Abstract
Objectives
Viral outbreaks are a frequent concern for humans. A great variety of drugs has been used to treat viral diseases, which are not always safe and effective and may induce adverse effects, indicating the need for new antiviral drugs extracted from natural sources. Propolis is a bee-made product exhibiting many biological properties. An overview of viruses, antiviral immunity, propolis safety and its immunomodulatory and antiviral action is reported, as well as perspectives for coronavirus disease 2019 (COVID-19) treatment. PubMed platform was used for data collection, searching for the keywords “propolis”, “virus”, “antiviral”, “antimicrobial” and “coronavirus”.
Key findings
Propolis is safe and exerts antiviral and immunomodulatory activity; however, clinical trials should investigate its effects on individuals with viral diseases, in combination or not with antiviral drugs or vaccines.
Summary
Regarding COVID-19, the effects of propolis should be investigated directly on the virus in vitro or on infected individuals alone or in combination with antiviral drugs, due to its immunomodulatory and anti-inflammatory action. Propolis administration simultaneously with vaccines should be analyzed, due to its adjuvant properties, to enhance the individuals’ immune response. The search for therapeutic targets may be useful to find out how propolis can help to control COVID-19.
Keywords: propolis, virus, antiviral action, antimicrobial action, coronavirus
Introduction
Viral outbreaks are a frequent concern for humans. Despite the most famous epidemics known to mankind, there have been outbreaks in the last decade of dengue virus (DENV) in Nepal and Hawaii,[1, 2] hepatitis viruses in India,[3] yellow fever virus (YFV) in Brazil,[4] norovirus (NV) in industrialized countries[5] and the pandemics of Influenza H1N1[6] ebola virus (EBOV)[7] and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[8]
A great variety of molecules and drugs have been used to treat viral diseases, such as interferon-α, ribavirin, cidofovir, acyclovir, ganciclovir and others that may control viral epidemics.[9, 10] However, these drugs are not always safe and effective and may induce adverse effects on humans such as kidney injury,[11] neurological damages[12] and others. Moreover, they may lead to antiviral drug resistance.[13] In addition, it has been reported that the misuse of antibiotics to treat viral respiratory diseases can lead to more problems, mainly bacterial drug-resistance.[14]
In this scenario, there is a demand for a broad spectrum of antiviral drugs[15] extracted from natural sources.[16] Indeed, the search for natural products with pharmacological properties has been reassessed by using herbal medicines,[17] some of them exhibiting antimicrobial activity and synergistic interaction with traditional drugs.[18]
Interest in bee products has gained traction in complementary medicine in the last decade.[19] Propolis is a resinous product made by bees from different parts of plants located around the hive. Propolis is highly utilized by the bees for protection of the hive preventing water infiltration, putrefaction of dead-intruders and maintaining local asepsis.[19, 20] Mankind also benefits from propolis, since it exhibits a variety of biological properties, such as antimicrobial, anti-inflammatory, immunomodulatory, antitumoral, antioxidant, among many others.[21] Regarding propolis antimicrobial action, it may exert antibacterial, antifungal and antiviral activity in isolation or in combination with pharmacological drugs.[22–25]
Propolis antiviral activity has been less documented compared to the number of articles dealing with its antibacterial action. Thus, this review aimed to collect/compile data about the antiviral properties of propolis and its mechanisms of action. This may be of interest for the emergence of new antiviral treatments, using propolis alone or in combination with antiviral drugs, depending on concentration and trying to reduce side effects. We also discussed propolis immunomodulatory action and perspectives regarding coronavirus disease 2019 (COVID-19) treatment.
To analyze the works dealing with propolis antiviral action up to now, PubMed platform (https://PubMed.ncbi.nlm.nih.gov/) was used for data collection, searching for the keywords “propolis”, “virus”, “antiviral”, “antimicrobial” and “coronavirus”. The articles were selected by title and content before data collection. We used only one database to avoid duplication of information. In addition, some platforms include abstracts of scientific events and we only included the published articles in an attempt to cite peer-reviewed papers available worldwide.
Viruses: General Characteristics
Viruses are an organized association of macromolecules that depend on a living cell to replicate. They are structurally simple and have no metabolic or motile activities. A genome (either DNA or RNA) and a shell of the protein named capsid compose an infectious virus particle, a virion. The enveloped viruses have an extra layer of protection: a lipid membrane. The function of this apparatus is to keep the genetic information safe until the virus reaches a susceptible cell to enter and exploit its physiological mechanisms of replication.[26] Using these structures, viruses can infect organisms of all domains of life.[27]
The capsid is a protein framework composed of multiple copies of identical subunits or even a few distinct proteins. The use of small equal subunits limits the acquisition of genetic information due to the small size of the viral nucleic acids. These proteins have the tendency to thrive in the most energetically favourable way to form the capsid, resulting in the most common designs found in nature, the helical tube and the icosahedral shell, each of these symmetrical structures having their specific physical properties.[28]
The lipid envelope present in some viruses is derived from membranes of the host cell, usually the plasma membrane. The acquisition of the envelope occurs by a budding process in which the membrane constricts towards the cytoplasm and wraps around the virus particle while it egresses from the cell.[29]
Viral cycle of infection and replication starts with the attachment of a viral particle to the cell surface. The presence of specific receptors in the cell surface that can bind to viral proteins makes the cell susceptible to infection.[26] After binding to receptors, viral entry proteins or the host cell receptors undergo conformational changes. Then, viruses can follow two main different strategies: non-enveloped viruses enter the cell by penetration, in which the cell membrane is partially disrupted and either the whole nucleocapsid enters the cell or the viral genome is released through the formed pore, whereas enveloped viruses fuse with the cell membrane.[30] After entry, the viral nucleic acid is transported to the host sites of replication.
Baltimore[31] proposed a classification of 6 types based on the viral nucleic acid and replication strategy, and a 7th type was added later. First, class I includes viruses with the double-stranded DNA, and the genome of these viruses is transported to the cell nucleus to be transcript in mRNA. Class II consists of viruses with a single-stranded DNA, and a complementary strand is made before replication. Viruses with double-stranded RNA are grouped in Class III: these viruses carry an RNA-dependent RNA polymerase that transcribes mRNA from the viral RNA. Class IV is composed of viruses with single-stranded RNA with a positive polarity that have identical base sequences to the mRNA. To replicate its genome, it must be directly translated and a complementary strand must be produced so that it can be transcripted in a new viral genome with a viral RNA polymerase. Class V: the RNA viruses are single-stranded with negative polarity, so they need to carry an RNA-dependent RNA polymerase to produce a complementary positive (mRNA) strand to be translated and transcripted. Retroviruses, single-stranded RNA viruses with an intermediate DNA compose the Class VI. These viruses carry an RNA-dependent DNA polymerase, a reverse transcriptase. Finally, class VII comprises double-stranded DNA viruses with an RNA intermediate in their replication cycle. The production of mRNA is similar to viruses from Class I.
After replication and protein synthesis, the final step in the viral infection cycle is the assembly and egress phase, and the synthesized viral proteins and nucleic acids are transported to specific sites for assembly. Lastly, the virion particles leave the cell by budding, controlled exocytosis or lysis of the cell host.[32] Due to its infectious and harmful cycle of replication, viruses cause many diseases in humans, activating hosts’ immune response.
An Overview of the Antiviral Immune Response
Innate immunity is the first defence against viruses and other infectious agents, playing a crucial role in their early recognition and triggering a pro-inflammatory condition. It is initiated when cellular proteins called pattern-recognition receptors (PRRs) recognize some microbial conserved structures or damaged cell products, known as pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), respectively.[33, 34] PAMPs may be lipopolysaccharide (LPS), peptidoglycan (PGN), mannose-binding lectin (MBL), DNA, RNA, lipoproteins and other molecules found in microorganisms. DAMPs include heat shock proteins (HSPs), mitochondrial DNA, ATP, high mobility group box 1 protein (HMGB1), among others. PRRs with potential antiviral response include Toll-like receptors (TLRs), C-type lectin receptors (CLRs), retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), NOD-like receptors (NLRs) and DNA sensors.[34–37]
After extracellular or intracellular PAMP-PRR binding, many signalling pathways can be activated involving adaptor molecules, kinases and transcription factors such as interferon regulatory factor 3 (IRF3), nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs) and others, which in turn induce the expression of proinflammatory cytokines, adhesion molecules, chemokines, immunoreceptors and interferons (IFNs) that may help the antiviral immunity. Besides phagocytosis of microbes, the release of inflammatory mediators, complement- or natural killer (NK) cell-mediated cellular lysis, as well as the synthesis of acute-phase proteins and other molecules will together arrange both the early host response to viral infection and the interface with the adaptive immunity.[33]
The main effector cells involved in innate immunity are monocytes/macrophages, neutrophils, dendritic cells (DCs) and NK cells. Macrophages and DCs are professional antigen-presenting cells (APCs) and their number and peripheral lifespan increase during infection, in an attempt to recruit other immune cells to prevent virus dissemination.[38–40]
Circulating monocytes and macrophages play an important role in the protection against viral infection, and the activation of these cells may occur through TLR-dependent and independent pathways.[33, 41] However, some viruses such as human immunodeficiency virus (HIV), cytomegalovirus (CMV) and human herpesvirus 8 (HHV-8) may evade and utilize these cells for dissemination and long-term persistence within tissues for virus replication.[42]
Neutrophils are clearly important in the initial antiviral defence due to their ability of phagocytosis and to destroy microorganisms. They secret inflammatory mediators and produce neutrophil extracellular traps (NET).[40] However, neutrophils can also exert a “Trojan horse” strategy, such as in the West Nile virus (WNV) infection, with not only neutrophil sequestration but also with a high viral replication within the cells, contributing to virus spread in the host.[40, 43]
The protective role of NK cells against virus-infected cells has been well documented. These cells exhibit several functions when stimulatory signals activate some receptors, leading to cytolysis of target cells (e.g. virus-infected cells) through its cytotoxic granules containing perforin and granzymes and mediation of antibody-dependent cellular cytotoxicity (ADCC). For example, early and robust activation of NK cells has been reported in arbovirus (WNV, DENV and Zika [ZIKV] virus) infection. Peripheral and tissue-resident NK cells can directly fight against infected cells (especially DCs) by triggering cytotoxicity or intense IFN production, contributing to viremia control.[44] NK cells may contribute to a decreased viremia in HIV patients treated early with antiretroviral therapy.[45] However, NK cells may be also susceptible to viral infections: herpes simplex virus (HSV) and Epstein-Barr virus (EBV) have shown to infect these cells.[46] Interestingly, an elevated number of NK cells was reported in the liver biopsies of patients who died from YFV.[44]
It is worth noting that deregulation or imbalance of the innate immune response may also occur in the pathophysiology of viral diseases. Since innate immunity exhibits lethal weapons to destroy invading pathogens, it may also damage the host tissue due to an overproduction of inflammatory mediators such as cytokines, NETs and reactive oxygen species (ROS).[40, 47]
Viral infections may cause the host immunopathological consequences. In hantavirus pathogenesis, DCs produce several cytokines that may lead to an increased activation of immune cells (NK, T and B cells), that contributes to vascular damage by attacking endothelial cells or by enhancing vascular permeability.[48] The severity of diseases caused by many types of influenza A virus (IAV), including pulmonary dysfunction and fatal cases, were associated with intense inflammatory status arising from the exacerbated action of neutrophils and DCs, the large number of infiltrating monocytes/macrophages and the cytokine milieu [49]. Likewise, in human papillomavirus (HPV)-mediated tumorigenesis, the cervix chronic inflammation can be related to the presence of growth factors, cyclooxygenase (COX), ROS, Interleukin-1 (IL-1β) and tumour necrosis factor (TNF-α).[50] In the “cytokine storm” caused by several viruses including SARS-CoV-2, an elevated cytokine production may occur and cause multi-organ damage, worsening the patient’s clinical condition.[47]
After pathogen phagocytosis and digestion by APCs, different fragments of the antigen are assembled and transported by the major histocompatibility complex class I and II (MHC-I and II) to the cell surface, to trigger the adaptive immune response.[38–40] The peptides bound to MHC are recognized by the T cell receptor (TCR) present on the surface of naïve T cells, leading to their activation and subsequently to the differentiation into specific T cell subsets, depending on the stimulus and the immunological environment.
CD4+ T cells play a pivotal role in orchestrating an effector immune response against a wide variety of viruses, due to their capacity to stimulate antibody production by B cells, enhance CD8+ T cells response and generate an immunologic memory. CD4+ T cells may also differentiate into distinct T helper (Th) cell subsets such as Th1, Th2, Th17 and T regulatory cells (Treg), with diverse cytokine profiles and functions.[51] Respectively, Th1 and Th2 are responsible for cellular and humoral immunity, and their main transcription factors are T-box-containing protein expressed in T cells (T-bet) and GATA-binding protein-3 (GATA-3). Th17 cells play both protective (i.e. maintaining mucosal homeostasis) and pro-inflammatory action, after triggering receptor-related orphan receptor gt (RORγT) activation. Treg cells, characterized by the expression of the transcription factor forkhead protein p3 (FoxP3), may exert a dual role in viral infections: these cells may control an exaggerated and destructive inflammation; on the other hand, they may inhibit virus-specific T cell responses, facilitating viral persistence and disease progression.[51, 52]
Thus, a proper balance between Th17/Treg cells is essential for the successful control of virus diseases. Treg cells may protect patients with chronic hepatitis C virus (HCV) infection from pathologic disorders and viral-induced inflammation, including those resulting from Th17 antiviral activity, limiting the extent of fibrosis.[53] Treg/Th17 ratio may also be used as a potential marker for predicting liver cirrhosis in patients with hepatitis B virus (HBV).[54] The clinical severity of respiratory syncytial virus (RSV) infection can also be determined by the pathogenic effect of Treg/Th17 imbalance, since Treg cells are efficient in viral clearance by coordinating the recruitment of CD8+ cytotoxic T cells to the lungs, avoiding an excessive RSV-specific T cell (CD4+ and CD8+) response. In a severe RSV infection, IL-17 causes an exaggerated mucus production, increases neutrophil infiltration in the lungs and seems to diminish effector CD8+ T cell response by exerting a negative regulation of T-bet activation, diminishing viral clearance.[55] In HIV infection, Th17 cells promote gut mucosa recovery and prevent microbial translocation, decreasing immune hyperactivation. However, these cells may increase viral replication by producing inflammatory cytokines, promoting a more activated and unbalanced microenvironment. The effects of Treg cells can be beneficial or harmful depending on the balance between mitigating HIV-induced immune hyperactivation and mounting an immune response to HIV and opportunistic pathogens.[56]
After MHC-I/antigen recognition, CD8+ T cells become cytotoxic T lymphocytes (CTLs) to kill infected cells by apoptosis (with the secretion of granzyme B and perforin, or apoptosis mediated by FasL/Fas) and produce cytokines such as IFN-γ and TNF-α to amplify the immune response.[52, 57] However, during chronic viral infections, the functions of CD8+ T cells are normally reduced in comparison to their potent effector and memory functions exerted in acute infections, and this T cell hyporesponsiveness is known as CD8+ exhaustion.[52] This phenomenon was observed in the lymphocytic choriomeningitis virus (LCMV) infection after chronic antigen stimulation, providing a possible explanation for the “loss of immunity” after persistent infections.[58] However, a better prognosis of the diseases may be observed when a robust CD8+ T cell response occurs, as in the case of HIV, HCV and HBV-infected patients.[52] In IAV infection, specific-CD8+ T cells produce both anti-inflammatory IL-10 and antiviral IFN-γ to fine-tune the CTL activity to an effective viral clearance, but in restrained levels to avoid tissue immunopathology driven by an inflammatory response.[57]
In addition to the cellular immune response, humoral immunity acts in controlling viral infection. After naïve B cells stimulation directly by antigens through the B cell receptors (BCR), these cells may act as APCs, processing and presenting peptides with MHC-II molecules, with further proliferation and differentiation into both memory B cells and antibody-secreting plasma cells. These cells produce antibodies or immunoglobulins (Ig) and mammals present five classes of antibodies based on their physicochemical, structural and immunological properties: IgA, IgD, IgE, IgG and IgM.[59] Virus-specific IgM is observed in a primary viral infection followed by IgG production and a more durable protective response.
Antibodies circulate freely and prevent the entry of the virus into the target cells to limit inflammation and tissue destruction, while later responses can help to eliminate the virus and protect the host from reinfections.[60] Neutralization of viruses means that viral infectivity was impaired due to antibody or complement components binding to the viral surface. The main mechanisms for complement-mediated neutralization of viral infectivity include virolysis, aggregation/opsonization and phagocytosis, via complement receptors (CRs) present on cells.[61] Such mechanisms were observed in the neutralization of human T-cell lymphotropic virus, (HTLV) and in SARS coronavirus,[62, 63] and the deposition of C3b was necessary for DENV neutralization.[64]
When DENV serotype-specific neutralizing antibodies are present, ADCC is important in secondary infections through another serotype, since cross-reactivity is commonly observed because the four DENV serotypes present 70% homology in their sequences, and this is an underlying mechanism of elevated immune-pathology associated with DENV severe forms.[44] In COVID-19, neutralizing antibodies block the viral infection because SARS-CoV-2 is recognized and phagocyted by alveolar macrophages, leading to viral clearance, minimal lung damage and patient recovery. In contrast, non-neutralizing antibodies may sometimes prove to be beneficial to the virus, by facilitating SARS-CoV-2 entry and replication in the target cell, through the phenomenon known as antibody-dependent enhancement (ADE), further exacerbating organ damage.[47] Additionally, in the case of natural HIV control, individuals known as “elite controls” exhibit an increased frequency of Gag-specific antibodies and a polyfunctional antibody effector response, although they maintain a large HIV-specific memory B cell pool, an important factor in the effectiveness of humoral immunity and long-term protection.[65]
Viruses are known to develop multiple strategies to escape from the immune system.[61] Several mechanisms have been described for evasion or downregulation of innate and adaptive immunity, including the modulation of PRR activation and the inhibition of antiviral molecules or transcription of genes related to the immune response.[39, 50] Besides, inhibition of CTL response associated with impairment of APC trafficking and maturation may occur, as well as deregulation of different T cell subpopulations.[66] The dysfunctional T and B response against non-mutated epitopes[67] and the ability to adapt to the host through mutations are related to immune evasion.
Viral resistance can be defined as a change in the viral genetic material and it becomes tolerant/resistant to the drug normally used for treatment. Antiviral substances act in different phases of viral replication aiming for inhibition. The chemotherapeutic agents can be divided into three categories: (1) virucides: which cause a direct viral inactivation, such as detergents, organic solvents and ultraviolet light; (2) antivirals: drugs that inhibit viral replication at specific levels and prevent its spread, but with restricted effectiveness against latent viruses; and (3) immunomodulators: agents that may enhance the host response to infections, including antibody secretion, interferon production or by intensifying cell-mediated immunity.[68]
The “direct virus-targeting antivirals” inhibit attachment, entry, protease, polymerase, integrase, methyltransferase and helicase. Some examples of “indirect virus-targeting antivirals” are blockers of replication and transcription (RTC) and ribonucleoprotein (RNP) complex. Regarding “host-targeting antivirals”, cyclophilin inhibitors, IFN and HIV-1 co-receptor antagonists are the most commonly used.[69] Non-pharmacological strategies can also be found, such as neutralizing antibodies and gene and cell therapy.[70] It is worth mentioning that antivirals should be chemically and metabolically stable, easily absorbed and should not be toxic, allergenic or mutagenic.[68]
Thus, taking into account the existence of antiviral immunity and, on the other hand, the possibility of viral resistance, it is imperative to discover new antiviral drugs. In the next chapters, the immunomodulatory action of propolis is discussed as well as its antiviral effectiveness.
Propolis: Safety/Side Effects and Immunomodulatory Action
Many medicines currently marketed come from natural products or their derivatives, representing more than half of the Food and Drug Administration (FDA)‐approved drugs and are a rich source for new drug discovery.[71] Several substances have been already described for their antiviral action, including propolis.[72]
Propolis is produced by bees from different parts of plants, such as leaves, buds, gums, flowers, bark and resinous exudates, to which bees add salivary enzymes, wax and pollen.[20, 73, 74] Its colour varies according to the botanical origin and can be green, red, brown and others. Characteristically, it has an aromatic and enjoyable smell. Regarding its etymology, the word propolis is originated from Greek and means “pro” = defence, and “polis” = city. Bees use propolis to defend the hive, to seal holes and cracks, to embalm dead invaders preventing their decomposition and diseases and to regulate the internal temperature.[75–77]
Propolis is a lipophilic, resinous and balsamic product, forming a complex matrix with poor bioavailability and absorption. In general, propolis consists of resins and balsams, beeswax, essential and aromatic oils, pollen and other substances, including organic debris. Its chemical composition is extremely complex and varies according to the geographic location and the local flora where it was produced. Several hundreds of components have been identified in propolis, and its biological activity is mainly associated with the presence of phenolic compounds, like flavonoids (e.g. quercetin, galangin, chrysin) and derivatives of hydroxycinnamic acids (e.g. caffeic acid, cinnamic acid, p-coumaric acid). Propolis also presents many other constituents, such as aromatic aldehydes, diterpenes, sesquiterpenes, esters, lignans, alcohols, amino acids, fatty acids, vitamins and minerals.[78–81]
In general, Asian and European propolis contain simple phenolic acids, in contrast, samples from tropical regions have more lignans. Caffeic acid phenethyl ester (CAPE) is the major component in samples from America, Asia and Europe. In the temperate zone of the Northern Hemisphere, bees collect propolis for only about 4 months, from late spring to early autumn, whereas in tropical countries, such as Brazil, propolis may be produced throughout the year. Brazilian green propolis is recognized for 3,5-diprenyl-4-hydroxycinnamic acid, artepillin C, prenylated cinnamic acids and caffeic acid derivatives. Seasonal variation in propolis chemical composition is not significant and is predominantly quantitative.[82, 83] Constituents of propolis can be extracted using different solvents such as water, ethanol, ether, acetone, toluene and trichloroethylene, and others.[84] Different extraction methods can result in different chemical profiles.[21] Freezing propolis does not lead to qualitative changes in its composition [85]. Since samples from different phytogeographic conditions may show different compositions, it is important to link the effects of a given propolis sample to its botanical sources and chemical components, to standardize research on propolis.[21]
Propolis has numerous biological activities and the most investigated ones include antimicrobial, antitumor, antioxidant, anti-inflammatory and immunomodulatory properties.[21] Nonetheless, different populations have used propolis in folk medicine for different purposes.[86] Due to its anti-putrefactive properties, Egyptians used propolis for mummification. Greeks and Romans used propolis to treat wounds, because of its antiseptic and cicatrizing action. Persians described the use of propolis for treating eczema, myalgia and rheumatism. The Incas used propolis as an antipyretic agent.[75] In the beginning of the 20th century, the first scientific reports on the biological activities and chemical composition of propolis emerged.[86, 87] Propolis is one of the few natural products that has maintained its popularity over time.[75] It is still currently in use due to its numerous biological and pharmacological properties and has stood out for its application in the pharmaceutical industry.[21]
Safety/side effects
In vitro assays have demonstrated that propolis and its dilutant display no cytotoxic effect on immune cells such as murine macrophages, human monocytes and dendritic cells (DCs), using concentrations up to 100 μg/ml.[88–93] A lung fibroblast cell line (V79) treated in vitro with propolis (6.25–25 μg/ml) and erythrocytes obtained from mice treated with propolis (7, 14 and 21 mg/kg) showed no genotoxic effects as observed in the micronucleus assays, demonstrating its safety.[94]
Propolis seems to be safe and non-toxic, with no adverse effects after its administration to humans or animals used for experimentation.[94, 95] Kumari et al.[96] described that propolis reduces DNA damage in mice treated with mitomycin C. Propolis is also able to reduce the toxic effects of several other substances, such as doxorubicin,[97] methotrexate,[98, 99] acetaminophen,[100] cerulean,[101] thioacetamide,[102] carbon tetrachloride,[103] methoxychlor,[104] mercury chloride,[105] diatrizoate,[106] ethylene glycol,[107] gentamicin,[108] paracetamol,[109] streptozotocin,[110] aluminum silicate,[111] cisplatin,[112] among others.
Fikri et al.[113] observed that a low dose of propolis (380 mg/kg) during pregnancy of mice did not alter parameters in fetal development as opposed to 1400 mg/kg, concluding that the lowest concentration is a safe dose to be used during pregnancy. In general, no behavioural and clinical toxicity has been seen in rats treated orally with propolis.[114] However, some side effects were observed in rats, such as sleep and shivering using 300 mg/kg/1 day, decreased ambulation and wheezing (300 mg/kg/1 day and 100 mg/kg/28 days) and diarrhoea (300 mg/kg/1 day, 10, 100 and 200 mg/kg/28 days).
Propolis did not alter lipids (cholesterol, HDL-cholesterol, total lipids, triglycerides) and the specific activity of aminotransferases (AST) and lactic dehydrogenase (LDH) after administration to rats in the short (1, 3 and 6 mg/kg/day for 30 days) or long-term (1 mg/kg/day for 90 and 150 days).[115]
In humans, propolis intake (226.8 mg/day for 8 weeks) by diabetic patients did not change parameters of glucose metabolism (homeostatic model assessment of insulin resistance – HOMA-IR, fasting plasma glucose, glycated haemoglobin, insulin), renal function (uric acid, estimated glomerular filtration rate – eGFR) and lipids (total cholesterol, LDL-cholesterol, HDL-cholesterol).[116] In HIV-infected individuals, no alterations were seen after propolis intake (500 mg/day for 3 months) in fasting blood glucose and lipids (total cholesterol, LDL-cholesterol, HDL-cholesterol) nor in hepatic (aspartate aminotransferase/AST and alanine aminotransferase/ALT activity) or renal components (urea, creatinine, uric acid) (unpublished data from our group).
Propolis (900 mg/day for 12 weeks) also exerted a positive action for people with diabetes mellitus, improving the glycemic (reducing fasting blood glucose and glycated haemoglobin) and lipid profile (limiting total cholesterol and LDL-cholesterol increase).[117] A dose of 1500 mg propolis/day for 8 weeks decreased fasting blood sugar, two-hour postprandial glucose, insulin, insulin resistance, glycated haemoglobin in diabetic patients.[118] Propolis intake by diabetic patients for 90 days (1000 mg/day) decreased AST/ALT activity and urea levels, promising to prevent hepatic and renal dysfunction in these individuals.[119] Silveira et al.[120] observed that the use of propolis in the long-term (500 mg/day for 12 months) is safe and well-tolerated by diabetic and non-diabetic patients, reducing proteinuria levels. Propolis intake (600 mg/day) for 4 weeks also reduced the concentration of creatinine and total bilirubin in smokers.[121] The use of propolis (15 drops/twice a day for 90 days) by patients with different chronic diseases demonstrated benefits by increasing HDL-cholesterol concentration and presenting an antioxidant effect, suggesting that propolis may improve the prognosis of several chronic diseases, although it also increased total cholesterol.[122]
Despite the absence of adverse effects, few cases of allergy and contact dermatitis to propolis have been described, especially in beekeepers.[123] However, beekeepers most often did not recognize the contact allergy and did not protect themselves adequately.[124] Gulbahar et al.[125] reported a case of hand dermatitis caused by propolis in a beekeeper who thought that his lesions were related to honeybee stings. Münstedt and Kalder[124] mentioned that beekeepers presenting contact allergy to propolis had a high prevalence of other allergies. The workers reported that the allergy appeared after an average of 9.5 years of beekeeping, and they believe that the substances used to clean their hands may be related to the development of the disease. Interestingly, some beekeepers presenting systemic reactions to propolis did not necessarily present these manifestations when they used propolis to treat other diseases.[124]
Contact sensitization to propolis is less frequent in children than in adults.[126] The reactions may occur due to some allergens in propolis like 1,1‐dimethylallyl caffeic acid ester, also named LB‐1.[127] Constituents of LB-1, like phenylethyl caffeate, can lead to strong reactions in propolis-sensitive patients, while other less frequent allergens – benzyl salicylate and benzyl cinnamate – led to a very weak or moderate reaction.[127] Nyman et al.[128] observed that three patients with positive reactions to caffeates also had strong reactions to propolis. Other components such as flavonoids, beeswax, essential oils, fatty acids and pollen exhibited a limited sensitization capability.[129]
Contact allergy to propolis seems to be more common among patients with adverse reactions to other products containing beeswax due to simultaneous exposure and sensitization to various constituents, such as beeswax, propolis, vegetable substances and fragrance markers.[128] The contact with an allergen, such as colophony, propolis and fragrance mixes may increase the risk of delayed-type hypersensitivity reaction to other compounds.[130] Although local cutaneous reactions are more common, Cho et al.[129] reported a case of generalized cutaneous manifestation after oral propolis ingestion, and Freedman et al.[131] described a systemic allergic dermatitis after topical or oral use of propolis.
Propolis may be considered safer than many synthetic medicines[20] and exerts protective effects on heart,[132] liver,[133] kidney,[106, 109] pancreas,[101] lung,[134] stomach[135] and other organs. In addition, a study conducted with healthy adults showed that propolis intake (375 mg/day for 15 days) did not cause any interaction with drugs commonly used by the population, such as caffeine, omeprazole, losartan, metoprolol, midazolam and fexofenadine.[136] Propolis intake (500 mg/day for 3 months) did not interfere during the therapeutic response of HIV-infected individuals under antiretroviral therapy, maintaining the undetectable HIV viral load and proper CD4+ T lymphocyte count (>500 cells/mm3) (unpublished data from our group).
Overall, all the works mentioned above indicate that propolis may be consumed by the public. Although propolis is considered safe, it is always prudent to seek medical advice before taking it as an immunomodulatory agent, as well as for supplementation or skin treatment.[78]
Propolis immunomodulatory action
For many years, research looking for information about propolis action in the immune system has been performed, using different experimental approaches in vitro and in vivo.[21, 137] Importantly, propolis may modulate the function of different cells involved in innate and adaptive immunity, such as macrophages, monocytes, neutrophils, NK cells, DCs and lymphocytes, enhancing their activity and mechanisms to fight against infectious agents.[21, 138]
Propolis stimulated ROS generation, TLR-2 and TLR-4 expression and the production of proinflammatory cytokines by murine macrophages,[88, 139, 140] and enhanced the fungicidal[141] and bactericidal[142] activity of these cells, indicating that propolis may activate the mechanisms involved in killing microorganisms.
Using animal cells in vitro, Tanaka et al.[143] verified that propolis inhibited IL-17 production and Th17 cell differentiation in DBA/1J mice. Propolis also inhibited IL-6 plus transforming growth factor (TGF-β)-induced Th17 cell differentiation and phosphorylation of the signal transducer and activator of transcription 3 (STAT3) in BALB/c mice.[144] Moreover, propolis treatment of naïve T cells from lymph nodes and spleen of C57BL/6 mice increased Th17 polarization to CD4+Foxp3+ cells.[145] In conclusion, these findings highlighted the propolis anti-inflammatory action.
The administration of propolis by gavage to Swiss mice for 4 days immediately after an injury caused by an intraperitoneal implant decreased TGF-β1 production and increased the number of neutrophils, macrophage accumulation and TNF-α and vascular endothelial growth factor (VEGF) production.[146] In an infectious disease, Miranda et al.[147] reported that BALB/c mice treated orally for 30 days with propolis and infected with Leishmania amazonensis presented a proinflammatory profile, with reduced IL-10 and TGF-β1 levels, and increased NF-κB, TNF-α and STAT3 expression.
Propolis anti-inflammatory action has also been reported in vivo. BALB/c male mice treated with propolis for 3 days presented a suppressed splenocyte proliferation and increased IFN-γ production.[148] An inhibitory effect on IL-1β, IL-6, IFN-γ, IL-2 and IL-10 production was seen in spleen cells from C57BL/6 mice treated with propolis for 14 days.[149] Daily oral administration of propolis to DBA/1J mice reduced the arthritis clinical scores and decreased IL-17, IFN-γ and IL-4 secretion by splenocytes.[143] To determine the effects of propolis on experimental asthma, C57BL/6 mice were sensitized with aluminium hydroxide adjuvant and ovalbumin (OVA) and, after the final sensitization, propolis was given for 17 or 22 days. Propolis treatment reduced inflammation, decreased mucus production, the total cell count, eosinophils and M2 macrophages count in the bronchoalveolar fluid, IL-5 levels and IL-13 gene expression in the lungs, and decreased IL-13 production in the lymph node cell culture.[145]
The histopathological analysis of lymphoid organs (thymus and spleen) was analysed after propolis administration to BALB/c mice submitted to immobilization stress, revealing that stressed mice showed an increase in germinal centres in the spleen, whereas propolis counteracted these alterations.[150]
Studies have also demonstrated the benefits of propolis to cells involved in human immunity. Propolis in noncytotoxic concentrations exerted immunomodulatory effects on cell receptors expression, cytokine production and fungicidal activity of human monocytes.[89] Propolis in combination with chlorhexidine decreased TNF-α and IL-6 production, activated the transcription factor NF-κB, and increased TLR-4 expression, IL-10 production and the bactericidal activity of these cells.[92] The treatment of peripheral blood mononuclear cells (PBMCs) with propolis stimulated IL-6 and IL-17 production and decreased IL-10 after challenge with Leishmania braziliensis.[151]
Investigating the effects of propolis produced in three different countries in Latin America (Brazil, Cuba and Mexico), Conti et al.[90] verified that samples with different chemical composition displayed different activities, i.e. pro- or anti-inflammatory action, because of the differences in their vegetal sources. Propolis from Morocco suppressed TNF-α and IL-6 and increased IL-10 production by LPS-stimulated PBMCs from healthy donors.[152]
Propolis modulated the maturation and function of DCs and may be useful in the initial steps of the immune response, providing a novel approach to the development of DC-based strategies and for the discovery of new immunomodulators.[91]
Cardoso et al.[93] investigated the involvement of phenolic acids in propolis action in human monocytes. These acids in isolation or in combination induced TLR-4 expression, favouring microorganisms’ recognition and the microbicidal activity of the cells. Nonetheless, the action of phenolic compounds did not correlate exactly to the propolis activity in all parameters, suggesting that it is difficult to state which compound(s) is(are) responsible for propolis effects.
As to antibody production and humoral immunity, Scheller et al.[153] were one of the pioneers in demonstrating that propolis may increase antibody production in sheep red blood cells (SRBC)-immunized mice, and a higher production was seen when propolis was administered for a short-term to the animals. Later, other authors have also shown that propolis or its isolated constituents may increase antibody production after immunization with different antigens.[154, 155]
Propolis in combination with vaccines induced higher amounts of antibodies than the vaccines themselves.[156–159] Regarding viral diseases, Fan et al.[160] verified that the epimedium polysaccharide-propolis flavone adjuvant-induced lymphocyte proliferation and antibody titer against the inactivated avian influenza and Newcastle disease vaccines in chickens. Peng et al.[161] compared four natural adjuvants co-administered with a porcine reproductive and respiratory syndrome virus (PRRSV) subunit vaccine, including propolis, Astragalus extract, Astragalus/Bacillus production and Freund’s adjuvant. Propolis and Astragalus extract enhanced humoral and cell-mediated immunity, but to a lower magnitude than Astragalus/Bacillus adjuvant and Freund’s adjuvant.
In summary, propolis and its constituents exert a remarkable immunomodulatory effect, and inhibitory or stimulatory activity may be observed, affecting several cells and components of the immune/inflammatory response such as neutrophil adhesion and transmigration, cytokines, chemokines, C-reactive protein, prostaglandin E2, signalling pathways, antibodies, among others.[102, 145, 162–165] Since propolis does exert both pro- and anti-inflammatory activity, depending on concentration, intake period and experimental conditions,[21] we strongly suggest that clinical trials should be performed to investigate the effects of propolis on individuals with viral diseases, in combination or not with antiviral drugs or vaccines.
Evidence for Propolis Antiviral Action
A review of works dealing with propolis and viruses
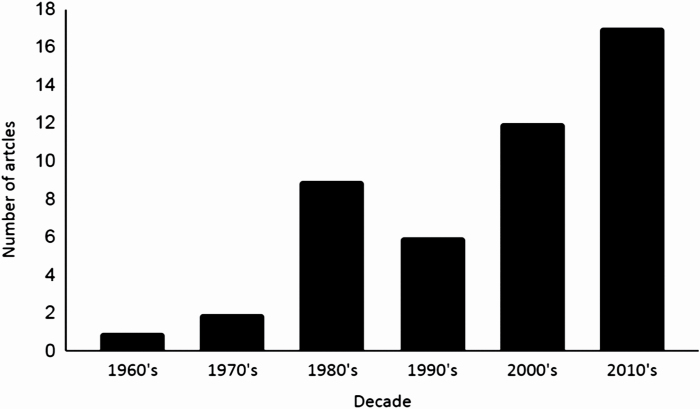
Research on propolis antiviral action started in the 60’s and the first work was published by N. Joirich in 1966 (“Bees and Medicine”, Medicina Publisher, Tashkent, 1966, pp. 194–195, in Russian). However, one cannot find this article on online platforms. By June 2020, the total number of works dealing with propolis extracts and viruses was 49, with the following number of manuscripts per year: 1966 (1 article), 1977 (1), 1978 (1), 1980 (2), 1981 (1), 1985 (4), 1987 (1), 1988 (1) and 1990 (1). An increased interest in researching this subject was registered after the 2000’s (Figure 1). In the last decade, the number of published articles found in the database was 17. Importantly, Serkedjieva et al. published the oldest article found in PubMed in 1992. In this work, there are citations of older articles related to propolis and viruses, but these works are not found on online databases.
Figure 1.
Published papers on the antiviral action of propolis by decades. The articles were selected according to the keywords aforementioned and by background title affinity (n = 49).
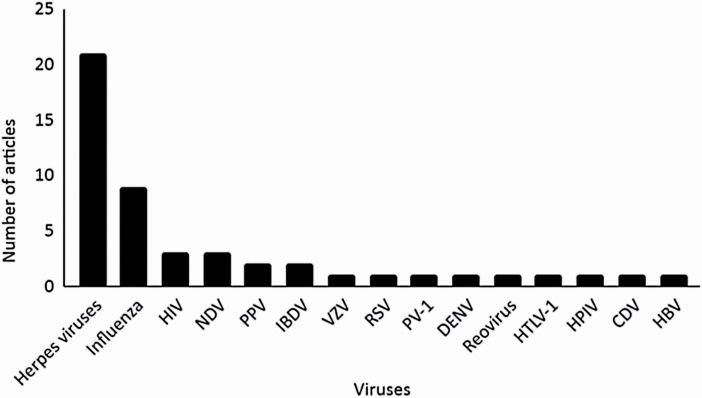
When sorting the data by virus type, an extensive attention was given to propolis action against herpesviruses, followed by studies focusing on influenza viruses (Figure 2). Some authors have researched the effects of propolis on more than one virus strain. It is worth mentioning that three works were discarded from Figure 2 due to lack of access.
Figure 2.
Number of published papers according to the virus type (n = 46). HIV, human immunodeficiency virus; NDV, Newcastle disease virus; PPV, porcine parvovirus; IBDV, infectious bursal disease virus; VZV, varicella zoster virus; RSV, respiratory syncytial virus; PV-1, poliovirus type 1; DENV, dengue virus; Reovirus, respiratory enteric orphan virus; HTLV-1, human T-cell leukemia-lymphoma virus type 1; HPIV, human parainfluenza virus; CDV, canine distemper virus; HBV, hepatitis B virus.
Methodologies to evaluate propolis antiviral action
Overall, the antiviral action of propolis was analyzed using in vitro assays, which are an excellent approach to determine the antiviral action of a substance and further initiate in vivo studies. Since viruses are obligatory intracellular pathogens, evaluation of the antiviral action of a given substance may be performed by cell infection in vitro, identifying the proper target cells for each virus and establishing cell culture conditions.
Cells can be treated with different concentrations of an antiviral agent at three different periods of incubation: before, simultaneously or after the virus infection. Data may be analyzed by polymerase chain reaction (PCR), calculating the percentage of viral inhibition and consequently the smallest relative RNA viral quantification. The real-time (RT)-PCR provides higher sensitivity and specificity to quantify viral nucleic acids, and an association between the concentrations of viral nucleic acid and cell culture infectivity may be established.[166] Viral infectivity may also be assessed by cytotoxic assays, including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and crystal violet methods, cytopathic effect (CPE) using an optical microscope, trypan blue and neutral red staining, neuraminidase inhibition and others.[167] Overall, the majority of works evaluating propolis antiviral activity employed PCR, immunoenzymatic assays, CPE, among others, to determine viral replication. Cytotoxic assays included cell count, reduction markers and the analysis of morphological changes. More recently, Silva-Beltrán et al.[72] employed MS2 and AV-08 bacteriophages to assess propolis activity against enterovirus. These bacteriophages have genetic and structural characteristics similar to enteric viruses and are considered a good model to evaluate new anti-enterovirus drugs in vitro. Table 1 shows the main characteristics of the in vitro tests assessing propolis antiviral action, and the most studied viruses are presented below.
Table 1.
Methodologies used in vitro to assess the antiviral activity of propolis. All the works below may be found on the PubMed platform. PFU, plaque-forming unit; RT-qPCR, real-time quantitative polymerase chain reaction; CPE, cytopathic effect; CCK-8, cell counting kit 8
| Virus | Cell line | Incubation (h) | Assay | Authors |
|---|---|---|---|---|
| Enteric viruses | MS2, AV-08 Bacteriophages | 24 | PFU | [72] |
| HSV-1 | CEM | 72 | ELISA (p24 antigen) |
[168] |
| HSV-1 | CEM | 72 | ELISA (p24 antigen) |
[169] |
| HSV-2 | RC-37 | 72 | Neutral red PFU |
[170] |
| HSV-2 | RC-37 | 72 | Neutral red PFU |
[171] |
| HSV-1; 2 | MDBK | 24 | Neutral red neutral assay |
[172] |
| H1N1, H3N2 and B/Lee | Embryonated hen’s eggs | 48–72 | Morphological changes Infectious titter |
[173] |
| H7N7 | CEF | 72 | CPE | [174] |
| H1N1 | MDCK | 72–96 | Trypan blue PFU |
[175] |
| H1N1 | MDCK | 96 | Trypan blue CPE |
[176] |
| HIV | H9 T | 96 | Cell count ELISA (p24 antigen) |
[177] |
| HIV | CD4+ lymphocytes | 168 | Trypan blue MTT ELISA (p24 antigen) |
[178] |
| PV1 | HEp-2 | 48 | Crystal violet RT-qPCR |
[179] |
| IBDV and reovirus | CEF | 120 | Crystal violet CPE |
[180] |
| NDV and IBDV | CEF | 120 | Crystal violet CPE |
[181] |
| CDV | Vero | 48 | MTT RT-qPCR |
[182] |
| HSV-1; 2 | HEp-2 | 72 | Trypan blue MTT RT-qPCR |
[183] |
| HSV-1, H1N1, HPIV and adenovirus | HEp‐2 | 48 | CPE | [184] |
| PPV | PK-15 | 48 | CCK-8 solution RT-qPCR |
[185] |
| PPV | PK-15 | 72 | MTT RT-qPCR |
[186] |
| RSV | HEp-2 | 72 | MTT | [187] |
| VZV | LEP | 216 | Neutral red PFU |
[188] |
Herpes simplex viruses
The prevalence of HSV-1 and HSV-2 was 47.8% and 11.9%, respectively, among people aged 14 to 49, mostly women, between 2015 and 2016.[189] The interest in studying propolis antiviral potential against HSV began in the 90’s when Amoros et al.[168] observed reduced HSV-1 infectivity in VERO cells treated with propolis balsam (72 µg/ml). In 1994, Amoros et al.[169] reported that poplar propolis extract acted on the viral DNA synthesis. Huleihel and Isanu[190] proposed that propolis affected viral absorption and the replication cycle of HSV-1. These authors found the LC50 (a lethal concentration that kills half of the cells) of 0.5% of propolis in VERO cells, whereas they used a 5% dose in vivo in rats and rabbits.
Nolkemper et al.[170] mentioned that aqueous (5 µg/ml) and ethanolic (4 µg/ml) extracts from Czech propolis damaged the HSV-2 envelope or camouflaged capside compounds. Schnitzler et al.[171] also tested ethanolic (4 µg/ml) and aqueous (5 µg/ml) extracts from Czech propolis against HSV-2 and observed an impaired virus absorption by RC-37 cells. Bankova et al.[172] reached the same conclusion using poplar propolis extract (100 µg/ml) against both types of HSV-1/2 and mentioned its interference in virus absorption and viral cycle replication in MDBK cells. Mazia et al.[191] observed damage to HSV-1 structures caused by green propolis ethanolic extract.
Finally, Sartori et al.[192] reported that brown propolis (50 mg/kg) reduced the damage caused by HSV-2 infection in BALB/c mice, due to its anti-inflammatory and antioxidant properties.
Taking together, the data suggested that propolis anti-herpes action might occur due to damage in the viral envelope and avoiding cellular absorption. In addition, the healing, antioxidant and anti-inflammatory effects of propolis can help to resolve the adverse effects of HSV infection in vivo.
Table 2 shows the works dealing with propolis and HSV.
Table 2.
Works related to propolis activity against herpes simplex viruses types 1 and 2 accessed on the PubMed platform. ND, not described
| HSV type | Propolis type | Proposed mode-action | Authors |
|---|---|---|---|
| HSV-1 | Propolis balsam | ND | [168] |
| HSV-1 | Poplar | Viral DNA synthesis | [169] |
| HSV-1 | ND | Virus absorption and viral replication cycle | [190] |
| HSV-2 | GH 2002 | Virion envelope structures or masking viral compounds | [170] |
| HSV-2 | ND | Virus adsorption to host cells | [171] |
| HSV-1; 2 | Poplar | Interference in the viral adsorption to the cells | [172] |
| HSV-1 | Green | Virion damage | [191] |
| HSV-2 | Brown | ND | [192] |
| HSV-1; 2 | Hatay propolis | ND | [183] |
| HSV-1 | PROPOLI ACTICHELATA | ND | [184] |
Influenza viruses
Influenza viruses cause infection to the respiratory tract as well as half a million deaths per year. These viruses regularly acquire mutation, complicating antiviral therapies.[193]
Serkedjieva and colleagues[173] authored the oldest work dealing with propolis and influenza viruses found in PubMed. The antiviral action of Bulgarian propolis on H3N2 (50 μg/ml) and H1N1 (100 μg/ml) was assessed in vitro, indicating that propolis and its components suppressed viral replication. In this work, there are older references from authors that evaluated propolis effects on H0N1, H1N1 and H3N2, but these works were not found on the PubMed platform.
Kujumgiev et al.[174] investigated propolis antimicrobial action against bacteria, fungi and H7N7, without mentioning a possible antiviral mechanism. Years later, propolis effect on H1N1 was studied in vitro with effective concentrations of 50% plaque reduction ranging from 60 to 111.635 μg/ml, and in vivo using DBA/2 mice, postulating that propolis extract (10 mg/kg) may act against H1N1 by increasing IFN-γ production and activating a Th1 response, orchestrated by T CD4+ lymphocytes.[175]
Recently, Governa et al.[176] investigated the action of poplar propolis extract (35 μg/ml) against H1N1 in vitro, verifying that it stimulated pro-inflammatory cytokines (IL-6 and IL-1β) secretion by PBMC and reduced neuraminidase, a key-protein for virus entry. Maybe propolis components could act as a neuraminidase inhibitor, which is an important class of antiviral agents.
Retroviruses
Propolis action was also investigated against harmful retroviruses to human health. For instance, propolis activity HIV that causes acquired immunodeficiency syndrome (AIDS) may occur by interfering in viral entry, probably by inhibiting the p24 antigen present in the HIV envelope. Propolis or its components were assayed in vitro against HIV using different T lymphocytes lineages: CEM,[194] H9[177] and CD4+ cells.[178] Furthermore, a virus from the HIV family, HTLV-1, which causes adult T-cell leukaemia, was susceptible to propolis and to its component CAPE, probably because it prevented the action of TAX oncogene in the activation of the transcription factor NF-kB.[195]
In addition to these viruses, Búfalo et al.[179] studied the effect of Brazilian green propolis against Poliovirus (PV-1), the causative agent of poliomyelitis in humans. HEp-2 cells were treated with propolis extract before, simultaneously or after cell infection, and its effects were more pronounced when the virus was within the cell, affecting the viral cycle.
Preclinical assays using experimental animals have been mapped out similarly to clinical trials, regarding the composition of experimental and control groups, excluding several types of interference found in humans. In addition, animal models allow obtaining data at the tissue level if the ethics committee approved euthanasia. Thus, in vivo models are still considered an important tool to investigate the effects of medications or natural products.[196] Although there are some works regarding the effects of propolis or its components on the main viruses of interest in human and veterinary medicine using animal models as described in the next item, most of the works were performed in vitro.
Propolis action against animal viruses
Propolis use as an antiviral agent has been investigated in animals for its therapeutic action or as an adjuvant in vaccines due to its immunomodulatory potential. It was demonstrated that poplar propolis extract was efficient in vitro against replication of the infectious bursal disease virus (IBDV) that causes infectious bursitis in chickens and turkeys.[180] A few years later, the same group evaluated propolis effects on IBDV and on Newcastle disease virus (NDV), another harmful virus to birds, using the Chick Embryo Fibroblasts (CEF) cell line.[181] Flavones of propolis were also evaluated against NDV, using CEF cell line in vitro and white roman chickens in vivo.[160] An increased antibody content was seen in propolis-treated chickens. Yuan et al.[197] also used roman chickens to assess the potential of propolis flavonoid liposome (PFL) against NDV in vivo, showing that PFL increased IgG and IgM titers, IFN-γ and IL-2 production and induced lymphocyte proliferation.
Another harmful virus is the canine distemper virus (CDV) that causes distemper – a disease that may cause mortality in domestic dogs. González-Búrquez et al.[182] analyzed the effect of propolis extract on CDV in vitro using VERO cells, suggesting that propolis or its components may exert an antiviral action.
Propolis antiviral potential still needs further investigation concerning viral diseases for animals.
Clinical trials
As far as we know, few works dealing with propolis and clinical trials against viral diseases can be found in the literature. Vynograd et al.[198] investigated three groups of male and female patients (n = 90) with recurrent infection caused by HSV-2. HSV viruses can cause infections characterized by pain and sores. These groups received propolis flavonoid ointment (300 mg/ml), acyclovir antiviral ointment or placebo ointment at the site of infection. An improved clinical picture such as healing and reduced incidence of infection was observed in the group receiving propolis flavonoid ointment compared to acyclovir and placebo groups.
The effectiveness of Herstat (3% propolis ointment ACF) was assessed in a randomised, double-blind placebo-controlled clinical trial conducted in Sweden. Patients with recurrence of herpes labialis were instructed to apply the medication five times/day as soon as they presented a cold sore. Patients who applied propolis ointment reported no pain before those who applied the placebo ointment. The propolis group appeared to heal the lesion more quickly than the placebo group in the first days of treatment. After treatment, patients from the propolis ointment group considered their treatment very effective (81.8%) and somewhat effective (18.2%).[199]
Soroy et al.[200] carried out a clinical trial in Indonesia using patented poplar propolis capsules (Propoelix) on patients with dengue hemorrhagic fever. They performed a double-blind, randomized, placebo-controlled trial. Patients (n = 63) were divided into two groups (Propoelix [200 mg] three times/day or placebo) and observed for 7 days. A decreased TNF-α production and increased platelets were seen in the propolis group, showing propolis benefit for the patients.
Coronaviruses, SARS-CoV-2, COVID-19 and Propolis Effects
In the current pandemic, SARS-CoV-2 that causes COVID-19 is leading to serious problems in the world economy,[201, 202] overwhelming the health systems[203, 204] and psychological problems not only due to the disease itself but also due to social distancing to avoid further contamination.[205, 206] The number of infected people and deaths has increased since the pandemic began in Wuhan, China, in late 2019. The numbers vary amongst countries because of different measures adopted to treat and control this disease.
It is crucial to understand its history, transmission, replication cycle and possible strategies to restrain the disease. The coronaviruses (CoVs) were first identified in the 60’s. They are enveloped viruses composed of a single-stranded positive-sense RNA[205] and belong to the Nidovirales order and Coronaviridae family. CoVs are divided into four genera, including Alpha-CoV, Beta-CoV, Gamma-CoV and Delta-CoV, with variable disease severity in humans and other vertebrates, causing respiratory, intestinal, liver or neurological diseases.[207–209]
A distinguishing feature of this viral family is the fact that they have the largest genome among all RNA viruses, including RNA viruses with segmented genomes, presenting about 27–32 kb, which code structural and non-structural proteins.[209, 210] Among the main proteins encoded by these viruses, the spike (S) protein will be further discussed. Protein S is a type I membrane glycoprotein, which plays an important role in the entry and replication of some CoVs in the cell, such as human coronavirus NL63 (HCoV-NL63), SARS-CoV and the new SARS-CoV-2, favouring the initial interaction with specific cell receptors and subsequent mediation of virus-cell fusion.[207]
CoV infections are commonly species-specific and transmission between hosts occurs mainly via the respiratory and faecal-oral routes, causing acute or persistent infections.[210] Currently, some CoVs have been identified capable of infecting humans (HCoVs): the HCoV-229E, HCoV-OC43, HCoV-NL63, HKU1 viruses, causing common colds and in some rare cases causing severe respiratory infection in babies, young children, elderly and immunosuppressed patients.[208]
SARS originated in southern China and caused an endemic in 2003. The primary source of the virus was the bat CoVs, transmitted through contact or meat consumption of pangolins. 8098 SARS cases have been reported globally including 774 deaths, with an extremely high human mortality rate ranging from 10 to 38%. SARS is spread from person to person through close contact with an infected person or through droplets expelled by an infected person’s coughing or sneezing, and most of the cases have been transmitted in hospitals. Symptoms included fever, headache, chills, muscle pain, dry cough and sometimes dyspnea. SARS-CoV mechanism of action involves a receptor for cell entry, the angiotensin-converting enzyme 2 (ACE2), which exists in soluble and membrane-bound forms. No cases of SARS-CoV infection have been reported since 2004.[211, 212]
In June 2012, the first case of infection attributed to the Middle East respiratory coronavirus syndrome (MERS-CoV) was registered in Saudi Arabia. Between 2012 and October 2018, this virus caused several sporadic outbreaks worldwide, registering 2266 MERS-CoV cases confirmed according to the World Health Organization (WHO) including 804 deaths and, in most cases, human-to-human transmission mainly in clinical settings.[213] MERS-CoV mechanism of infection is based on the interaction of protein S with the dipeptidyl peptidase 4 (DPP4) receptor, present in non-ciliated bronchial epithelial cells and type II pneumocytes.[211] According to phylogenetic analyzes, MERS-CoV originated from bat CoVs and was transmitted by camels, through contact or meat consumption of infected animals.[211, 214, 215] The typical symptoms of the MERS infection were common cold, presenting a rapid evolution to pneumonia and respiratory distress syndrome. The symptoms most reported were high fever, cough, dyspnea, chills, chest pain, myalgia, diarrhoea, nausea and vomiting, rhinorrhea and others, progressing to severe pneumonia with renal and respiratory failure.[214]
At the end of 2019, a new coronavirus (SARS-CoV-2) capable of infecting humans emerged and caused COVID-19, with the first cases in Wuhan, Hubei province in China, spreading quickly to all Chinese provinces and to other countries in the world, resulting in the declaration of a global pandemic by the WHO on March 11, 2020.[203] The transmission of the virus occurs by dissemination from person to person through aerosols, touch, contact with contaminated secretions through the air, and there are still reports of faecal-oral transmission.[216–218]
Unlike SARS-CoV and MERS-CoV, the community can sustain SARS-CoV-2 transmission. Once the host is infected, he/she can transmit the virus to an average of 2–3 uninfected individuals. The incubation period is reported to be 1 to 14 days and its clinical picture can vary between (1) asymptomatic, (2) symptoms of a common flu syndrome such as cough, tiredness and fever, (3) or more severe symptoms such as dyspnea and hypoxemia, usually in the elderly (>50 years) and in those with cardiac and respiratory disorders. It can progress to pneumonia, acute respiratory distress syndrome, multiple organ failure and the mortality rate varies from 3 to 4%.[219, 220]
Based on the phylogenetic analysis of the complete genome, the new coronavirus shares 80% of its genome similarity to SARS-CoV and 96% to RaTG13 bat coronavirus, reinforcing the theory that bats could have been the primary source for the virus.[218, 221] However, studies have shown that pangolin CoVs also shared about 91.02% of its genomic identity with SARS-CoV-2, raising doubts of the real SARS-CoV-2 origin.[210]
The mechanism of action of SARS-CoV-2 infection is analogous to that of SARS-CoV, starting with the cleavage of protein S mediated by host cell proteases, in particular transmembrane protease serine 2 (TMPRSS2), in two functional subunits responsible for binding to the host cell receptor: S1 and S2. S1 subunit comprises the receptor-binding domain (RBD) and interacts with the ACE2 peptidase domain of the host cell, which is mainly present in the lower respiratory epithelium specifically on the surface of type II pneumocytes (cells of the pulmonary alveoli). Therefore, the pathological process of the disease occurs mainly in these tissues but also in the cells of the small intestine mucosa, in the mouth and tongue, facilitating viral entry into the host.[222, 223]
After S1 binding to the ACE2 host receptor, S2 is exposed and cleaved by host furins and proteases, and is responsible for membrane fusion.[221, 222] The virion releases its RNA strand in the cytosol and is translated into protein, and this genetic material replicates, forming new virions, with only one virion particle capable of producing hundreds of new virions and each one is capable of infecting a new cell.[220]
During SARS-CoV-2 infection, alveolar cells stop performing their normal activity producing the surfactant to clean the airways, resulting in a progressive accumulation of debris and fluids in the lungs, causing the acute respiratory distress syndrome (ARDS).[224]
The diagnosis of SARS-CoV-2 has been performed by analysis of respiratory secretions using molecular tests for viral RNA detection by RT-qPCR and serological tests to detect IgG, IgM and IgA antibodies against SARS-CoV-2 nucleoprotein in serum or plasma. Chest computed tomography (CT) might be helpful for the diagnosis, as well as routine laboratory tests, such as blood count.[225] Although CT is not indicated as the only diagnostic test, it is a valuable tool to monitor the progression of the disease and to detect possible complications.
Regarding the antiviral immunity, Robbiani et al.[226] analyzed 149 COVID-19 convalescent individuals recruited in the Rockefeller University Hospital in New York, USA, verifying they did not have high levels of neutralizing antibodies. Similar observations were reported by Long et al.[227], assessing 37 asymptomatic individuals in the Wanzhou District, China, who were diagnosed with RT–PCR-confirmed SARS-CoV-2 infection. These authors also mentioned that asymptomatic individuals had a weaker immune response and reduced antibody levels in the early convalescent phase, raising implications for immunity strategies and serological surveys.
Liu et al.[228] analyzed the prevalence of IgG and IgM from individuals who tested positive for SARS-CoV-2 both in serological and RT-PCR tests and from healthcare individuals without COVID-19 diagnosis in Wuhan, China. Although a significant number of healthcare individuals had been infected with this virus, SARS-CoV-2-specific IgG was detected only in a few of them. The authors also mentioned no long-lasting protective antibodies against this virus.
However, although the scientific community is worried regarding the duration of antibody-mediated immunity, important findings revealed that SARS-CoV-2 induces a memory T cell response in antibody-seronegative and antibody-seropositive individuals with asymptomatic or mild COVID-19.[229] These authors reported that acute phase SARS-CoV-2-specific T cells displayed a highly activated cytotoxic phenotype that correlated with various clinical markers of disease severity, whereas convalescent-phase SARS-CoV-2-specific T cells were polyfunctional and displayed a stem-like memory phenotype. Importantly, SARS-CoV2-specific T cells were detectable in antibody-seronegative family members and in individuals with a history of asymptomatic or mild COVID-19. These findings indicated that SARS-CoV-2 elicits an adequate memory T cell response and may prevent recurrent episodes of severe COVID-19 even in seronegative individuals.
Currently, treatment has been based on a wide spectrum of antiviral drugs, as well as immunomodulatory/anti-inflammatory drugs (e.g. corticosteroids), serotherapy under study (blood antibodies taken from healed individuals), anticoagulants (for thromboprophylaxis), monoclonal antibodies and others according to the clinical conditions of patients.[207] Some vaccines in advanced phases are being evaluated in clinical trials, produced by Sinovac Life Sciences Co., Ltd. (China); University of Oxford/AstraZeneca (United Kingdom); China National Pharmaceutical Group (Sinopharm) by the Beijing Institute of Biological Products and the Wuhan Institute of Biological Products; Moderna Therapeutics/National Institute of Allergy and Infectious Diseases (NIAID, USA); BioNTech/Fosun Pharma/Pfizer (USA); Gamaleya Research Institute (Russia); Janssen Pharmaceutical Companies (Belgium); Bharat Biotech (India); CanSino Biological Inc./Beijing Institute of Biotechnology (China) and Novavax, Inc. (USA).[230]
Works dealing with propolis action against coronaviruses are scarce. The key-components of propolis, such as flavonoids, have already been assayed against coronavirus showing some inhibitory effect.[231] However, one may speculate that some components present in the vast majority of propolis samples around the world may display an anti-coronavirus action. Among these components, the flavonoid quercetin stands out. Quercetin can help against SARS and MERS-CoV infection by modulating unfolded protein response, preventing the complete viral cycle. Furthermore, CAPE exhibits an anti-p21-activated kinase (PAKs) property,[232] which is an important enzyme for several human viruses’ entry and replication [233]. Osés et al.[234] reported that propolis components (catechin, p-coumaric acid and flavanols) displayed an ACE inhibitory activity. Thus, we recommend the investigation of propolis effect on SARS-CoV-2 replication in vitro.
Since propolis exerts both antiviral and anti-inflammatory activity, it should be also analyzed for the treatment of COVID-19, to see its capability for interfering in the viral cycle and for controlling the cytokine storm. Moreover, the synergism between propolis components and current antiviral drugs should be assessed. We postulate that propolis may benefit the patients in different ways: (1) maybe due to a direct action, affecting cell entry and viral cycle; (2) by exerting an anti-inflammatory action and controlling the cytokine storm; (3) enhancing not only antibody production but also cell-mediated immunity.
Conclusions
Although viruses may elicit an acquired immune response, we still face big challenges in combating viral infections, since intracellular agents may develop different strategies to escape from the host immunity. Moreover, viral infections usually cause chronic diseases causing difficulties in the fight against them. Regarding COVID-19, little is known about humoral and cell-mediated immunity, since this is a very recent pandemic. Although a ton of information is being generated, it is still difficult to have a clear picture of host immunity, concerning the role of antibodies not only for diagnosis but also for individual’s protection. Cellular immunity seems to be preserved and many vaccines are being produced rapidly. Some crucial questions are still not fully answered: how durable is immunity and protection after infection with SARS-CoV-2? Will the primary immune response protect in subsequent reinfections? Is there a cross-reaction of SARS-CoV-2 with other antibodies? What is the mutation rate of this virus?
Antiviral agents have been employed and, while vaccines are still being produced, natural products should be assayed in an attempt to discover new antiviral drugs. As mentioned above, propolis antiviral action has been evaluated in vitro, while few in vivo assays and clinical trials have been carried out to explore its potential to stimulate host immunity to fight against viruses. Specific to COVID-19, the effects of propolis should be firstly investigated directly on the virus in vitro or on infected individuals alone or in combination with antiviral drugs, due to its immunomodulatory and anti-inflammatory action. Bachevski et al.[232] also recommended propolis as a prophylactic product for high-risk groups, such as individuals in close contact with infected patients.
The search for therapeutic targets may be useful to find out how propolis can help to control COVID-19 and bioinformatics approaches can add new insights. Using a homology-based structural model of TMPRSS2 and molecular docking, Kumar et al.[235] investigated the binding potential of CAPE, Withaferin-A (Wi-A) and Withanone (Wi-N) to TPMRSS2. Despite their binding affinity towards TMPRSS2, Wi-A and Wi-N seemed to be more efficient in binding and interacting with this protease. Maruta and He[236] reported another approach for virus control: the discovery of PAK1 blockers. Among mammalian kinases called PAKs (RAC/CDC42-activated kinases), PAK1 is the major “pathogenic” kinase and its abnormal activation is responsible for some diseases including viral infection. PAK1 may also suppress both T and B cells, affecting antibody production. CAPE found in propolis were shown to inhibit RAC, which activates PAK1. These authors also mentioned a large-scale clinical trial using propolis for COVID-19 patients in the Netherlands (https://osaka20420.blogspot.com/2020/04/propolis-therapy-of-covid-19-letter.html). Thus, we recommend the investigation of propolis effect against SARS-CoV-2 replication in vitro. A possible inhibitory action of propolis in ACE activity could be investigated in vivo as well.
Researchers have tested the use of propolis for different purposes and the number of bioproducts containing propolis (nanoparticles, mouthwashes, gels, chewing gums, ointments) and patents have increased,[21] indicating its promising therapeutic applications. Since propolis is nontoxic and practically without side effects, patients should ask for the medical recommendation to include propolis in combination with the antiviral agents or with the new vaccines when available. Previous research from our group revealed that propolis might exert a synergistic action in combination with some antibiotics.[22, 237, 238] Thus, we strongly suggest the evaluation of propolis effectiveness in combination with different antiviral drugs to obtain a new treatment for viral diseases. In this context, Altindis et al.[239] investigated in vitro the effects of propolis and olive leaf extract (OLE) alone or in combination with acyclovir regarding their antiviral activity against HSV-1. This combination exerted an efficient antiviral effect and caused no CPE, suggesting that reduced doses and side effects of acyclovir could be achieved by a simultaneous administration of propolis and OLE. Propolis could be used for COVID-19 prevention (prophylactic effect) or even in combination with the recommended treatment of this disease (therapeutic effect) due to its biological properties. We also recommend the evaluation of propolis administration simultaneously with vaccines, due to its adjuvant properties, to enhance the individuals’ immune response. The addition of propolis to antivirals and vaccines should be investigated in clinical trials aiming to use lower doses of the drugs and reduce side effects; moreover, the use of capsules with standardized extraction and defined concentrations of propolis should be considered. Propolis antiviral and immunomodulatory activity and proposals for anti-SARS-CoV-2 approaches are shown in Figure 3.
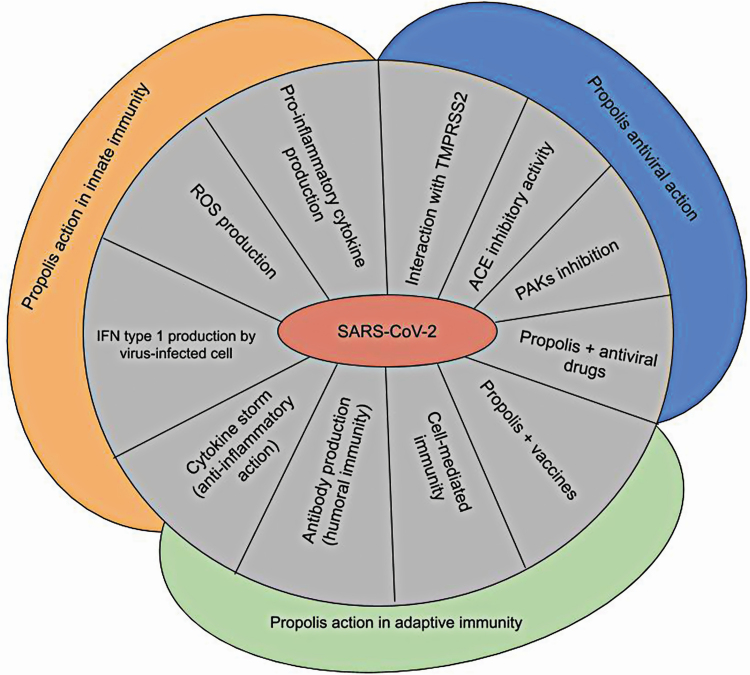
Figure 3.
Propolis antiviral and immunomodulatory activity and proposals for anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) approaches. Propolis and its constituents can induce (1) pro-inflammatory cytokine production, (2) reactive oxygen species (ROS) generation by innate immune cells and (3) interferons (IFN) type I production by virus-infected cells in the onset of viral infection. (4) Antibody production (humoral immunity) and (5) cell-mediated immunity may be enhanced by propolis. Propolis may be investigated in combination with (6) vaccines and (7) antivirals. Propolis anti-inflammatory action may help to control (8) the cytokine storm. (9) p21-activated kinases (PAKs) and (10) angiotensin-converting enzyme (ACE) inhibition. (11) transmembrane protease serine 2 (TMPRSS2) interaction with propolis constituents can be considered in the control of SARS-CoV-2.
Last but not least, this review was designed in an attempt to positively contribute to this pandemic scenario, to inspire new research that may help with different aspects of COVID-19, controlling the virus and benefiting people who are infected or not.
Acknowledgements
Authors are thankful for Prof. Christiaan Moora for assisting with the English. Authors also wish to express their solidarity to those who have lost their beloved ones due to COVID-19.
Author Contributions
Authors equally contributed to this work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interests.
Data Availability Statement
Original data generated in the course of the study or to third-party data analyzed in the article were properly cited.
References
- 1. Gupta BP, Tuladhar R, Kurmi Ret al. . Dengue periodic outbreaks and epidemiological trends in Nepal. Ann Clin Microbiol Antimicrob 2018; 17(1): 1–6. 10.1186/s12941-018-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lew RJ, Tsai WY, Wang WK. Dengue outbreaks in Hawai‘i after WWII – a review of public health response and scientific literature. Hawaii J Med Public Health 2018; 77(12): 315–8. [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar T, Shrivastava A, Kumar A, et al. . Viral hepatitis surveillance – India, 2011–2013. MMWR Morb Mortal Wkly Rep 2015; 64(28): 758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Possas C, Lourenço-de-Oliveira R, Tauil PLet al. . Yellow fever outbreak in Brazil: the puzzle of rapid viral spread and challenges for immunisation. Mem Inst Oswaldo Cruz 2018; 113(10): 1–12. 10.1590/0074-02760180278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nasheri N, Vester A, Petronella N. Foodborne viral outbreaks associated with frozen produce. Epidemiol Infect 2019; 147: 1–8. 10.1017/S0950268819001791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009; 459(7249): 931–9. 10.1038/nature08157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murray MJ. Ebola virus disease: a review of its past and present. Anesth Analg 2015; 121(3): 798–809. 10.1213/ANE.0000000000000866 [DOI] [PubMed] [Google Scholar]
- 8. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020; 109: 102433. 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Razonable RR. Antiviral drugs for viruses other than Human Immunodeficiency Virus. Mayo Clin Proc 2011; 86(10): 1009–26. 10.4065/mcp.2011.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vilas Boas LCP, Campos ML, Berlanda RLAet al. . Antiviral peptides as promising therapeutic drugs. Cell Mol Life Sci 2019; 76(18): 3525–42. 10.1007/s00018-019-03138-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 2005; 45(5): 804–17. 10.1053/j.ajkd.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 12. Abers MS, Shandera WX, Kass JS. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs 2014; 28(2): 131–45. 10.1007/s40263-013-0132-4 [DOI] [PubMed] [Google Scholar]
- 13. Schnitzler P. Essential oils for the treatment of Herpes Simplex Virus infections. Chemotherapy 2019; 64(1): 1–7. 10.1159/000501062 [DOI] [PubMed] [Google Scholar]
- 14. Semret M, Schiller I, Jardin BAet al. . Multiplex respiratory virus testing for antimicrobial stewardship: a prospective assessment of antimicrobial use and clinical outcomes among hospitalized adults. J Infect Dis 2017; 216(8): 936–44. 10.1093/infdis/jix288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu JD, Meng W, Wang XJet al. . Broad-spectrum antiviral agents. Front Microbiol 2015; 6: 1–15. 10.3389/fmicb.2015.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rothan HA, Bahrani H, Rahman NAet al. . Identification of natural antimicrobial agents to treat dengue infection: In vitro analysis of latarcin peptide activity against dengue virus. BMC Microbiol 2014; 14(1): 1–10. 10.1186/1471-2180-14-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 2015; 14(2): 111–29. 10.1038/nrd4510 [DOI] [PubMed] [Google Scholar]
- 18. Ayaz M, Ullah F, Sadiq Aet al. . Synergistic interactions of phytochemicals with antimicrobial agents: potential strategy to counteract drug resistance. Chem-Biol Interact 2019; 308: 294–303. 10.1016/j.cbi.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 19. Pasupuleti VR, Sammugam L, Ramesh Net al. . Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev 2017; 2017: 1–21. 10.1155/2017/1259510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toreti VC, Sato HH, Pastore GMet al. . Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid Based Complement Altern Med 2013; 2013: 1–13. 10.1155/2013/697390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res 2016; 30(6): 894–905. 10.1002/ptr.5605 [DOI] [PubMed] [Google Scholar]
- 22. Orsi RO, Fernandes A Jr, Bankova Vet al. . The effects of Brazilian and Bulgarian propolis in vitro against Salmonella Typhi and their synergism with antibiotics acting on the ribosome. Nat Prod Res 2012; 26(5): 430–7. 10.1080/14786419.2010.498776 [DOI] [PubMed] [Google Scholar]
- 23. Gucwa K, Kusznierewicz B, Milewski Set al. . Antifungal activity and synergism with azoles of Polish propolis. Pathogens 2018; 7(2): 1–22. 10.3390/pathogens7020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grecka K, Kuś PM, Okińczyc Pet al. . The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules 2019; 24(9): 1–24. 10.3390/molecules24091732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ong TH, Chitra E, Ramamurthy Set al. . Cationic chitosan-propolis nanoparticles alter the zeta potential of S. epidermidis, inhibit biofilm formation by modulating gene expression and exhibit synergism with antibiotics. PLoS ONE 2019; 14(2): 1–13. 10.1371/journal.pone.0213079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marsh M, Helenius A. Virus entry: open sesame. Cell 2006; 124(4): 729–40. 10.1016/j.cell.2006.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raoult D, Forterre P. Redefining viruses: lessons from Mimivirus. Nat Rev Microbiol 2008; 6(4): 315–9. 10.1038/nrmicro1858 [DOI] [PubMed] [Google Scholar]
- 28. Caspar DLD, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol 1962; 27: 1–24. 10.1101/SQB.1962.027.001.005 [DOI] [PubMed] [Google Scholar]
- 29. Votteler J, Sundquist WI. Virus budding and the ESCRT pathway. Cell Host Microbe 2013; 14(3): 232–41. 10.1016/j.chom.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dimitrov DS. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol 2004; 2(2): 109–22. 10.1038/nrmicro817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baltimore D. Expression of animal virus genomes. Bacteriol Rev 1971; 35(3): 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brandenburg B, Zhuang X. Virus trafficking – learning from single-virus tracking. Nat Rev Microbiol 2007; 5(3): 197–208. 10.1038/nrmicro1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22(2): 240–73. 10.1128/CMR.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow J, Franz KM, Kagan JC. PRRs are watching you: localization of innate sensing and signaling regulators. Virology 2015; 479-480: 104–9. 10.1016/j.virol.2015.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34(5): 637–50. 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 36. Kell AM, Gale M Jr. RIG-I in RNA virus recognition. Virology 2015; 479-480: 110–21. 10.1016/j.virol.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bermejo-Jambrina M, Eder J, Helgers LCet al. . C-Type lectin receptors in antiviral immunity and viral escape. Front Immunol 2018; 9: 1–12. 10.3389/fimmu.2018.00590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol 2017; 44: 43–51. 10.1016/j.coi.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vono M, Lin A, Norrby-Teglund Aet al. . Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017; 129(14): 1991–2001. 10.1182/blood-2016-10-744441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naumenko V, Turk M, Jenne CNet al. . Neutrophils in viral infection. Cell Tissue Res 2018; 371(3): 505–16. 10.1007/s00441-017-2763-0 [DOI] [PubMed] [Google Scholar]
- 41. Malmgaard L, Melchjorsen J, Bowie AGet al. . Viral activation of macrophages through TLR-dependent and -independent pathways. J Immunol 2004; 173(11): 6890–8. 10.4049/jimmunol.173.11.6890 [DOI] [PubMed] [Google Scholar]
- 42. Nikitina E, Larionova I, Choinzonov Eet al. . Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci 2018; 19(9): 1–25. 10.3390/ijms19092821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bai F, Kong KF, Dai Jet al. . A paradoxical role for neutrophils in the pathogenesis of West Nile Virus. J Infect Dis 2010; 202(12): 1804–12. 10.1086/657416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maucourant C, Petitdemange C, Yssel Het al. . Control of acute arboviral infection by natural killer cells. Viruses 2019; 11(2): 1–15. 10.3390/v11020131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gondois-Rey F, Chéret A, Mallet Fet al. . A mature NK profile at the time of HIV primary infection is associated with an early response to cART. Front Immunol 2017; 8: 1–12. 10.3389/fimmu.2017.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ali A, Gyurova, IE, Waggoner SN. Mutually assured destruction: the cold war between viruses and natural killer cells. Curr Opin Virol 2019; 34: 130–9. 10.1016/j.coviro.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tay MZ, Poh CM, Rénia L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20(6): 363–74. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schönrich G, Raftery MJ. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol 2019; 9: 1–14. 10.3389/fcimb.2019.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lamichhane PP, Samarasinghe AE. The role of innate leukocytes during influenza virus infection. J Immunol Res 2019; 2019: 1–17. 10.1155/2019/8028725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nunes RAL, Morale MG, Silva GÁFet al. . Innate immunity and HPV: friends or foes. Clinics 2018; 73: 1–7. 10.6061/clinics/2018/e549s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28(1): 445–89. 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zuniga EI, Macal M, Lewis GMet al. . Innate and adaptive immune regulation during chronic viral infections. Annu Rev Virol 2015; 2(1): 573–597. 10.1146/annurev-virology-100114-055226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rios DA, Valva P, Casciato PCet al. . Chronic hepatitis C liver microenvironment: role of the Th17/Treg interplay related to fibrogenesis. Sci Rep 2017; 7(1): 1–9. 10.1038/s41598-017-13777-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lan YT, Wang ZI, Tian Pet al. . Treg/Th17 imbalance and its clinical significance in patients with hepatitis B-associated liver cirrhosis. Diagn Pathol 2019; 14(1): 1–9. 10.1186/s13000-019-0891-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mangodt TC, Van Herck MA, Nullens Set al. . The role of Th17 and Treg responses in the pathogenesis of RSV infection. Pediatr Res 2015; 78(5): 483–91. 10.1038/pr.2015.143 [DOI] [PubMed] [Google Scholar]
- 56. Valverde-Villegas JM, Matte MCC, Medeiros RMet al. . New insights about Treg and Th17 cells in HIV infection and disease progression. J Immunol Res 2015; 2015: 1–14. 10.1155/2015/647916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duan S, Thomas PG. Balancing immune protection and immune pathology by CD8+ T-Cell responses to influenza infection. Front Immunol 2016; 7: 1–16. 10.3389/fimmu.2016.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wherry EJ, Blattman JN, Murali-Krishna Ket al. . Viral persistence alters CD8 T-Cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003; 77(8): 4911–27. 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mazzini L, Martinuzzi D, Hyseni Iet al. . Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J Immunol Methods 2021; 489: 1–10. 10.1016/j.jim.2020.112937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lam JH, Smith FL, Baumgarth N. B cell activation and response regulation during viral infections. Viral Immunol 2020; 33(4): 294–306. 10.1089/vim.2019.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Agrawal P, Nawadkar R, Ojha Het al. . Complement evasion strategies of viruses: an overview. Front Microbiol 2017; 8: 1–19. 10.3389/fmicb.2017.01117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ikeda F, Haraguchi Y, Jinno Aet al. . Human complement component C1q inhibits the infectivity of cell-free HTLV-I. J Immunol 1998; 161(10): 5712–19. [PubMed] [Google Scholar]
- 63. Ip WKE, Chan KH, Law HKet al. . Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis 2005; 191(10): 1697–704. 10.1086/429631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Avirutnan P, Hauhart RE, Marovich MAet al. . Complement-mediated neutralization of dengue virus requires mannose-binding lectin. mBio 2011; 2(6): 1–11. 10.1128/mBio.00276-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moris A, Pereira M, Chakrabarti L. A role for antibodies in natural HIV control. Curr Opin HIV AIDS 2019; 14(4): 265–72. 10.1097/COH.0000000000000554 [DOI] [PubMed] [Google Scholar]
- 66. Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol 2019; 9: 1–18. 10.3389/fonc.2019.00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Collins DR, Gaiha GD, Walker BD. CD8 + T cells in HIV control, cure and prevention. Nat Rev Immunol 2020; 20(8): 471–82. 10.1038/s41577-020-0274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saxena SK, Saxena S, Saxena Ret al. . Emerging trends, challenges and prospects in antiviral therapeutics and drug development for infectious diseases. Electron J Biol 2010; 6: 26–31. [Google Scholar]
- 69. Lou Z, Sun Y, Rao Z. Current progress in antiviral strategies. Trends Pharmacol Sci 2014; 35(2): 86–102. 10.1016/j.tips.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Giacomelli A, de Rose S, Rusconi S. Clinical pharmacology in HIV cure research – what impact have we seen? Expert Rev Clin Pharmacol 2019; 12(1): 17–29. 10.1080/17512433.2019.1561272 [DOI] [PubMed] [Google Scholar]
- 71. Xie T, Song S, Li Set al. . Review of natural product databases. Cell Prolif 2015; 48(4): 398–404. 10.1111/cpr.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Silva-Beltrán NP, Balderrama-Carmona AP, Umsza-Guez MAet al. . Antiviral effects of Brazilian green and red propolis extracts on Enterovirus surrogates. Environ Sci Pollut Res 2020; 27(23): 28510–7. 10.1007/s11356-019-07458-z [DOI] [PubMed] [Google Scholar]
- 73. Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol 1998; 36(4): 347–63. 10.1016/S0278-6915(97)00145-2 [DOI] [PubMed] [Google Scholar]
- 74. Bankova V. Recent trends and important developments in propolis research. Evid Based Complement Altern Med 2005; 2: 29–32. 10.1093/ecam/neh059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Altern Med 2015; 2015: 1–29. 10.1155/2015/206439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ghisalberti EL. Propolis: a review. Bee World 1979; 60(2): 59–84. 10.1080/0005772X.1979.11097738 [DOI] [Google Scholar]
- 77. Salatino A, Teixeira ÉW, Negri G. Origin and chemical variation of Brazilian propolis. Evid Based Complement Altern Med 2005; 2(1): 33–8. 10.1093/ecam/neh060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Braakhuis A. Evidence on the health benefits of supplemental propolis. Nutrients 2019; 11(11): 1–15. 10.3390/nu11112705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bankova VS, Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie 2000; 31(1): 3–15. 10.1051/apido:2000102 [DOI] [Google Scholar]
- 80. Huang S, Zhang CP, Wang Ket al. . Recent advances in the chemical composition of propolis. Molecules 2014; 19(12): 19610–32. 10.3390/molecules191219610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Castro SL. Propolis: biological and pharmacological activities. Therapeutic uses of this bee-product. Annu Rev Biomed Sci 2006; 3(0): 49–83. 10.5016/1806-8774.2001v3p49 [DOI] [Google Scholar]
- 82. Bankova V, Boudourova-Krasteva G, Popov Set al. . Seasonal variations of the chemical composition of Brazilian propolis. Apidologie 1998; 29(4): 361–7. 10.1051/apido:19980406 [DOI] [Google Scholar]
- 83. Boudourova-Krasteva G, Bankova V, Sforcin JMet al. . Phenolics from Brazilian propolis. Z Naturforsch, C 1997; 52(9–10): 676–9. 10.1515/znc-1997-9-1016 [DOI] [Google Scholar]
- 84. Cunha IBS, Sawaya ACHF, Caetano FMet al. . Factors that influence the yield and composition of Brazilian propolis extracts. J Braz Chem Soc 2004; 15(6): 964–70. 10.1590/S0103-50532004000600026 [DOI] [Google Scholar]
- 85. Conti BJ, Bankova V, Sforcin JM. Chemical composition of the same Brazilian propolis sample analyzed in 1997 and in 2012: no freezing effect. Nat Prod Commun 2015; 10(7): 1279–80. 10.1177/1934578X1501000736 [DOI] [PubMed] [Google Scholar]
- 86. Anjum SI, Ullah A, Khan KAet al. . Composition and functional properties of propolis (bee glue): a review. Saudi J Biol Sci 2019; 26(7): 1695–703. 10.1016/j.sjbs.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Helfenberg, KD. The analysis of beeswax and propolis. Chem Ztg 1908; 31: 987–98. [Google Scholar]
- 88. Orsatti CL, Missima F, Pagliarone ACet al. . Propolis immunomodulatory action in vivo on Toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phytother Res 2010; 24(8): 1141–6. 10.1002/ptr.3086 [DOI] [PubMed] [Google Scholar]
- 89. Búfalo MC, Bordon‐Graciani AP, Conti BJet al. . The immunomodulatory effect of propolis on receptors expression, cytokine production and fungicidal activity of human monocytes. J Pharm Pharmacol 2014; 66(10): 1497–504. 10.1111/jphp.12279 [DOI] [PubMed] [Google Scholar]
- 90. Conti BJ, Santiago KB, Búfalo MCet al. . Modulatory effects of propolis samples from Latin America (Brazil, Cuba and Mexico) on cytokine production by human monocytes. J Pharm Pharmacol 2015; 67(10): 1431–8. 10.1111/jphp.12431 [DOI] [PubMed] [Google Scholar]
- 91. Conti BJ, Santiago KB, Cardoso EOet al. . Propolis modulates miRNAs involved in TLR-4 pathway, NF-κB activation, cytokine production and in the bactericidal activity of human dendritic cells. J Pharm Pharmacol 2016; 68(12): 1604–12. 10.1111/jphp.12628 [DOI] [PubMed] [Google Scholar]
- 92. Santiago KB, Conti BJ, Cardoso EOet al. . Immunomodulatory/anti-inflammatory effects of a propolis-containing mouthwash on human monocytes. Pathog Dis 2016; 74(8): ftw081. 10.1093/femspd/ftw081 [DOI] [PubMed] [Google Scholar]
- 93. Cardoso EO, Conti BJ, Santiago KBet al. . Phenolic compounds alone or in combination may be involved in propolis effects on human monocytes. J Pharm Pharmacol 2017; 69(1): 99–108. 10.1111/jphp.12660 [DOI] [PubMed] [Google Scholar]
- 94. Rocha BA, Bueno PCP, Vaz MMOLLet al. . Evaluation of a propolis water extract using a reliable RP-HPLC methodology and in vitro and in vivo efficacy and safety characterisation. Evid Based Complement Altern Med 2013; 2013: 1–12. 10.1155/2013/670451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sforcin JM, Novelli ELB, Funari SRC. Absence of seasonal effect on the immunomodulatory action of Brazilian propolis on natural killer activity. Journal of Venomous Animals and Toxins 2002; 8(1): 19–29. 10.1590/S0104-79302002000100003 [DOI] [Google Scholar]
- 96. Kumari S, Nayak G, Lukose STet al. . Indian propolis ameliorates the mitomycin C-induced testicular toxicity by reducing DNA damage and elevating the antioxidant activity. Biomed Pharmacother 2017; 95: 252–63. 10.1016/j.biopha.2017.08.065 [DOI] [PubMed] [Google Scholar]
- 97. Rizk SM, Zaki HF, Mina MA. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem Toxicol 2014; 67: 176–86. 10.1016/j.fct.2014.02.031 [DOI] [PubMed] [Google Scholar]
- 98. Ulusoy HB, Öztürk İ, Sönmez MF. Protective effect of propolis on methotrexate-induced kidney injury in the rat. Ren Fail 2016; 38(5): 744–50. 10.3109/0886022X.2016.1158070 [DOI] [PubMed] [Google Scholar]
- 99. Abdul-Hamid M, Salah M. Intervention of ginger or propolis ameliorates methotrexate-induced ileum toxicity. Toxicol Ind Health 2016; 32(2): 313–22. 10.1177/0748233713500833 [DOI] [PubMed] [Google Scholar]
- 100. Nirala SK, Bhadauria M. Propolis reverses acetaminophen induced acute hepatorenal alterations: a biochemical and histopathological approach. Arch Pharm Res 2008; 31(4): 451–61. 10.1007/s12272-001-1178-5 [DOI] [PubMed] [Google Scholar]
- 101. Büyükberber M, Savaş MC, Bağci Cet al. . The beneficial effect of propolis on cerulein-induced experimental acute pancreatitis in rats. Turk J Gastroenterol 2009; 20: 122–8. [PubMed] [Google Scholar]
- 102. Ahmed KM, Saleh EM, Sayed EMet al. . Anti-inflammatory effect ofdifferent propolis extracts in thioacetamide-induced hepatotoxicity in male rat. Aust J Basic Appl Sci 2012; 6: 29–40. [Google Scholar]
- 103. Bhadauria M. Propolis prevents hepatorenal injury induced by chronic exposure to carbon tetrachloride. Evid Based Complement Altern Med 2012; 2012: 1–12. 10.1155/2012/235358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. El-Sharkawy EE, Kames AO, Sayed SMet al. . The ameliorative effect of propolis against methoxychlor induced ovarian toxicity in rat. Exp Toxicol Pathol 2014; 66(9): 415–21. 10.1016/j.etp.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 105. Ercis K, Aydoğan S, Atayoğlu ATet al. . Effect of propolis on erythrocyte rheology in experimental mercury intoxication in rats. Environ Sci Pollut Res 2015; 22(16): 12534–43. 10.1007/s11356-015-4512-9 [DOI] [PubMed] [Google Scholar]
- 106. Baykara M, Silici S, Özçelik Met al. . In vivo nephroprotective efficacy of propolis against contrast-induced nephropathy. Diagn Interv Radiol 2015; 21(4): 317–21. 10.5152/dir.2015.14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. El Menyiy N, Al Waili N, Bakour Met al. . Protective effect of propolis in proteinuria, crystaluria, nephrotoxicity and hepatotoxicity induced by ethylene glycol ingestion. Arch Med Res 2016; 47(7): 526–34. 10.1016/j.arcmed.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 108. Aldahmash BA, El-Nagar DM, Ibrahim KE. Reno-protective effects of propolis on gentamicin-induced acute renal toxicity in swiss albino mice. Nefrología 2016; 36(6): 643–52. 10.1016/j.nefroe.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 109. El Menyiy N, Al-Waili N, El Ghouizi Aet al. . Evaluation of antiproteinuric and hepato-renal protective activities of propolis in paracetamol toxicity in rats. Nutr Res Pract 2018; 12(6): 535–40. 10.4162/nrp.2018.12.6.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nna VU, Bakar ABA, Mohamed M. Malaysian propolis, metformin and their combination, exert hepatoprotective effect in streptozotocin-induced diabetic rats. Life Sci 2018; 211: 40–50. 10.1016/j.lfs.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 111. Omar NA, Abu-Almaaty AH, Abd El-Aziz YMet al. . Impacts of Egyptian propolis extract on rat cerebellum intoxicated by aluminum silicate: histopathological studies. Environ Sci Pollut Res 2019; 26(21): 22061–8. 10.1007/s11356-019-05469-4 [DOI] [PubMed] [Google Scholar]
- 112. Yuluğ E, Türedi S, Yıldırım Öet al. . Biochemical and morphological evaluation of the effects of propolis on cisplatin induced kidney damage in rats. Biotech Histochem 2019; 94(3): 204–13. 10.1080/10520295.2018.1543895 [DOI] [PubMed] [Google Scholar]
- 113. Fikri AM, Sulaeman A, Handharyani Eet al. . The effect of propolis administration on fetal development. Heliyon 2019; 5(10): 1–7. 10.1016/j.heliyon.2019.e02672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mohammadzadeh S, Shariatpanahi M, Hamedi Met al. . Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem 2007; 103(4): 1097–103. 10.1016/j.foodchem.2006.10.006 [DOI] [Google Scholar]
- 115. Mani F, Damasceno HCR, Novelli ELBet al. . Propolis: effect of different concentrations, extracts and intake period on seric biochemical variables. J Ethnopharmacol 2006; 105(1): 95–8. 10.1016/j.jep.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 116. Fukuda T, Fukui M, Tanaka Met al. . Effect of Brazilian green propolis in patients with type 2 diabetes: a double-blind randomized placebo-controlled study. Biomed Rep 2015; 3(3): 355–60. 10.3892/br.2015.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Samadi N, Mozaffari-Khosravi H, Rahmanian Met al. . Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: a randomized, double-blind clinical trial. J Integr Med 2017; 15(2): 124–34. 10.1016/S2095-4964(17)60315-7 [DOI] [PubMed] [Google Scholar]
- 118. Afsharpour F, Hashemipour S, Khadem-Haghighian Het al. . Effects of Iranian propolis on glycemic status, inflammatory factors, and liver enzyme levels in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. JNSD 2017; 3: 9–14. [Google Scholar]
- 119. Zakerkish M, Jenabi M, Zaeemzadeh Net al. . The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Sci Rep 2019; 9(1): 1–11. 10.1038/s41598-019-43838-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Silveira MAD, Teles F, Berretta AAet al. . Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. BMC Nephrol 2019; 20(1): 1–12. 10.1186/s12882-019-1337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Koo HJ, Lee KR, Kim HSet al. . Detoxification effects of aloe polysaccharide and propolis on the urinary excretion of metabolites in smokers. Food Chem Toxicol 2019; 130: 99–108. 10.1016/j.fct.2019.05.029 [DOI] [PubMed] [Google Scholar]
- 122. Mujica V, Orrego R, Pérez Jet al. . The role of propolis in oxidative stress and lipid metabolism: a randomized controlled trial. Evid Based Complement Altern Med 2017; 2017: 1–11. 10.1155/2017/4272940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Oryan A, Alemzadeh E, Moshiri A. Potential role of propolis in wound healing: biological properties and therapeutic activities. Biomed Pharmacother 2018; 98: 469–83. 10.1016/j.biopha.2017.12.069 [DOI] [PubMed] [Google Scholar]
- 124. Münstedt K, Kalder M. Contact allergy to propolis in beekeepers. Allergol Immunopathol (Madr) 2009; 37(6): 298–301. 10.1016/j.aller.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 125. Gulbahar O, Ozturk G, Erdem Net al. . Psoriasiform contact dermatitis due to propolis in a beekeeper. Ann Allergy Asthma Immunol 2005; 94(4): 509–11. 10.1016/S1081-1206(10)61123-4 [DOI] [PubMed] [Google Scholar]
- 126. Francuzik W, Geier J, Schubert Set al. . A case-control analysis of skin contact allergy in children and adolescents. Pediatr Allergy Immunol 2019; 30(6): 632–7. 10.1111/pai.13069 [DOI] [PubMed] [Google Scholar]
- 127. Pereira RF, Bártolo PJ. Traditional therapies for skin wound healing. Adv Wound Care. 2016; 5(5):208–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nyman GSA, Tang M, Inerot Aet al. . Contact allergy to beeswax and propolis among patients with cheilitis or facial dermatitis. Contact Dermatitis 2019; 81(2): 110–6. 10.1111/cod.13306 [DOI] [PubMed] [Google Scholar]
- 129. Cho E, Lee JD, Cho SH. Systemic contact dermatitis from propolis ingestion. Ann Dermatol 2011; 23(1): 85–8. 10.5021/ad.2011.23.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shi Y, Nedorost S, Scheman Let al. . Propolis, colophony, and fragrance cross-reactivity and allergic contact dermatitis. Dermatitis 2016; 27(3): 123–6. 10.1097/DER.0000000000000186 [DOI] [PubMed] [Google Scholar]
- 131. Freedman J, Griggs J, De Padova MPet al. . What’s the “buzz” about propolis? Propolis-induced systemic contact dermatitis. Contact Dermatitis 2019; 80(1): 65–7. 10.1111/cod.13131 [DOI] [PubMed] [Google Scholar]
- 132. Daleprane JB, Abdalla DS. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. Evid Based Complement Altern Med 2013; 2013: 1–8. 10.1155/2013/175135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Su KY, Hsieh CY, Chen YWet al. . Taiwanese green propolis and propolin G protect the liver from the pathogenesis of fibrosis via eliminating TGF-β-induced Smad2/3 phosphorylation. J Agric Food Chem 2014; 62(14): 3192–201. 10.1021/jf500096c [DOI] [PubMed] [Google Scholar]
- 134. Yangi B, Cengiz Ustuner M, Dincer Met al. . Propolis protects endotoxin induced acute lung and liver inflammation through attenuating inflammatory responses and oxidative stress. J Med Food 2018; 21(11): 1096–105. 10.1089/jmf.2017.0151 [DOI] [PubMed] [Google Scholar]
- 135. Costa P, Somensi LB, Silva RCMVAFet al. . Role of the antioxidant properties in the gastroprotective and gastric healing activity promoted by Brazilian green propolis and the healing efficacy of artepillin C. Inflammopharmacol 2020; 28(4): 1009–25. 10.1007/s10787-019-00649-7 [DOI] [PubMed] [Google Scholar]
- 136. Cusinato DAC, Martinez EZ, Cintra MTet al. . Evaluation of potential herbal-drug interactions of a standardized propolis extract (EPP-AF®) using an in vivo cocktail approach. J Ethnopharmacol 2019; 245: 1–10. 10.1016/j.jep.2019.112174 [DOI] [PubMed] [Google Scholar]
- 137. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 2011; 133(2): 253–60. 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 138. Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol 2007; 113(1): 1–14. 10.1016/j.jep.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 139. Orsi RO, Funari SRC, Soares AMVCet al. . Immunomodulatory action of propolis on macrophage activation. J Venom Anim Toxins 2000; 6(2): 205–19. 10.1590/S0104-79302000000200006 [DOI] [Google Scholar]
- 140. Bachiega TF, Orsatti CL, Pagliarone ACet al. . The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phytother Res 2012; 26(9): 1308–13. 10.1002/ptr.3731 [DOI] [PubMed] [Google Scholar]
- 141. Murad JM, Calvi SA, Soares AMVCet al. . Effects of propolis from Brazil and Bulgaria on fungicidal activity of macrophages against Paracoccidioides brasiliensis. J Ethnopharmacol 2002; 79(3): 331–4. 10.1016/S0378-8741(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 142. Orsi RO, Sforcin JM, Funari SRCet al. . Effects of Brazilian and Bulgarian propolis on bactericidal activity of macrophages against Salmonella Typhimurium. Int Immunopharmacol 2005; 5(2): 359–68. 10.1016/j.intimp.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 143. Tanaka M, Okamoto Y, Fukui Tet al. . Suppression of interleukin 17 production by Brazilian propolis in mice with collagen-induced arthritis. Inflammopharmacol 2012; 20(1): 19–26. 10.1007/s10787-011-0088-2 [DOI] [PubMed] [Google Scholar]
- 144. Okamoto Y, Tanaka M, Fukui Tet al. . Brazilian propolis inhibits the differentiation of Th17 cells by inhibition of interleukin-6-induced phosphorylation of signal transducer and activator of transcription 3. Immunopharmacol Immunotoxicol 2012; 34(5): 803–9. 10.3109/08923973.2012.657304 [DOI] [PubMed] [Google Scholar]
- 145. Piñeros AR, Lima MH, Rodrigues Tet al. . Green propolis increases myeloid suppressor cells and CD4+Foxp3+ cells and reduces Th2 inflammation in the lungs after allergen exposure. J Ethnopharmacol 2020; 252: 1–9. 10.1016/j.jep.2019.112496 [DOI] [PubMed] [Google Scholar]
- 146. Lima LD, Andrade SP, Campos PPet al. . Brazilian green propolis modulates inflammation, angiogenesis and fibrogenesis in intraperitoneal implant in mice. BMC Complement Altern Med 2014; 14(1): 1–9. 10.1186/1472-6882-14-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Miranda MM, Panis C, Cataneo AHDet al. . Nitric oxide and Brazilian propolis combined accelerates tissue repair by modulating cell migration, cytokine production and collagen deposition in experimental leishmaniasis. PLoS ONE 2015; 10(5): 1–19. 10.1371/journal.pone.0125101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sá-Nunes A, Faccioli LH, Sforcin JM. Propolis: lymphocyte proliferation and IFN-γ production. J Ethnopharmacol 2003; 87(1): 93–7. 10.1016/S0378-8741(03)00121-1 [DOI] [PubMed] [Google Scholar]
- 149. Missima F, Pagliarone AC, Orsatti CLet al. . The effect of propolis on Th1/Th2 cytokine expression and production by melanoma-bearing mice submitted to stress. Phytother Res 2010; 24(10): 1501–7. 10.1002/ptr.3142 [DOI] [PubMed] [Google Scholar]
- 150. Missima F, Sforcin JM. Green Brazilian propolis action on macrophages and lymphoid organs of chronically stressed mice. Evid Based Complement Altern Med 2008; 5(1): 71–5. 10.1093/ecam/nel112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Thomazelli APFS, Tomiotto-Pellissier F, Silva SSet al. . Brazilian propolis promotes immunomodulation on human cells from American Tegumentar Leishmaniasis patients and healthy donors infected with L. braziliensis. Cell Immunol 2017; 311: 22–7. 10.1016/j.cellimm.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 152. Touzani S, Embaslat W, Imtara Het al. . In vitro evaluation of the potential use of propolis as a multitarget therapeutic product: physicochemical properties, chemical composition, and immunomodulatory, antibacterial, and anticancer properties. Biomed Res Int 2019; 2019: 1–11. 10.1155/2019/4836378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Scheller S, Gazda G, Pietsz Get al. . The ability of ethanol extract of propolis to stimulate plaque formation in immunized mouse spleen cells. Pharmacol Res Commun 1988; 20(4): 323–8. 10.1016/S0031-6989(88)80068-7 [DOI] [PubMed] [Google Scholar]
- 154. Park JH, Lee JK, Kim HSet al. . Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int Immunopharmacol 2004; 4(3): 429–36. 10.1016/j.intimp.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 155. Sforcin JM, Orsi RO, Bankova V. Effect of propolis, some isolated compounds and its source plant on antibody production. J Ethnopharmacol 2005; 98(3): 301–5. 10.1016/j.jep.2005.01.042 [DOI] [PubMed] [Google Scholar]
- 156. Chu WH. Adjuvant effect of propolis on immunisation by inactivated Aeromonas hydrophila in carp (Carassius auratus gibelio). Fish Shellfish Immunol 2006; 21(1): 113–7. 10.1016/j.fsi.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 157. Fischer G, Conceição FR, Leite FPLet al. . Immunomodulation produced by a green propolis extract on humoral and cellular responses of mice immunized with SuHV-1. Vaccine 2007; 25(7): 1250–6. 10.1016/j.vaccine.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 158. Sena-Lopes Â, Bezerra FSB, Neves RNet al. . Chemical composition, immunostimulatory, cytotoxic and antiparasitic activities of the essential oil from Brazilian red propolis. PLoS ONE 2018; 13(2): 1–16. 10.1371/journal.pone.0191797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bezerra FSB, Rezende AFS, Silva MTOet al. . The combination of Brazilian red propolis and recombinant protein rCP01850 in the immunoprophylaxis of Corynebacterium pseudotuberculosis infection in mice. Microb Pathog 2020; 149: 1–6. 10.1016/j.micpath.2020.104354 [DOI] [PubMed] [Google Scholar]
- 160. Fan Y, Liu J, Wang Det al. . Epimedium polysaccharide and propolis flavone can synergistically inhibit the cellular infectivity of NDV and improve the curative effect of ND in chicken. Int J Biol Macromol 2011; 48(3): 439–44. 10.1016/j.ijbiomac.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 161. Peng J, Yuan Y, Shen Set al. . Immunopotentiation of four natural adjuvants co-administered with a highly pathogenic porcine reproductive and respiratory syndrome virus glycoprotein 5 subunit. Virus Genes 2016; 52(2): 261–9. 10.1007/s11262-016-1299-9 [DOI] [PubMed] [Google Scholar]
- 162. Khayyal MT, El‐Ghazaly MA, El‐Khatib ASet al. . A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam Clin Pharmacol 2003; 17(1): 93–102. 10.1046/j.1472-8206.2003.00117.x [DOI] [PubMed] [Google Scholar]
- 163. Hori JI, Zamboni DS, Carrão DBet al. . The inhibition of inflammasome by Brazilian propolis (EPP-AF). Evid Based Complement Altern Med 2013; 2013: 1–11. 10.1155/2013/418508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Bueno-Silva B, Kawamoto D, Ando-Suguimoto ESet al. . Brazilian Red propolis attenuates inflammatory signaling cascade in LPS-activated macrophages. PLoS ONE 2015; 10(12): 1–14. 10.1371/journal.pone.0144954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Franchin M, Freires IA, Lazarini JGet al. . The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur J Med Chem 2018; 153: 49–55. 10.1016/j.ejmech.2017.06.050 [DOI] [PubMed] [Google Scholar]
- 166. Min BS, Noh YJ, Shin JHet al. . Assessment of the quantitative real-time polymerase chain reaction using a cDNA standard for human group A rotavirus. J Virol Methods 2006; 137(2): 280–6. 10.1016/j.jviromet.2006.06.028 [DOI] [PubMed] [Google Scholar]
- 167. Food and Drug Administration (FDA). Guidance for Industry; Antiviral Product Development—Conducting and Submitting Virology Studies to the Agency. Rockville, MD: United States Department of Health and Human Services, Rockville, MD. 2006: 1–14. [Google Scholar]
- 168. Amoros M, Simões CM, Girre Let al. . Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J Nat Prod 1992; 55(12): 1732–40. 10.1021/np50090a003 [DOI] [PubMed] [Google Scholar]
- 169. Amoros M, Lurton E, Boustie Jet al. . Comparison of the anti-herpes simplex virus activities of propolis and 3-methyl-but-2-enyl caffeate. J Nat Prod 1994; 57(5): 644–7. 10.1021/np50107a013 [DOI] [PubMed] [Google Scholar]
- 170. Nolkemper S, Reichling J, Sensch KHet al. . Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine 2010; 17(2): 132–8. 10.1016/j.phymed.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 171. Schnitzler P, Neuner A, Nolkemper Set al. . Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res 2010; 24(S1): S20–8. 10.1002/ptr.2868 [DOI] [PubMed] [Google Scholar]
- 172. Bankova V, Galabov AS, Antonova Det al. . Chemical composition of propolis extract ACF® and activity against herpes simplex virus. Phytomedicine 2014; 21(11): 1432–8. 10.1016/j.phymed.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 173. Serkedjieva J, Manolova N, Bankova Vet al. . Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids). J Nat Prod 1992; 55(3): 294–7. 10.1021/np50081a003 [DOI] [PubMed] [Google Scholar]
- 174. Kujumgiev A, Tsvetkova I, Serkedjieva Yet al. . Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol 1999; 64(3): 235–40. 10.1016/S0378-8741(98)00131-7 [DOI] [PubMed] [Google Scholar]
- 175. Shimizu T, Hino A, Tsutsumi Aet al. . Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antivir Chem Chemother 2008; 19(1): 7–13. 10.1177/095632020801900102 [DOI] [PubMed] [Google Scholar]
- 176. Governa P, Cusi MG, Borgonetti Vet al. . Beyond the biological effect of a chemically characterized poplar propolis: antibacterial and antiviral activity and comparison with flurbiprofen in cytokines release by LPS-stimulated human mononuclear cells. Biomedicines 2019; 7(4): 1–10. 10.3390/biomedicines7040073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ito J, Chang FR, Wang HKet al. . Anti-AIDS Agents. 48. Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J Nat Prod 2001; 64(10): 1278–81. 10.1021/np010211x [DOI] [PubMed] [Google Scholar]
- 178. Gekker G, Hu S, Spivak Met al. . Anti-HIV-1 activity of propolis in CD4+ lymphocyte and microglial cell cultures. J Ethnopharmacol 2005; 102(2): 158–63. 10.1016/j.jep.2005.05.045 [DOI] [PubMed] [Google Scholar]
- 179. Búfalo MC, Figueiredo AS, Sousa JPBet al. . Anti-poliovirus activity of Baccharis dracunculifolia and propolis by cell viability determination and real-time PCR. J Appl Microbiol 2009; 107(5): 1669–80. 10.1111/j.1365-2672.2009.04354.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. El Hady FKA, Hegazi AG. Egyptian Propolis: 2. Chemical composition, antiviral and antimicrobial activities of East Nile Delta propolis. Z Naturforsch C 2002; 57(3–4): 386–94. 10.1515/znc-2002-3-431 [DOI] [PubMed] [Google Scholar]
- 181. El Hady FKA, Hegazi AG, Wollenweber E. Effect of Egyptian propolis on the susceptibility of LDL to oxidative modification and its antiviral activity with special emphasis on chemical composition. Z Naturforsch C 2007; 62(9–10): 645–55. 10.1515/znc-2007-9-1004 [DOI] [PubMed] [Google Scholar]
- 182. González-Búrquez MJ, González-Díaz FR, García-Tovar CGet al. . Comparison between in vitro antiviral effect of Mexican propolis and three commercial flavonoids against canine distemper virus. Evid Based Complement Altern Med 2018; 2018: 1–9. 10.1155/2018/7092416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yildirim A, Duran GG, Duran Net al. . Antiviral Activity of Hatay propolis against replication of Herpes Simplex Virus type 1 and type 2. Med Sci Monit 2016; 22: 422–30. 10.12659/MSM.897282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Drago L, De Vecchi E, Nicola Let al. . In vitro antimicrobial activity of a novel propolis formulation (Actichelated propolis). J Appl Microbiol 2007; 103(5): 1914–21. 10.1111/j.1365-2672.2007.03421.x [DOI] [PubMed] [Google Scholar]
- 185. Ma X, Guo Z, Zhang Zet al. . Ferulic acid isolated from propolis inhibits porcine parvovirus replication potentially through Bid-mediate apoptosis. Int Immunopharmacol 2020; 83: 106379. 10.1016/j.intimp.2020.106379 [DOI] [PubMed] [Google Scholar]
- 186. Ma X, Guo ZShen Zet al. . The anti-porcine parvovirus activity of nanometer propolis flavone and propolis flavone in vitro and in vivo. Evid Based Complement Altern Med 2015; 2015: 1–10. 10.1155/2015/472876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Takeshita T, Watanabe W, Toyama Set al. . Effect of Brazilian propolis on exacerbation of respiratory syncytial virus infection in mice exposed to tetrabromobisphenol A, a brominated flame retardant. Evid Based Complement Altern Med 2013; 2013: 1–9. 10.1155/2013/698206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Labská K, Plodková H, Pumannová Met al. . Antiviral activity of propolis special extract GH 2002 against Varicella zoster virus in vitro. Pharmazie 2018; 73(12): 733–6. 10.1691/ph.2018.8672 [DOI] [PubMed] [Google Scholar]
- 189. McQuillan G, Kruszon-Moran D, Flagg EWet al. . Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14-49: United States, 2015-2016. NCHS Data Brief 2018; ( 304): 1–7. [PubMed] [Google Scholar]
- 190. Huleihel M, Isanu V. Anti-herpes simplex virus effect of an aqueous extract of propolis. Isr Med Assoc J 2002; 4(11 Suppl): 923–7. [PubMed] [Google Scholar]
- 191. Mazia RS, Pereira RRA, Francisco LMBet al. . Formulation and evaluation of a mucoadhesive thermoresponsive system containing Brazilian green propolis for the treatment of lesions caused by herpes simplex type I. J Pharm Sci 2016; 105(1): 113–21. 10.1016/j.xphs.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 192. Sartori G, Pesarico AP, Pinton Set al. . Protective effect of brown Brazilian propolis against acute vaginal lesions caused by herpes simplex virus type 2 in mice: involvement of antioxidant and anti-inflammatory mechanisms. Cell Biochem Funct 2012; 30(1): 1–10. 10.1002/cbf.1810 [DOI] [PubMed] [Google Scholar]
- 193. Ohno M, Sekiya T, Nomura Net al. . Influenza virus infection affects insulin signaling, fatty acid-metabolizing enzyme expressions, and the tricarboxylic acid cycle in mice. Sci Rep 2020; 10(1): 1–12. 10.1038/s41598-020-67879-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Harish Z, Rubinstein A, Golodner Met al. . Suppression of HIV-1 replication by propolis and its immunoregulatory effect. Drugs Exp Clin Res 1997; 23(2): 89–96. [PubMed] [Google Scholar]
- 195. Shvarzbeyn J, Huleihel M. Effect of propolis and caffeic acid phenethyl ester (CAPE) on NFκB activation by HTLV-1 Tax. Antivir Res 2011; 90(3): 108–15. 10.1016/j.antiviral.2011.03.177 [DOI] [PubMed] [Google Scholar]
- 196. Dothel G, Vasina V, Barbara Get al. . Animal models of chemically induced intestinal inflammation: predictivity and ethical issues. Pharmacol Ther 2013; 139(1): 71–86. 10.1016/j.pharmthera.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 197. Yuan J, Liu J, Hu Yet al. . The immunological activity of propolis flavonoids liposome on the immune response against ND vaccine. Int J Biol Macromol 2012; 51(4): 400–5. 10.1016/j.ijbiomac.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 198. Vynograd N, Vynograd I, Sosnowski Z. A comparative multi-centre study of the efficacy of propolis, acyclovir and placebo in the treatment of genital herpes (HSV). Phytomedicine 2000; 7(1): 1–6. 10.1016/S0944-7113(00)80014-8 [DOI] [PubMed] [Google Scholar]
- 199. Hoheisel O. The effects of Herstat (3% propolis ointment ACF) application in cold sores: a double-blind placebo-controlled clinical trial. J Clin Diagnostic Res 2000; 4: 65–75. [Google Scholar]
- 200. Soroy L, Bagus S, Yongkie IPet al. . The effect of a unique propolis compound (PropoelixTM) on clinical outcomes in patients with dengue hemorrhagic fever. Infect Drug Resist 2014; 7: 323–9. 10.2147/IDR.S71505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Fernandes N. Economic Effects of Coronavirus Outbreak (COVID-19) on the World Economy. Rochester, NY: Social Science Research Network, 2020. 10.2139/ssrn.3557504 [DOI] [Google Scholar]
- 202. Zhang D, Hu M, Ji Q. Financial markets under the global pandemic of COVID-19. Finance Res Lett 2020; 36: 1–6. 10.1016/j.frl.2020.101528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Li R, Pei S, Chen Bet al. . Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020; 368(6490): 489–493. 10.1126/science.abb3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323(16): 1545–6. 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 205. Hellewell J, Abbott S, Gimma Aet al. . Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health 2020; 8(4): e488–96. 10.1016/S2214-109X(20)30074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Pfefferbaum B, North CS. Mental health and the COVID-19 pandemic. N Engl J Med 2020; 383(6): 510–2. 10.1056/NEJMp2008017 [DOI] [PubMed] [Google Scholar]
- 207. Cascella M, Rajnik M, Cuomo Aet al. . Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2020. Available at: http://www.ncbi.nlm.nih.gov/books/NBK554776/. Accessed September 14, 2020. [PubMed] [Google Scholar]
- 208. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020; 92(4): 418–23. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Shanmugaraj B, Siriwattananon K, Wangkanont Ket al. . Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol 2020; 38(1): 10–8. 10.12932/AP-200220-0773 [DOI] [PubMed] [Google Scholar]
- 210. Masters PS. The Molecular Biology of Coronaviruses. In: Advances in Virus Research, Vol 66. Cambridge, MA: Academic Press, 2006: 193–292. 10.1016/S0065-3527(06)66005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019; 17(3): 181–92. 10.1038/s41579-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 2020; 12(3): 1–15. 10.3390/v12030254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bleibtreu A, Bertine M, Bertin Cet al. . Focus on Middle East respiratory syndrome coronavirus (MERS-CoV). Med Mal Infect 2020; 50(3): 243–51. 10.1016/j.medmal.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Mohd HA, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol J 2016; 13(1): 1–7. 10.1186/s12985-016-0544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chafekar A, Fielding BC. MERS-CoV: understanding the latest human coronavirus threat. Viruses 2018; 10(2): 1–22. 10.3390/v10020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Yang Y, Peng F, Wang Ret al. . The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun 2020; 109: 1–16. 10.1016/j.jaut.2020.102434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wang D, Hu B, Hu Cet al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323(11): 1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Wang W, Xu Y, Gao Ret al. . Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323(18): 1843–4. 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Hsu LY, Chia PY, Lim JF. The novel coronavirus (SARS-CoV-2) pandemic. Ann Acad Med Singap 2020; 49(3): 105–7. [PubMed] [Google Scholar]
- 220. Sharma A, Tiwari S, Deb MKet al. . Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents 2020; 56(2): 1–13. 10.1016/j.ijantimicag.2020.106054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Yan R, Zhang Y, Li Yet al. . Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367(6485): 1444–8. 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Andersen KG, Rambaut A, Lipkin WIet al. . The proximal origin of SARS-CoV-2. Nat Med 2020; 26(4): 450–2. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Walls AC, Park YJ, Tortorici MAet al. . Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020; 181(2): 281–92.e6. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Perico L, Benigni A, Remuzzi G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. NEF 2020; 144(5): 213–21. 10.1159/000507305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Nicol T, Lefeuvre C, Serri Oet al. . Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J Clin Virol 2020; 129: 1–7. 10.1016/j.jcv.2020.104511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Robbiani DF, Gaebler G, Muecksch Fet al. . Convergent antibody responses to SARS-CoV-2 infection in convalescent individuals. Nature 2020; 584: 437–42. 10.1038/s41586-020-2456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Long QX, Tang XJ, Shi QLet al. . Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26(8): 1200–4. 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 228. Liu T, Wu S, Tao Het al. . Prevalence of IgG antibodies to SARS-CoV-2 in Wuhan - implications for the ability to produce long-lasting protective antibodies against SARS-CoV-2. medRxiv 2020: 2020.06.13.20130252. 10.1101/2020.06.13.20130252 [DOI] [Google Scholar]
- 229. Sekine T, Perez-Potti A, Rivera-Ballesteros Oet al. . Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020; 183: 158–68. 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. World Health Organization (WHO). Draft landscape of COVID-19 candidate vaccines. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed September 14, 2020.
- 231. Pagani L. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica 1990; 13: 207–13. [PubMed] [Google Scholar]
- 232. Bachevski D, Damevska K, Simeonovski Vet al. . Back to the basics: propolis and COVID-19. Dermatol Ther 2020; 33(4): 1–3. 10.1111/dth.13780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Van den Broeke C, Radu M, Chernoff Jet al. . An emerging role for p21-activated kinases (Paks) in viral infections. Trends Cell Biol 2010; 20(3): 160–9. 10.1016/j.tcb.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Osés SM, Marcos P, Azofra Pet al. . Phenolic profile, antioxidant capacities and enzymatic inhibitory activities of propolis from different geographical areas: needs for analytical harmonization. Antioxidants 2020; 9(1): 1–16. 10.3390/antiox9010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Kumar V, Dhanjal JK, Bhargava Pet al. . Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J Biomol Struct Dyn 2020; 16: 1–13. 10.1080/07391102.2020.1775704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Maruta H, He H. PAK1-blockers: potential therapeutics against COVID-19. Med Drug Discov 2020; 6: 1–5. 10.1016/j.medidd.2020.100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Orsi RO, Sforcin JM, Funari SRCet al. . Synergistic effect of propolis and antibiotics on the Salmonella Typhi. Braz J Microbiol 2006; 37(2): 108–12. 10.1590/S1517-83822006000200002 [DOI] [Google Scholar]
- 238. Orsi RO, Fernandes A Jr, Bankova Vet al. . Antibacterial effects of Brazilian and Bulgarian propolis and synergistic effects with antibiotics acting on the bacterial DNA and folic acid. Natural Product Research 2012; 26(4): 344–9. 10.1080/14786411003754355 [DOI] [PubMed] [Google Scholar]
- 239. Altındiş M, Aslan FG, Uzuner Het al. . [Comparison of antiviral effect of olive leaf extract and propolis with acyclovir on herpes simplex virus type 1]. Mikrobiyol Bul 2020; 54(1): 79–94. 10.5578/mb.69019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated in the course of the study or to third-party data analyzed in the article were properly cited.