Global spread of coronavirus disease 2019 (COVID-19) has created an unprecedented infectious disease crisis worldwide.1 To date, non-pharmaceutical interventions implemented in response to the SARS-CoV-2 epidemic are the main option to curb the spread of the virus. After new evidence appeared about the aerosol transmission of SARS-CoV-22 and that face mask use would be associated with a mean risk reduction of virus transmission of 43% in the non-healthcare setting,3 the World Health Organization has recommended its use. In France, despite evidence of a risk of rebound of the epidemic by the end of the summer if most individuals did not use face masks,4 self-reported data from a nationwide survey5 of 2000 individuals representative of the French general adult population (based on age, sex, socio-economic status and place of residence) indicate that about 40% adhered to this measure after the end of the lockdown (11 May), 52% in June, 61% in July and 69% in late August. This increase may be explained by the French government’s decision to make face mask use mandatory in indoor venues and at the workplace, following the observed increase in the number of COVID-19 cases from July in France. However, low adherence to face mask use of the population may have led the virus to widely circulate.1
In this report, we sought to examine whether an early implementation of face mask use with an achievable adherence of 80% in the population would have reduced cumulative COVID-19 incidence, mortality, and the number of hospital and intensive care unit (ICU) admissions, as compared to the observed epidemiological situation. We considered an adherence rate of 80% as achievable because this rate was achieved at the time of the second lockdown in late October.5 This knowledge is crucial when the hypothesis of repeated rebounds of the epidemic until its end, and particularly when protection measures are relaxed, cannot be ruledout.
To this end, we report results from a stochastic agent-based microsimulation model of the epidemic in France, which showed adequate calibration and validation as fully described elsewhere.4 Briefly, this model includes (i) a realistic synthetic population generated with demographic characteristics, medical comorbidities and household structure representative of the French general population; (ii) a social contact network among the individuals, each with a geolocalized activity sequence over the day, taking into account co-location probability and duration, including contacts with family members, extended family members or friends (at home or at bars and restaurants), contacts at school or at work, and during public transport or grocery shopping or cultural activities and (iii) a disease model, which translates the edge weights in the social contact network into infection probability of the edge over the day. The model included 194 parameters related to French population characteristics (n = 140), social contacts (n = 33) and SARS-CoV-2 characteristics (n = 21). Parameter values on population characteristics were based on data from the French National Statistical Institute and Santé Publique France. Parameters related to social contacts were based on prior studies (n = 11) or assumptions when no data were available (n = 22). Finally, parameters on disease characteristics were based on data from the Direction de la Recherche, des Études, de l’Évaluation et des Statistiques, Institut Pasteur and London Imperial College, except for two unknown key parameters of the epidemic: contamination risk and proportion of undiagnosed COVID-19 cases, which were simultaneously estimated through model calibration. The model parameters are summarized in Supplementary Table 1 and fully described elsewhere.4 Source code for the model has been deposited in a recognized public source code repository (GitHub, https://github.com/henrileleu/covid19).
We compared the scenario of the observed epidemiological situation including data from the French general population survey on face mask use4 to a hypothetical one in which 80% of individuals would have been using face masks from the date of the end of the lockdown, i.e. 11 May. In both scenarios, we assumed that adherence to face mask use would be 80% from 1 September, the date when its use was mandatory in France and would remain at this level throughout the epidemic. This assumed rate, although slightly overestimated, was validated by data from the French general population survey on face mask use,5 which reported a 72% rate of mask adherence in mid-September and an 80% rate achieved in late October. No other changes were made to the model parameters previously published,4 except the reduction of COVID-19 mortality in ICUs by 50% since June, following the improvement in care and the use of dexamethasone in ICUs.6,7
The results are summarized in Figure 1. We projected that between 1 July and 23 September, early use of face masks with an 80% adherence rate would have reduced the cumulative incidence by 678 000 (95% prediction interval, 662 000–694 000), the number of hospital admissions by 9000 (95% prediction interval, 8900–9200], the number of ICU admissions by 1330 (95% prediction interval, 1300–1360) and the number of deaths by 450 (95% prediction interval, 436–464), compared to the observed epidemiological situation in France. Based on our model, extrapolations after 23 September suggest that these differences would further increase over time despite a high (i.e. 80%) adherence to face mask use from 1 September in both scenarios, with a difference of 2 477 000 (95% prediction interval, 2 452 000–2 503 000) COVID-19 cases, 40 400 (95% prediction interval, 40 100–40 700) hospital admissions, 5780 (95% prediction interval, 5720–5830) ICU admissions, and 2555 (95% prediction interval, 2526–2584) deaths by 1 February. Our findings suggest that high adherence to face mask use is a highly effective measure to curb the viral spread and mitigate its consequences, particularly when this measure is adopted early by most people. These findings may be relevant in a context where the perspective of COVD-19 vaccination programmes might lead to reduced adherence to protection measures.
Figure 1.
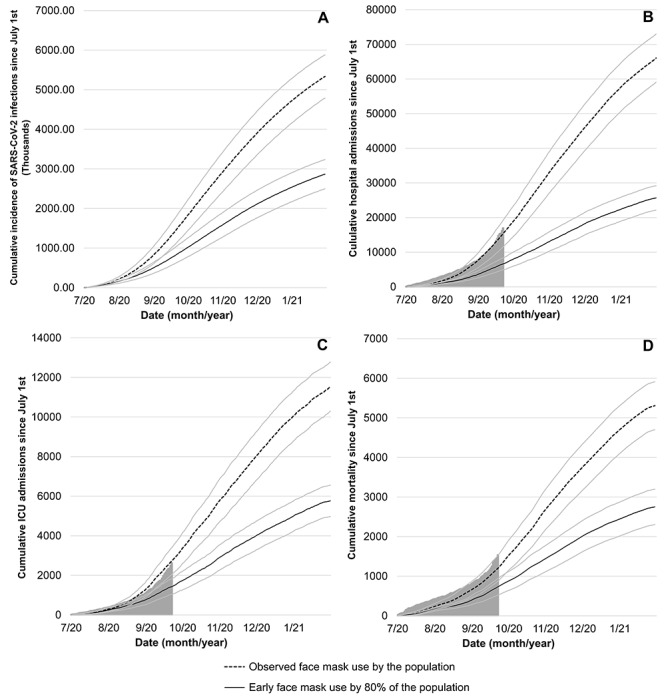
Model predicted cumulative incidence of SARS-COV-2 infection (A), COVID-19-related hospital admissions (B), ICU admissions (C) and deaths (D) in France from 1 July 2020 to 1 February 2021. The grey area represents the actual observed data for France. The solid grey lines represent the uncertainty range (95% prediction range) stemming from the uncertainty in the parameter values. (A) no data are available for new infections, only for diagnosed cases
Highlights
Results from a stochastic agent-based microsimulation model suggest that early implementation of face mask use with an achievable adherence of 80% in the population would have reduced cumulative COVID-19 incidence, mortality, and hospital-bed occupancy, as compared to the observed epidemiological situation in France, supporting the importance of this protection measure.
Author contribution
N.H. drafted the manuscript, critically revised the model and contributed to the analyses; H.L. designed the model, performed the analyses and drafted the manuscript; M.B. critically revised the model and manuscript for scientific content; M.S.R. and F.L. critically revised the manuscript for scientific content. All authors contributed to and have approved the final manuscript.
Supplementary Material
Funding
This work did not receive any external funding. The corresponding author (N.H.) and the last author (H.L.) had full access to all the data in the study and have final responsibility for the decision to submit for publication.
Conflict of interest: None declared.
References
- 1. Hoertel N, Blachier M, Blanco C et al. Facing the COVID-19 epidemic in NYC: a stochastic agent-based model of various intervention strategies. Med Rxiv 2020. doi: 10.1101/2020.04.23.20076885. [DOI] [Google Scholar]
- 2. Morawska L, Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int 2020; 139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chu DK, Akl EA, Duda S et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet 2020; 395:1973–87. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoertel N, Blachier M, Blanco C et al. A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nat Med 2020; 26:1417–21. doi: 10.1038/s41591-020-1001-6. [DOI] [PubMed] [Google Scholar]
- 5. Santé Publique France . Covid-19: une enquête pour suivre l’évolution des comportements et de la santé mentale pendant l’épidémie. Covid-19 Une Enq Pour Suivre L’évolution Comport Santé Ment Pendant Lépidémie. 2020.
- 6. Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA 2020; 324:1292–5. [DOI] [PubMed] [Google Scholar]
- 7. Hoertel N, Sánchez M, Vernet R et al. Dexamethasone use and mortality in hospitalized patients with coronavirus disease 2019: a Multicenter retrospective observational study. MedRxiv 2020. doi: 10.1101/2020.10.23.20218172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


