Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is transmitted mainly via respiratory droplets. A key question in the coronavirus disease 2019 pandemic is whether SARS-CoV-2 could be transmitted via the airborne route as well. We report for the first time SARS-CoV-2 nosocomial infections despite using surgical masks and physical distancing. This report may provide possible evidence for airborne transmission of SARS-CoV-2.
Keywords: aerosol, airborne transmission, COVID-19, SARS-CoV-2, surgical masks
SARS-CoV-2 is transmitted mainly via respiratory droplets. We report for the first time SARS-CoV-2 nosocomial infections, despite using surgical masks and physical distancing. It may provide a possible evidence for airborne transmission of the virus.
Respiratory viruses spread among humans via contact, respiratory droplets, and aerosols. The transmission form of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is thought to be mainly via respiratory droplets from infected individuals [1, 2]. Nevertheless, airborne transmission of SARS-CoV-2 can occur during aerosol-generating procedures (AGPs). There are several reports on coronavirus disease 2019 (COVID-19) outbreaks in indoor crowded places, suggesting a possibility of airborne transmission of the virus. However, to date, COVID-19 transmission via this route has not been confirmed [3].
Face masks and respirators are important in reducing transmission of respiratory viruses. Generally, face masks are recommended for preventing diseases transmitted via respiratory droplets (>5 µm), which are emitted mostly while coughing or sneezing and do not transmit over long distances. Respirators are recommended for preventing aerosol transmission (<5 µm), which can remain in the air for several hours and transmit infections over long distances [4, 5].
According to the World Health Organization (WHO) recommendations, health care workers (HCWs) should wear medical masks in addition to other personal protective equipment (PPE) while providing direct care to COVID-19 patients and use a respirator (N95, FFP2/3) during AGPs [6].
In this report, we describe a single-source outbreak of COVID-19 from an asymptomatic patient to 9 HCWs and room contacts in a general pediatric ward, despite meeting the current guidelines for PPE and wearing surgical masks.
METHODS
Schneider Children’s Medical Center (SCMC) is a tertiary, university-affiliated, pediatric medical center in Israel.
Per the Centers for Disease Control and Prevention (CDC) guidelines, soon after the beginning of COVID-19 pandemic, HCWs in the hospital were instructed to wear masks (standard 3-layer surgical masks, approved by the Israeli Ministry of Health) at all times and to keep physical distancing from other HCWs, patients, and families when medically possible [7].
According to the local hospital’s policy, all children admitted to our hospital are screened for SARS-CoV-2. We use the Allplex 2019-nCoV assay kit, with a detection limit of 100 RNA copies/reaction. Droplet and contact precautions are taken when COVID-19 screening results are pending. COVID-19-negative patients are managed with standard precautions for PPE and surgical masks. COVID-19-positive patients or parents are hospitalized in separate rooms and managed with airborne precautions. Every new diagnosis of COVID-19 in patients or staff is followed by thorough epidemiological investigation regarding possible sources and contacts.
Patient Consent Statement
This study does not include factors necessitating patient consent. The work has been approved by the local institutional review board committee of Rabin and Schneider medical centers.
CASES DESCRIPTION
A 3-year-old boy was admitted to a general pediatric ward in SCMC for steroid treatment due to electrical status epilepticus in sleep. He was screened for COVID-19, and a negative result was given (d-4). The hospitalization was uneventful, and he was discharged on d0 as planned. On d4, 2 days after having symptoms compatible with COVID-19, his mother received a positive result. In the next few days, 9 patients were identified as COVID-19 positive; 6 of them were HCWs (4 physicians, 1 nurse, and 1 dietician). All participated in the same medical round, 3 of them without any direct contact with the child. All HCWs reported wearing PPE as guided, including surgical masks. No close contacts or family members of the HCWs were diagnosed with COVID-19. The child’s mother wore a surgical mask constantly as well. The 3 other infected patients were 2 children and 1 mother staying in the same room. On d6 after discharge, that child was also positive for COVID-19.
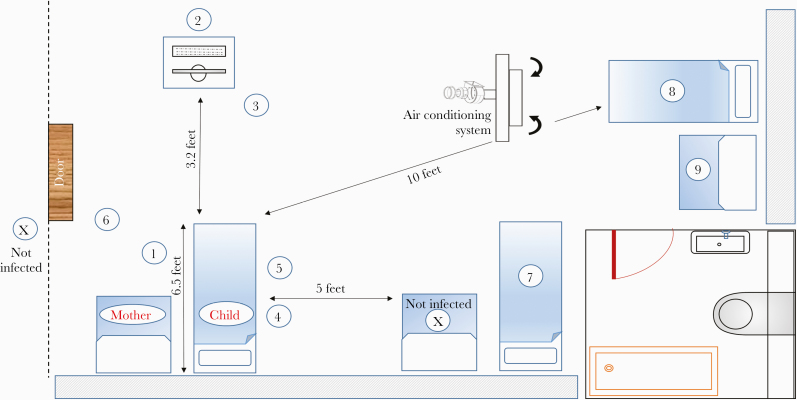
Figure 1 illustrates the room, the distances between index patients, and adds details about the ventilation spaces, airflow, air conditioning, and rate of air exchange.
Figure 1.
Room illustration. Index cases 1–9 are drawn according to their location in the room. The distances and airflow directions are detailed above. There is a special air conditioning system that diverts air only outside the room. The ventilation characteristics are 3–4 air changes per hour. The average temperature in the room is 23–24°C (73–75°F), and the humidity is 40%–55%.
Enclosed is a brief review of all contacts infected:
Cases 1–3—Morning Rounds
Three pediatricians (1 interim) were found to be positive for COVID-19 on d5 and d6. Two were symptomatic, and 1 was diagnosed due to general screening of the ward’s staff. Epidemiologic investigation revealed that the only known exposure of each of them was to the family mentioned above, during morning rounds (d0). Two of them were at a distance of about 6 feet (~2 meters) and did not have direct or indirect contact with the child or his mother. The third pediatrician examined the child, without any mucosal exposure or other AGP. All reported wearing surgical masks continuously. The whole meeting with the family lasted less than 10 minutes.
Cases 4–5—Obtaining Blood Samples
Another pediatrician and a nurse were found to be positive for COVID-19 on d6 and d7, after having mild COVID-19 symptoms. Similarly, their only known exposure was to the same family, when obtaining blood samples from the child (d0). The mother stayed in the room during the whole procedure, which lasted ~10 minutes. The HCWs reported using PPE including wearing surgical masks as guided.
Case 6—Dietician Counseling
The child had a low-carbohydrate diet due to his medical condition. A dietician consulted on the case twice (d-1 and d0), each time for ~15 minutes. Physical distancing was observed, and both the mother and the dietician reported wearing surgical masks. On d5, the dietician became symptomatic and tested positive for COVID-19.
Cases 7–9—Other Inpatients Infected
We mapped additional inpatients who were hospitalized in the same room and dates of the index patient. Out of 4 room contacts, 3 (2 children and 1 mother) tested positive for the virus. They were all at a distance of more than 6 feet apart from each other and reported no direct contact with the child or his mother. However, in the hospital inpatient setting, a smaller distance or a direct contact cannot be completely ruled out.
As a part of the outbreak investigation, SARS-CoV-2 tests were performed for the entire ward’s staff, patients, and escorts. No additional COVID-19 infections were documented.
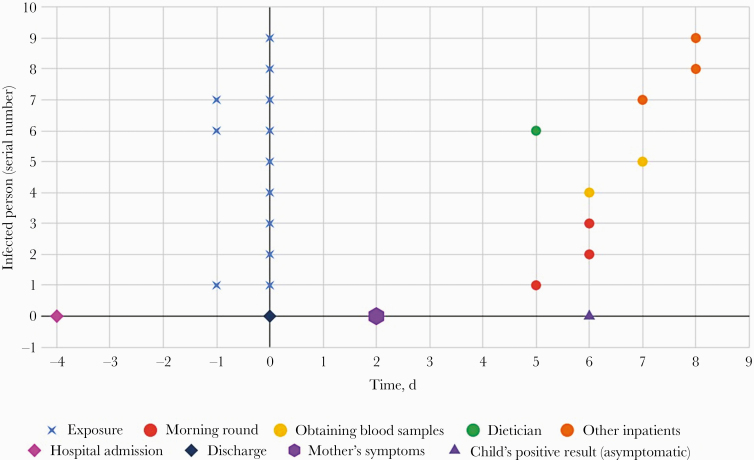
Figure 2 is a time graph showing the admission time, index exposures, and time frame of positive results.
Figure 2.
A time graph of admission time, indexes exposures, and positive results. Each line represents an index case. The blue X represents the exposure time. The circle represents the time of coronavirus disease 2019 diagnosis (for symptomatic patients, the positive result was received on the same day the symptoms began).
DISCUSSION
In this report, we described a superspreading event of 9 COVID-19 infections from 1 family despite using PPE and wearing surgical masks. Overall, 6 HCWs were infected, of whom at least 3 did not have direct contact with the child or his mother. None of the other 3 HCWs performed any AGPs or other procedures involving mucosal membranes. Also, they did not have any direct contact with the child’s mother. During morning rounds, the child was asymptomatic and did not cough, sneeze, cry, or even talk. The other infected inpatients were at a distance of more than 6 feet away from the asymptomatic COVID-19 patient. No other patients, escorts, or HCWs were infected.
To the best of our knowledge, this is the first description of nosocomial infection outbreak with SARS-CoV-2 despite using surgical masks.
Few studies have described the possibility of airborne transmission of SARS-CoV-2, but the current guidelines focus mainly on droplet precautions [8]. Apparently, the distinction between airborne and respiratory droplet transmission is not dichotomous. There are pathogens that spread mostly via respiratory droplets but can spread via the airborne route as well [4, 9]. Aerosol transmission is plausible when (1) virus-containing aerosols are generated from infected individuals, (2) the virus remains infective in the aerosols, and (3) the target tissues are accessible to the aerosol with enough load [10]. It seems like all of the above are possible for the current COVID-19 pandemic. Understanding the routes of SARS-CoV-2 transmission may prove a crucial breakthrough in controlling this pandemic.
We assume that the facts that the infected individuals wore surgical masks and that most of them were at a distance of 6 feet or more without any direct contact with the COVID-19 patient cannot be explained only by droplets or contact transmission of the virus. It may imply airborne transmission of SARS-CoV-2 and strengthen the assumption of infection via aerosol particles in superspreading events.
Another possibility for transmission is via fomites. It seems like the virus could survive on surfaces, despite the demonstrated rapid infectivity decline from clothes, paper, and cotton [11]. Nevertheless, fomite transmission could not explain the infections of indexes 2, 3, and 6 (2 pediatricians and a dietician, respectively), where there was neither physical contact nor shared equipment.
As some of this report’s authors were taking part in medical rounds and got infected, we can declare that there was full compliance with PPE guidelines.
COVID-19 has a low incidence in children (around 1%–2% of cases). The role of children in transmission of SARS-CoV-2 requires further investigation. Yet, few studies have described lower transmission rates in children compared with adults. As described in a meta-analysis from Australia, children are unlikely to be to primary sources (index cases) of documented household clusters [11, 12].
Therefore, the probable suspected source of the outbreak is the mother. The child seems less likely to be the source due to his age, the negative COVID-19 test on admission, the time frame of becoming positive, and his asymptomatic course of illness. We suggest phonation (by the mother) was the trigger for aerosol formation in this case, as has been described before in other superspreading events, such as in a call center in Korea, at choir practice in the United States, and at a restaurant in China [13].
This report is limited by its reliance on retrospective evidence of those involved, although all individuals declared adhering PPE guidelines in different individual epidemiological investigations. Furthermore, the COVID-19 pandemic is widely spreading, so it is possible that some of the cases were infected elsewhere. However, the fact that all cases occurred in 1 ward at the same time and the negative SARS-CoV-2 screening tests of the unexposed ward’s staff, patients, and escorts, including close contacts of the HCWs, are highly suggestive of an outbreak from 1 superspreader person.
In conclusion, we described COVID-19 infections in a pediatric ward despite adherence to guidelines for physical distancing and wearing surgical masks. Our reported cases may provide evidence for airborne transmission of SARS-CoV-2. More studies are needed to further our understanding of nosocomial transmission and the potential impact of airborne transmission on the COVID-19 pandemic.
Acknowledgments
We would like to acknowledge our dedicated department’s staff, especially those who became infected with SARS-CoV-2.
Financial support. No funding was secured for this study.
Potential conflict of interest. No author has a potential conflict of interest in this manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Somsen GA, van Rijn C, Kooij S, et al. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir Med 2020; 8:658–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020; 55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO). Transmission of SARS-CoV-2: implications for infection prevention precautions. 9 July 2020. Available at: https://apps.who.int/iris/rest/bitstreams/1286634/retrieve. Accessed 13 October 2020.
- 4. MacIntyre CR, Chughtai AA. Facemasks for the prevention of infection in healthcare and community settings. BMJ 2015; 350:h694. [DOI] [PubMed] [Google Scholar]
- 5. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee . 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35:S65–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Advice on the use of masks in the context of COVID-19 - interim guidance (5 June 2020). Available at: https://reliefweb.int/sites/reliefweb.int/files/resources/Advice%20on%20the%20use%20of%20masks%20in%20the%20context%20of%20COVID-19.pdf. Accessed 13 October 2020.
- 7. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. Update 15 July 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed 13 October 2020.
- 8. Morawska L, Milton DK. It is time to address airborne transmission of COVID-19. Clin Infect Dis 2020; 71:2311–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brosseau LM, Jones R. COMMENTARY: health workers need optimal respiratory protection for Ebola. Center for Infectious Disease Research and Policy (CIDRAP), 2014. Available at: www.cidrap.umn.edu/news-perspective/2014/09/commentary-health-workers-need-optimal-respiratory-protection-ebola. Accessed 13 October 2020.
- 10. Tang S, Mao Y, Jones RM, et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ Int 2020; 144:106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhillon P, Breuer M, Hirst N. COVID-19 breakthroughs: separating fact from fiction. FEBS J 2020; 287:3612–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Y, Bloxham CJ, Hulme KD, et al. A meta-analysis on the role of children in SARS-CoV-2 in household transmission clusters [published online ahead of print December 6, 2020]. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson N, Corbett S, Tovey E. Airborne transmission of COVID-19. Guidelines and governments must acknowledge the evidence and take steps to protect the public. BMJ 2020; 370:m3206. [DOI] [PubMed] [Google Scholar]