Abstract
Background
Nearly a year into the COVID-19 pandemic, we still lack effective anti-SARS-CoV-2 drugs with substantial impact on mortality rates except for dexamethasone. As the search for effective antiviral agents continues, we aimed to review data on the potential of repurposing antiparasitic drugs against viruses in general, with an emphasis on coronaviruses.
Methods
We performed a review by screening in vitro and in vivo studies that assessed the antiviral activity of several antiparasitic agents: chloroquine, hydroxychloroquine (HCQ), mefloquine, artemisinins, ivermectin, nitazoxanide (NTZ), niclosamide, atovaquone and albendazole.
Results
For HCQ and chloroquine we found ample in vitro evidence of antiviral activity. Cohort studies that assessed the use of HCQ for COVID-19 reported conflicting results, but randomized controlled trials (RCTs) demonstrated no effect on mortality rates and no substantial clinical benefits of HCQ used either for prevention or treatment of COVID-19. We found two clinical studies of artemisinins and two studies of NTZ for treatment of viruses other than COVID-19, all of which showed mixed results. Ivermectin was evaluated in one RCT and few observational studies, demonstrating conflicting results. As the level of evidence of these data is low, the efficacy of ivermectin against COVID-19 remains to be proven. For chloroquine, HCQ, mefloquine, artemisinins, ivermectin, NTZ and niclosamide, we found in vitro studies showing some effects against a wide array of viruses. We found no relevant studies for atovaquone and albendazole.
Conclusions
As the search for an effective drug active against SARS-CoV-2 continues, we argue that pre-clinical research of possible antiviral effects of compounds that could have antiviral activity should be conducted. Clinical studies should be conducted when sufficient in vitro evidence exists, and drugs should be introduced into widespread clinical use only after being rigorously tested in RCTs. Such a search may prove beneficial in this pandemic or in outbreaks yet to come.
Keywords: Chloroquine, artemisinins, nitazoxanide, COVID-19, corona, SARS, viruses
Introduction
The COVID-19 pandemic has already incurred a shocking price in terms of lives lost, worldwide economic recession, political tensions and psychological stress.1,2 This pandemic’s effects on high-risk populations are still being studied.3 Since time is critical, and no treatment except dexamethasone has been proven to decrease mortality,4 there is now a worldwide rush to find an effective antiviral agent.
Repurposing of existing antivirals is ongoing but results from most studies thus far have been poor, with many agents showing promise in small groups of relatively young patients, who are less likely to succumb to COVID-19. The few controlled trials that have been performed are somewhat disappointing; the protease inhibitor lopinavir/ritonavir has failed to demonstrate clear superiority over a placebo in one randomized controlled trial (RCT). A combination of lopinavir, ritonavir, ribavirin and interferon beta-1b was found to be effective in alleviating symptoms and shortening viral shedding, but such complex regimen is unlikely to become a mainstay of treatment.5
Another antiviral compound, remdesivir, was initially shown to be modestly effective in shortening illness duration in an RCT.6 However, recent clinical trials including the World Health Organization solitary trail (that did not include a placebo group), did not show any mortality benefits.7,8 The drug is administered only intravenously, probably does not decrease mortality and is unavailable to most clinicians worldwide.
Under these circumstances, the use of older antiparasitic agents, based on in vitro antiviral activity, animal studies or preliminary data on COVID-19 patients, is an interesting option. In this review, we describe the available evidence on the activity of antiparasitic agents, including antimalarial drugs, on viruses and specifically on SARS-CoV-2 and reviewed interventional and observational studies that assessed the clinical effect of those therapies for COVID-19.
Methods
Type of studies
In this review, we screened in vivo and in vitro studies that focus on antiviral activities of several antiparasitic agents, including specific activity against COVID-19 and other coronavirus-related infections [Middle East respiratory syndrome (MERS), SARS and SARS-CoV-1]. Antiparasitic agents include HCQ, chloroquine, mefloquine, artemisinins, ivermectin, nitazoxanide (NTZ) and niclosamide, atovaquone and albendazole. The structures and uses of these drugs are given in Table 2. We included studies published during the COVID-19 pandemic in English. We have included RCTs, non-randomized comparative cohorts, case series and case reports containing data on the antiviral activity of individual drugs. In vitro studies were included if detailing an experiment with a relevant drug and a specific virus.
Table 2.
Mechanism of action of antiparasitic drugs when used as antiviral agents
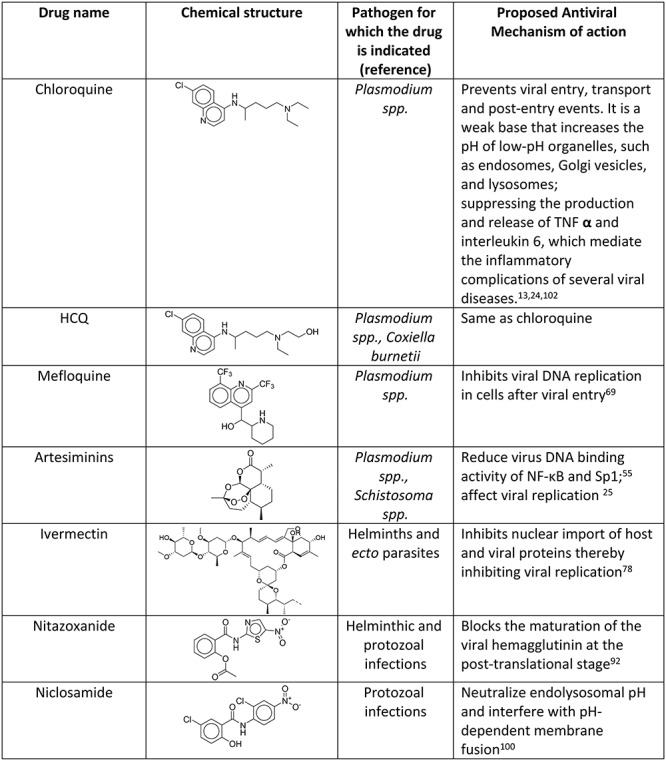
|
Types of outcome measures
Clinically relevant outcomes such as mortality rates, hospital admission rates, lengths of hospital stay and disease severity were recorded. In addition, virologic parameters such as viral clearance time, viral shedding duration and viral load when relevant were also described.
Search methods for identification of studies
We conducted a systematic electronic literature screen using the PubMed search engine by searching one or more of the following terms: ‘COVID’, ‘MERS’, ‘SARS’ or ‘antiviral’, combined with the names of several antiparasitic agents (chloroquine, HCQ, mefloquine, artemisinins, NTZ, niclosamide, ivermectin, albendazole and atovaquone). We used references of retrieved papers, including reviews or systematic reviews to identify further studies. Two reviewers independently screened all studies published before 15 November 2020. We excluded all retrieved articles that did not fulfil the inclusion criteria. In cases of disagreement, a third reviewer acted as arbitrator.
Results
The numbers of all in vitro and in vivo articles that were included are shown in Table 1. Our main findings are summarized in Tables 2 and 3. As we found neither in vitro nor in vivo studies for albendazole or atovaquone, these two agents are not included in this review.
Table 1.
Articles screened, reviewed and included in the review
| Druga | Total abstract screened | In vitro and animal model studies included | In vivo studies included |
|---|---|---|---|
| Chloroquine and HCQ | 1191 | 10 | 21 |
| Artemisinins | 116 | 14 | 2 |
| Mefloquine | 78 | 3 | 0 |
| Ivermectin | 93 | 7 | 5 |
| NTZ | 88 | 14 | 3 |
| Niclosamide | 72 | 17 | 0 |
aNo articles were found for albendazole and atovaquone.
Table 3.
Activity of selected antiparasitic agents against different coronaviruses
| Drug | In-vitro study | Animal Model | Clinical trial | comments |
|---|---|---|---|---|
| HCQ26,27,30–41,43–49 | SARS-CoV-2 | SARS-CoV-2 | Conflicting results | Widely used worldwide, conflicting results in observational studies, no benefit in RCTs. |
| Chloroquine21–25,29 | SARS-CoV-1 SARS-CoV2 MERS-CoV | None | None | None |
| Artemisinins66–68 | SARS-CoV-1 | None | None | In silico study suggests possible activity against SARS-CoV-2 |
| Mefloquine71,72 | Pangolin& Feline coronaviruses | None | None | |
| Ivermectin34,78,81 | SARS-CoV-2 | None | None | Conflicting results. A synergistic effect with HCQ has been suggested. |
| NTZ23,94–96 | MERS-CoV-1 Canine coronavirus SARS-CoV-2 | None | One clinical trial | Benefit unclear |
| Niclosamide97–99 | SARS-CoV-1, MERS-CoV | None | None |
Chloroquine and HCQ
Chloroquine and HCQ are 4-aminoquinoline organic compounds that have long been used for the treatment and prevention of malaria, as antibacterial therapy for Q fever and as immune modulators. These two drugs are very similar in structure, and their mechanism of action appears identical. HCQ is well tolerated and is generally regarded to have a better safety profile than chloroquine.
Most adverse reactions of HCQ are mild and include gastrointestinal adverse effects, skin rash and photosensitivity. Large cumulative dosages, commonly administered over a prolonged period of time, have been associated with irreversible toxic retinopathy. Cardiac toxicity is rare but may be life threatening.9 According to current knowledge, significant QT prolongation occurs in 3–13% of patients, with increasing incidence with age and among patients that are co-treated with azithromycin. Although torsades de pointes has not been recorded in small cohorts of COVID-19 patients, it is possible that fatal arrhythmias will be encountered if the drug will be used extensively.10–12
Chloroquine is active in vitro and in animal models against several viruses, including human immunodeficiency virus (HIV),13 hepatitis C virus (HCV),14 influenza type H1N1,15 H5N1,16 flaviviruses such as dengue virus and Zika,17 MERS virus18–20 and SARS-CoV-1.21–25
In early in vitro studies, chloroquine was found effective in blocking SARS-CoV-2 infection at low concentrations.23 Recent in vitro and physiologically based pharmacokinetic modelling studies found higher potency for HCQ compared to chloroquine in reducing SARS-CoV-2.26,27
Clinical experience with HCQ and chloroquine
From the onset of the COVID-19 pandemic, HCQ was administered in most affected countries. A dose of 400 mg twice daily followed by 200 mg twice daily of HCQ was predicted to achieve an antiviral effect based on pharmacokinetic (PK) modelling, although dosage and treatment duration varied between guidelines.27 In the current review, we did not specify the exact doses that were used; we refer the reader to a previous systematic review for more specific details.28
In some non-randomized comparative studies or small cohorts, a shortening of clinical disease, a faster viral clearance and a decrease in the likelihood to develop pneumonia were reported after the use of chloroquine or HCQ.29–32 The combination of HCQ and azithromycin was also suggested to be beneficial in non-randomized small non-comparative studies.30,33 Possible synergistic effects with ivermectin were also suggested.34
Other observational studies demonstrated no significant reduction in mortality rates after HCQ treatment.35–39 In one study, all-cause mortality risk was significantly higher among HCQ-treated patients compared to patients that were not treated with HCQ [adjusted hazard ratio 1.83; 95% confidence interval (CI) 1.16–2.89; P = 0.009].40
Recently, the results of three large scale RCTs were published. The ‘RECOVERY Collaborative group’ trial compared 1561 patients treated with HCQ to 3155 patients that received standard of care. Death within 28 days occurred in 421 patients (27.0%) in the HCQ group compared to 790 (25.0%) in the usual-care group (rate ratio, 1.09; 95% CI, 0.97–1.23; P = 0.15). Furthermore, patients in the HCQ group that were not undergoing mechanical ventilation at baseline had a higher frequency of invasive mechanical ventilation or death (30.7 vs 26.9%; risk ratio, 1.14; 95% CI, 1.03–1.27).41 The WHO solitary study compared 954 patients treated with HCQ to 909 patients receiving its control. Death occurred in 10.9% of patients receiving HCQ and in 9.2% receiving its control (rate ratio, 1.19; 95% CI, 0.89–1.59; P = 0.23). The use of HCQ did not reduced initiation of ventilation or hospitalization duration.7 The third study evaluated 479 hospitalized adults with respiratory symptoms from COVID-19. It demonstrated no significant differences in the distribution of the Day 14 clinical status score (measured using a 7-category ordinal scale) for patients receiving HCQ compared with placebo [adjusted odds ratio (OR), 1.02].42
Few smaller RCTs were also published. Two of them demonstrated no significant differences in the virological cure rates between HCQ treatment and the standard of care groups.43,44 In three others, no mortality and clinical status benefits were observed using HCQ (or HCQ and Azithromycin) compared with standard of care treatment.45–48
One large RCT assessed the role of HCQ in post-exposure prevention of COVID-19. It demonstrated no significant difference in the new cases of COVID-19 between the HCQ treatment (800 mg once, followed by 600 mg in 6–8 hours, then 600 mg daily for 4 additional days) and the placebo groups.49
A specific alert cautioning against HCQ use without appropriate monitoring was issued by the Food and Drug Administration (FDA).50 Similarly, the WHO recently discontinued the Solidarity Trial citing lack of any benefit of HCQ.51
Artemisinins
It is produced from the plant Artemisia annua; artemisinin and its derivatives have become the main weapon in the fight against malaria. The antimalarial effect of artemisinins is probably related to the formation of reactive oxygen species, although its full mechanism of action is unknown.52 Modulation of heme oxygenase-1 by artesunate has been also suggested as a treatment that lowers mortality and improves sepsis-related lung injury in an animal model.53
The effect of artemisinins when used as antiviral agents has been assessed for several viruses.54,55 Artemisinin and artesunate inhibited the viral production of hepatitis B virus and Epstein-Barr virus.56 Artemisinins, either alone or synergistically with other antiviral agents, was shown to be active against Cytomegalovirus,57–60 inhibited replication of John Cunningham (JC) virus.61 and BK virus62 and has been used anecdotally to treat HHV6 myocarditis.63
There has been one notable demonstration of widespread artemisinin use as an ‘accidental’ antiviral. During the 2014 Ebola epidemic in Liberia, all patients were prescribed either artesunate–amodiaquine or artemether–lumefantrine regimens empirically for suspected co-infection with malaria. Those who were treated with artesunate–amodiaquine had a 31% lower risk of death. This effect was stronger among patients without malaria.64,65 This was, however, a non-randomized trial, and unknown confounding factors, such as cases of low-level Plasmodium parasitemia that might have been missed, may have biased the results.
A. annua extract exhibited activity against SARS CoV-1 as measured by a cytopathic effect reduction (that does not prove an effect on the virus itself).66 In another study, artesunate did not show an inhibitory activity against SARS CoV-1 in a cell line.67 A recent in silico study showed a potential interaction between artemisinins and the SARS-CoV-2 Spike protein Lys353 and Lys31 binding hotspots, but did not directly assess antiviral activity.68 These in vitro and in silico studies, and the fact that artesunate leads to a decrease in sepsis-related lung injury in an animal model, suggest that artemisinins may prove to be interesting candidates for further testing as anti-COVID-19 agents.53
Mefloquine
Mefloquine is an antimalarial similar in structure to quinine. It is active through the destruction of the asexual forms of the Plasmodium parasite. In the past, it has been used for patients with progressive multifocal encephalopathy due to in vitro anti-JC virus activity.69 A RCT failed to demonstrate any benefit, and its use as an antiviral has been largely abandoned.70 In vitro, mefloquine demonstrates antiviral activity against coronaviruses, similar to earlier reports with JC virus. In a cell culture, mefloquine inhibits the cytopathic effect of two coronaviruses closely related to SARS CoV-2: the pangolin coronavirus GX_P2V/pangolin/2017/Guangxi and feline coronavirus.71,72 To the best of our knowledge, no RCT is currently assessing the possible use of mefloquine for COVID-19.
Ivermectin
Ivermectin is used to treat many parasitic infections such as filariasis, soil-transmitted helminths, scabies and head lice, and it is relatively safe. The discoverers of avermectin, its precursor, received the 2015 Nobel Prize for their achievement.73 Its antiparasitic effect involves an increase in parasite cell membrane permeability, a mechanism naturally irrelevant to viruses. It has been shown, however, to display some in vitro activity against dengue virus, chikungunya virus and other flaviviruses.74–76 One RCT that was conducted in Thailand and published only as an abstract demonstrated shorter duration of NS1 antigenemia in patients with dengue fever; however, it failed to demonstrate any clinical benefit.77
In vitro studies suggest that ivermectin leads to a ~5000-fold reduction in SARS-CoV-2 RNA at 48 hours, likely through inhibiting IMPα/β1-mediated nuclear import of viral proteins.78 It remains unclear, however, if relevant concentrations can be achieved with current human or veterinary drug formulations, and what the in vivo effect would be. A synergistic effect of ivermectin and HCQ has also been suggested.34
Recently, few countries in Latin America used ivermectin as a routine, yet unproven, treatment of COVID-19. In northern Bolivia 350 000 doses were given to health care workers and in Peru around 20 000 bottles of animal-grade ivermectin were sold on the black market as a treatment for COVID-19.79 A recent RCT evaluated the effect of ivermectin or ivermectin and doxycycline therapy on hospitalized COVID-19 patients. No significant difference in fever, cough or sore throat were observed between placebo and treatment groups after 7 days. Earlier virological clearance was observed in the ivermectin group compared to placebo, but not in the ivermectin and doxycycline group.80
A retrospective cohort study demonstrated lower mortality in the ivermectin group compared with the standard of care (15.0 vs 25.2%, OR 0.52, 95% CI 0.29–0.96, P = 0.03) and lower mortality of patients with severe pulmonary disease treated with ivermectin (38.8 vs 80.7%, OR 0.15, CI 0.05–0.47, P = 0.001), but with no significant differences in the rates of successful weaning from mechanical ventilation (36.1 vs 15.4%, OR 3.11 (0.88–11.00), P = 0.07).81 Other small, non-randomized trials, which are potentially biased, have reported conflicting results regarding the benefit of ivermectin.82–84
As the level of evidence of these data is likely low, the efficacy of ivermectin against COVID-19 remains to be proven. However, based on current evidence, it is unlikely that Ivermectin will be a game changer in COVID-19 treatment.
Nitazoxanide
NTZ is an antiparasitic drug used for the treatment of Giardia and Cryptosporidium infections, and the research of its possible repurposing for treatment of several viruses is ongoing. These viruses include, among others, parainfluenza, respiratory syncytial virus, canine coronavirus, rhinovirus and flaviviruses, including HCV, hepatitis B virus, HIV and influenza.85–90 NTZ has an in vitro synergistic effect against influenza A virus when combined with oseltamivir and zanamivir.91 NTZ acts against influenza viruses by blocking the maturation of the viral hemagglutinin at the post-translational level.92 Tizoxanide, the active circulating metabolite of NTZ, is capable of inhibiting the replication of several strains of influenza A and B-16 strains of influenza A/H1N1, H3N2, H3N2v, H3N8, H5N9, H7N1 and one strain of influenza B.89 In an RCT that included 624 patients with influenza virus infection, NTZ was associated with a reduction in the duration of symptoms compared to a placebo.90 In another RCT conducted among patients with acute respiratory illness that required hospital admission in Mexico (6.6% of whom were infected with various coronaviruses), NTZ was not associated with any benefits compared to placebo.93 Patients in this study, however, had multiple causative agents, both bacterial and viral, so the measure of effect of NTZ on specific pathogens was impossible to assess.
NTZ exhibits antiviral activity against MERS-CoV and other coronaviruses by inhibiting the expression of the viral N protein,94,95 and inhibits the SARS-CoV-2 at a low-micromolar concentration.23 One small prospective non-controlled study that assessed NTZ treatment of in and outpatients with COVID-19 was performed. Among 20 women (pregnant or in immediate puerperium) that were treated with NTZ, two women died. Five hospitalized patients showed a positive outcome with two patients weaned from mechanical ventilation. A total of 16 outpatients treated with NTZ were cured.96 The low number of patients and the relatively high mortality rate do not support the use of this agent against COVID-19. However, larger studies are needed in order to provide more solid data.
Niclosamide
Niclosamide (2′,5-dichloro-4′-nitrosalicylanilide) is another FDA approved drug with anticestodal activity that was discovered in 1958. It has an in vitro antiviral effect against a broad range of viruses such as SARS-CoV-1,97,98 MERS-CoV,99 Zika virus, Japanese encephalitis virus, HIV, HCV and human adenovirus.100,101 The broad antiviral activity of niclosamide is attributed to its ability to neutralize endo-lysosomal pH and interfere with pH-dependent membrane fusion, which is an essential step in viral entry mechanisms. The antiviral effect of niclosamide should be further assessed in vitro before any consideration of clinical utility could be made.
Discussion
The current COVID-19 pandemic has strained national and international resources. The research community has produced a vast amount of new research. However, efforts were at times fragmented and the methodology of many clinical trials that were performed was of low quality. Several months into the outbreak, the number of large, well-conducted RCTs that have been published is still small, despite the millions of infected people worldwide. The only antiviral drug with proven benefit, remdesivir, has been shown to shorten symptom duration in a large RCT that included >1000 patients. A non-significant trend towards lower mortality rates has been observed in this trial although absolute risk reduction was small in the standard of infectious diseases. Remdesivir is therefore only modestly effective, and the search for more active anti-SARS-CoV-2 continues.6
We included in this review several antiparasitic agents that have been shown to have some in vitro or in vivo antiviral activities. We elaborated on drug activity against different viruses, including the new SARS-CoV-2 and its suggested mechanisms of action (Tables 2 and 3).
Most drugs in Table 2 were shown to reduce the viral replication stage by affecting cellular organelles. However, intensive in vitro/in vivo studies are required to establish the detailed mechanism of action of these agents against viruses, particularly against SARS-CoV-2. Combination therapies of drugs with effects on the various stages of the viral life cycle should be also considered.
The limitations of this review are those of the studies themselves. In vitro studies have used very different methodologies, and at least some were authored by researchers with potential conflict of interests. Some clinical studies were limited to small cohorts that were prone to selection bias, i.e. inclusion of patients with relatively good prognosis. The rate of publication of well-conducted RCTs, as mentioned before, has been disappointedly slow, and recent studies have been retracted from major journals due to methodological or ethical issues.
The only antiparasitic agent that has been included in major RCTs is HCQ. Although initial observational trials suggested benefit of HCQ, larger high-quality trials provide ample evidence that HCQ should not be used neither for treatment nor for prevention of COVID-19. Its inclusion in clinical trials using differing protocols is ethical as no excess mortality was shown in the Solidarity trials.10 We call for the continuation of pre-clinical research of existing compounds with potential antiviral effect against SARS-COV-2. However, as in vitro results and virologic tests often correlate poorly with actual efficacy, we argue that drugs should be introduced into widespread clinical use only after being rigorously tested in RCTs. Such a search may prove beneficial in this pandemic, or in future outbreaks yet to come.
Funding
No funding was received for this work.
Author statements
All authors have read and approved the contents of this manuscript.
Conflict of interests
None declared.
Author contributions
SR—data collection, literature search and writing of manuscript (MS); AN—study design, data collection and writing of MS; AD—data analysis and writing of MS; NP—data collection and literature search; ES—study design and writing of MS.
Contributor Information
S Rakedzon, Division of Internal Medicine, Rambam Health Care Campus, Haifa, Israel.
A Neuberger, Bruce Rappaport Faculty of Medicine, Technion, Haifa, Israel; Division of Internal Medicine, Rambam Health Care Campus, Haifa, Israel; Division of Internal Medicine, Unit of Infectious Diseases, Rambam Healthcare Campus, Haifa, Israel.
A J Domb, Institute of Drug Research, School of Pharmacy-Faculty of Medicine & Institute of Criminology - Faculty of Law. The Hebrew University of Jerusalem, Jerusalem, Israel.
N Petersiel, Division of Internal Medicine, Unit of Infectious Diseases, Rambam Healthcare Campus, Haifa, Israel.
E Schwartz, Sheba Medical Center, Geographic Medicine and Tropical Diseases, Ramat Gan, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
References
- 1. Flaherty GT, Nasir N. Reiseangst: travel anxiety and psychological resilience during and beyond the COVID-19 pandemic. J Travel Med 2020; 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chu IY, Alam P, Larson HJ, Lin L. Social consequences of mass quarantine during epidemics: a systematic review with implications for the COVID-19 response. J Travel Med 2020; 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pirjani R et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Travel Med 2020; 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med 2020; 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hung IF, Lung KC, Tso EYK et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395:1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beigel JH, Tomashek KM, Dodd LE et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Consortium, W.H.O.S.T et al. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Zhang D, du G et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plantone D, Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig 2018; 38:653–71. [DOI] [PubMed] [Google Scholar]
- 10. Mercuro NJ, Yen CF, Shim DJ et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5:1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeJong C, Wachter RM. The risks of prescribing hydroxychloroquine for treatment of COVID-19-first, do no harm. JAMA Intern Med 2020; 180:1118–9. [DOI] [PubMed] [Google Scholar]
- 12. Saleh M, Gabriels J, Chang D et al. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol 2020; 13:e008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savarino A, Lucia MB, Rastrelli E et al. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J Acquir Immune Defic Syndr 2004; 35:223–32. [DOI] [PubMed] [Google Scholar]
- 14. Mizui T, Yamashina S, Tanida I et al. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J Gastroenterol 2010; 45:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ooi EE, Chew JS, Loh JP, Chua RC. In vitro inhibition of human influenza A virus replication by chloroquine. Virol J 2006; 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan Y, Zou Z, Sun Y et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 2013; 23:300–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiryaev SA, Mesci P, Pinto A et al. Repurposing of the anti-malaria drug chloroquine for Zika virus treatment and prophylaxis. Sci Rep 2017; 7:15771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Wilde AH, Jochmans D, Posthuma CC et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014; 58:4875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dyall J, Coleman CM, Hart BJ et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother 2014; 58:4885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dyall J, Gross R, Kindrachuk J et al. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs 2017; 77:1935–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vincent MJ, Bergeron E, Benjannet S et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 2004; 323:264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang M, Cao R, Zhang L et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis 2006; 6:67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Alessandro S et al. The use of antimalarial drugs against viral infection. Microorganisms 2020; 8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yao X, Ye F, Zhang M et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghazy RM, Almaghraby A, Shaaban R et al. A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci Rep 2020; 10:22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020; 14:72–3. [DOI] [PubMed] [Google Scholar]
- 30. Lagier JC, Million M, Gautret P et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med Infect Dis 2020; 36:101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu B, Li C, Chen P et al. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci 2020; 63:1515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arshad S, Kilgore P, Chaudhry ZS et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis 2020; 97:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gautret P, Lagier JC, Parola P et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis 2020; 34:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patri A, Fabbrocini G. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol 2020; 82:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg ES, Dufort EM, Udo T et al. Association of Treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA 2020; 323:2493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paccoud O, Tubach F, Baptiste A et al. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molina JM, Delaugerre C, le Goff J et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect 2020; 50:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geleris J, Sun Y, Platt J et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020; 382:2411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahevas M et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 2020; 369:m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magagnoli J et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med (N Y) 2020; 1:114–27 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Group, R.C et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020; 383:2030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Self WH, Semler MW, Leither LM et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA 2020; 324:2165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang W et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020; m1849:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen J, Liu D, Liu L et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020; 49:215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cavalcanti AB, Zampieri FG, Rosa RG et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med 2020; 383:2041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Skipper CP, Pastick KA, Engen NW et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med 2020; 173:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abd-Elsalam S, Esmail ES, Khalaf M et al. Hydroxychloroquine in the treatment of COVID-19: a Multicenter randomized controlled study. Am J Trop Med Hyg 2020; 103:1635–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48. Lyngbakken MN, Berdal JE, Eskesen A et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun 2020; 11:5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boulware DR, Pullen MF, Bangdiwala AS et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020; 383:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. FDA . FDA Cautions Against Use of Hydroxychloroquine or Chloroquine for COVID-19 Outside of the Hospital Setting or a Clinical Trial due to Risk of Heart Rhythm Problems. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or (Accessed date 02 December 2020).
- 51. World Health Organization . WHO Discontinues Hydroxychloroquine and Lopinavir/Ritonavir Treatment Arms for COVID-19. https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 (Accessed date 15 July 2020).
- 52. Meshnick SR. Artemisinin mechanisms of action, resistance and toxicity. Int J Parasitol 2002; 32:1655–60. [DOI] [PubMed] [Google Scholar]
- 53. Cao TH, Jin SG, Fei DS et al. Artesunate protects against sepsis-induced lung injury via Heme Oxygenase-1 modulation. Inflammation 2016; 39:651–62. [DOI] [PubMed] [Google Scholar]
- 54. Raffetin A, Bruneel F, Roussel C et al. Use of artesunate in non-malarial indications. Med Mal Infect 2018; 48:238–49. [DOI] [PubMed] [Google Scholar]
- 55. Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJG, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis 2008; 47:804–11. [DOI] [PubMed] [Google Scholar]
- 56. Romero MR, Efferth T, Serrano MA et al. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an "in vitro" replicative system. Antiviral Res 2005; 68:75–83. [DOI] [PubMed] [Google Scholar]
- 57. Drouot E, Piret J, Boivin G. Artesunate demonstrates in vitro synergism with several antiviral agents against human cytomegalovirus. Antivir Ther 2016; 21:535–9. [DOI] [PubMed] [Google Scholar]
- 58. Flobinus A, Taudon N, Desbordes M et al. Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J Antimicrob Chemother 2014; 69:34–40. [DOI] [PubMed] [Google Scholar]
- 59. Wolf DG, Shimoni A, Resnick IB et al. Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation. Antiviral Res 2011; 90:183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chou S, Marousek G, Auerochs S, Stamminger T, Milbradt J, Marschall M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antiviral Res 2011; 92:364–8. [DOI] [PubMed] [Google Scholar]
- 61. Sharma BN, Marschall M, Rinaldo CH. Antiviral effects of artesunate on JC polyomavirus replication in COS-7 cells. Antimicrob Agents Chemother 2014; 58:6724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharma BN, Marschall M, Henriksen S, Rinaldo CH. Antiviral effects of artesunate on polyomavirus BK replication in primary human kidney cells. Antimicrob Agents Chemother 2014; 58:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hakacova N, Klingel K, Kandolf R, Engdahl E, Fogdell-Hahn A, Higgins T. First therapeutic use of Artesunate in treatment of human herpesvirus 6B myocarditis in a child. J Clin Virol 2013; 57:157–60. [DOI] [PubMed] [Google Scholar]
- 64. DeWald LE, Johnson JC, Gerhardt DM et al. In vivo activity of amodiaquine against Ebola virus infection. Sci Rep 2019; 9:20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gignoux E, Azman AS, de Smet M et al. Effect of artesunate-amodiaquine on mortality related to Ebola virus disease. N Engl J Med 2016; 374:23–32. [DOI] [PubMed] [Google Scholar]
- 66. Li SY, Chen C, Zhang HQ et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 2005; 67:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen F, Chan KH, Jiang Y et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 2004; 31:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sehailia, M. Chemat, S. Antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19. J Biomol Struct Dyn, 2020; 1–11. [DOI] [PMC free article] [PubMed]
- 69. Brickelmaier M, Lugovskoy A, Kartikeyan R et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother 2009; 53:1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clifford DB, Nath A, Cinque P et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol 2013; 19:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fan HH, Wang LQ, Liu WL et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl) 2020; 133:1051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McDonagh P, Sheehy PA, Norris JM. Identification and characterisation of small molecule inhibitors of feline coronavirus replication. Vet Microbiol 2014; 174:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tambo E, Khater EI, Chen JH, Bergquist R, Zhou XN. Nobel prize for the artemisinin and ivermectin discoveries: a great boost towards elimination of the global infectious diseases of poverty. Infect Dis Poverty 2015; 4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tay MY, Fraser JE, Chan WKK et al. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res 2013; 99:301–6. [DOI] [PubMed] [Google Scholar]
- 75. Mastrangelo E, Pezzullo M, de Burghgraeve T et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother 2012; 67:1884–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Varghese FS, Kaukinen P, Gläsker S et al. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antiviral Res 2016; 126:117–24. [DOI] [PubMed] [Google Scholar]
- 77. Ouedraogo AL, Bastiaens GJH, Tiono AB et al. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clin Infect Dis 2015; 60:357–65. [DOI] [PubMed] [Google Scholar]
- 78. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mega ER. Latin America's embrace of an unproven COVID treatment is hindering drug trials. Nature 2020; 586:481–2. [DOI] [PubMed] [Google Scholar]
- 80. Ahmed S et al. A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis 2020; 103:214–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rajter JC et al. Use of Ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON study. Chest 2020; 159:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Camprubí D, Almuedo-Riera A, Martí-Soler H et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PLoS One 2020; 15:e0242184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Szente Fonseca SN, de Queiroz Sousa A, Wolkoff AG et al. Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: comparative analysis. Travel Med Infect Dis 2020; 38:101906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khan MSI, Khan MSI, Debnath CR et al. Ivermectin treatment May improve the prognosis of patients with COVID-19. Arch Bronconeumol 2020; 56:828–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beigel JH, Nam HH, Adams PL et al. Advances in respiratory virus therapeutics - a meeting report from the 6th isirv antiviral group conference. Antiviral Res 2019; 167:45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stachulski AV, Swift K, Cooper M et al. Synthesis and pre-clinical studies of new amino-acid ester thiazolide prodrugs. Eur J Med Chem 2017; 126:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shakya A, Bhat HR, Ghosh SK. Update on nitazoxanide: a multifunctional chemotherapeutic agent. Curr Drug Discov Technol 2018; 15:201–13. [DOI] [PubMed] [Google Scholar]
- 88. Piacentini S, la Frazia S, Riccio A et al. Nitazoxanide inhibits paramyxovirus replication by targeting the fusion protein folding: role of glycoprotein-specific thiol oxidoreductase ERp57. Sci Rep 2018; 8:10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rossignol JF. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res 2014; 110:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Haffizulla J, Hartman A, Hoppers M et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 2014; 14:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Belardo G, Cenciarelli O, La Frazia S, Rossignol JF, Santoro MG. Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro. Antimicrob Agents Chemother 2015; 59:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rossignol JF. Thiazolides: a new class of antiviral drugs. Expert Opin Drug Metab Toxicol 2009; 5:667–74. [DOI] [PubMed] [Google Scholar]
- 93. Gamiño-Arroyo AE, Guerrero ML, McCarthy S et al. Efficacy and safety of nitazoxanide in addition to standard of care for the treatment of severe acute respiratory illness. Clin Infect Dis 2019; 69:1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rabaan AA, Bazzi AM, Al-Ahmed SH, Al-Tawfiq JA. Molecular aspects of MERS-CoV. Front Med 2017; 11:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rossignol JF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health 2016; 9:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Meneses Calderón J, Figueroa Flores MR, Paniagua Coria L et al. Nitazoxanide against COVID-19 in three explorative scenarios. J Infect Dev Ctries 2020; 14:982–6. [DOI] [PubMed] [Google Scholar]
- 97. Wu CJ, Jan JT, Chen CM et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother 2004; 48:2693–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wen CC, Kuo YH, Jan JT et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem 2007; 50:4087–95. [DOI] [PubMed] [Google Scholar]
- 99. Gassen NC, Niemeyer D, Muth D et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun 2019; 10:5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu J, Shi PY, Li H, Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis 2020; 6:909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev Anti Infect Ther 2006; 4:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis 2003; 3:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


