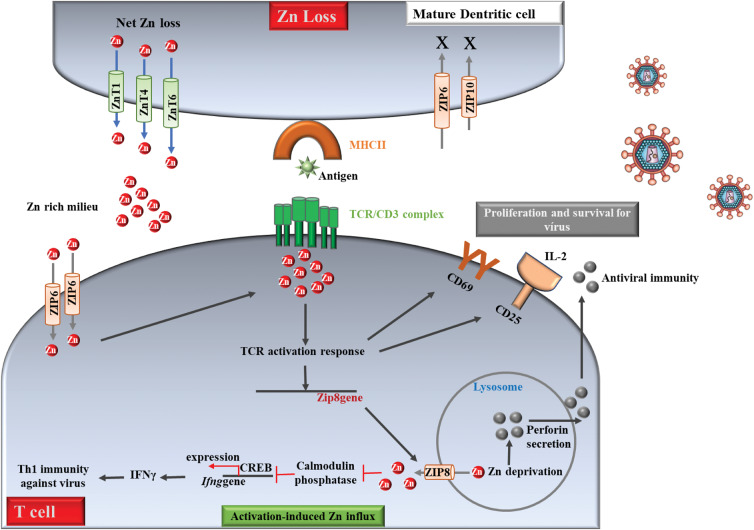
Fig. 2.
Zinc regulation in T cell mediated antiviral defences. Activation of T cells is essential for cytokine production and clearance of viruses. Zn is exported from dentritic cells (DCs) to drive Zn loss. This process matures them and increases surface MHCII. When T cells establish physical contact with an antigen-presenting MHCII, Zn is imported from the extracellular space via ZIP6. The Zn lost from DCs into the extracellular milieu can potentially be drawn into T cells. Imported Zn accumulates in the sub-synaptic region at the point of DC-T cell contact to drive the TCR activation response leading to an increase in CD69 and CD25. These molecules support survival and proliferation of T cells required to elicit T cell antiviral immunity. Activation enhances Zip8 expression, which may be negatively controlled by mi-RNAs. ZIP8 imports lysosomal Zn into the cytosol. Cytosolic Zn inhibits Ca2P/calmodulin phosphatase to sustain CREB mediated IFNγ production to bolster Th1 immunity against microbes. Lysosomal Zn deprivation caused by ZIP8 induces perforin secretion from lysosomes that is essential in CD8 T cell responses to viral infection. Red dots, Zn ions; solid arrows and lines, established links; dotted arrows and lines, predicted links.