for COVID-19 in 2 weeks. His semen analysis revealed no evidence of virus in the semen. After his recovery, the couple was keen to re-start their in vitro fertilization procedure soon, because of their advancing age and diminished ovarian reserve of the woman.
One-month after his recovery from the infection, we started the second stimulation cycle. Three M-II oocytes were collected. To our surprise, the semen analysis of the man taken 43 days after his recovery showed severe ‘oligo-astheno-teratozoospermia’, with severe sperm DNA damage. There were no acrosomes with dummy and fragmented heads (Table 1, Figure 1B). On Day 3, three embryos of 7–8 cells having Grade-2 quality (poor quality) were formed. The embryos showed severe fragmentation with poor implantation potential (Figure 1D). They were also frozen. We then started antioxidant and multivitamin treatment of coenzyme Q10, lycopene, docosahexaenoic acid, folic acid, selenium and zinc to the man and continued it for 3 months.
Four months post-recovering from COVID-19, the third cycle was planned. Four M-II oocytes were collected. The semen analysis of the man taken 135 days after his recovery showed an improvement in sperm count and motility, but the morphology remains poor with typical characteristics of ‘teratozoospermia’. The sperm DNA also remains severely damaged (Table 1). On Day 3, only two embryos were formed with eight cells of Grade-2 quality (poor quality) showing severe fragmentation (Figure 1E). However, due to the severe fragmentation and poor quality, these embryos also cannot be transferred to the woman.
Discussion
To date, there is no convincing proof to validate the long-term impact of COVID-19 infection on important biomarkers of male fertility such as sperm count, motility, morphology and sperm DNA damage among patients recovered from this new infection.1 In the present case, we report for the first time that COVID-19 can lead to long-term detrimental effects on cardinal male fertility parameters, particularly the sperm DNA and morphology. Owing to the possible repercussions of angiotensin-II upregulation at different stages of spermatozoon’s journey consequent to the infection, the intrinsic apoptotic pathway might get stimulated resulting in sperm senescence and cell death. Further, its prolonged exposure may result in premature acrosomal exocytosis and reduction in sperm motility, respectively.2,3 Nevertheless, the presence of transmembrane protease, serine 2 (TMPRSS2) in the sperm plasma membrane creates additional evidence of spermatozoa, being a vulnerable target of this infection.4 In the present case, we saw even a mild infection can result in long-term alterations in sperm morphology and sperm DNA integrity of men and can take a much longer time (>4 months) than what has been previously thought (70–90 days) for returning to the basal state of sperm parameters5 or might ultimately induce male infertility. Additionally, delaying fertility treatments due to COVID-19 may result in failure to conceive as the age of the couple has been established as an important predictor of pregnancy potential in assisted reproductive technology outcomes. Further studies are needed to evaluate the post-COVID-19-associated complications on male fertility and revamp assisted reproductive technology procedures for patients who have recovered from this new infection.
Acknowledgments
We thank the patient for granting permission to publish this information. We also thank Mohammed Ahmedullah Qureshi, Embryologist, Angels Fertility Center, and Dr A. Srinivasa Rao, Principal, Bhaskar Pharmacy College for their constant support.
Funding
The authors received no specific funding for this work.
Conflict of interest. None declared.
References
- 1. Seymen CM. The other side of COVID-19 pandemic: Effects on male fertility. J Med Virol 2020; 93:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aitken RJ. COVID-19 and human spermatozoa-potential risks for infertility and sexual transmission? Andrology 2020; 9:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olaniyan OT, Dare A, Okotie GE, Adetunji CO, Ibitoye BO, Bamidele OJ, et al. Testis and blood-testis barrier in Covid-19 infestation: role of angiotensin-converting enzyme 2 in male infertility. J Basic Clin Physiol Pharmacol 2020; 31. https://www.degruyter.com/view/journals/jbcpp/ahead-of-print/article-10.1515-jbcpp-2020-0156/article-10.1515-jbcpp-2020-0156.xml (31 December 2020, date last accessed). [DOI] [PubMed] [Google Scholar]
- 4. Ji H-L, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 2020; 100:1065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril 2007; 88:970.e1–e7. [DOI] [PubMed] [Google Scholar]
- 6. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Human Reproduction Update 2010; 16:231–45. 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]