Abstract
Multisystem inflammatory syndrome in children (MIS-C) has been observed in temporal association with coronavirus disease 2019 (COVID-19), typically within 2 to 6 weeks of illness or exposure. We present a case of MIS-C occurring 16 weeks after initial COVID-19 illness to highlight the prolonged period of risk for developing MIS-C.
Keywords: multisystem inflammatory syndrome in children, myocarditis, novel coronavirus 2019, severe acute respiratory syndrome coronavirus 2
We describe the presentation, diagnostic evaluation, and management of an adolescent presenting with a multisystem inflammatory process sixteen weeks following COVID-19. We highlight an expanded risk period, longer then commonly reported, for MIS-C to bring heightened awareness to its possibility.
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was publicly reported, and the novel coronavirus disease 2019 (COVID-19) quickly spread throughout the world. Early cases in children suggested a typically mild illness compared with adults. However, in April 2020, reports emerged describing a severe, systemic, hyperinflammatory process presenting in children in the weeks following peak community spread [1]. The Centers for Disease Control and Prevention (CDC) defined multisystem inflammatory syndrome in children (MIS-C) as fever, laboratory evidence of inflammation, and a minimum of 2 organ system involvement in the setting of recent or current SARS-CoV-2 infection or possible exposure to COVID-19 within 4 weeks without a plausible alternative diagnosis in a patient of 21 years of age or younger [2]. At this time, in the United States, there have been over 2 million cases of COVID-19 in children. As of December 4, 2020, the CDC reports 1288 cases of MIS-C, and a disproportionate majority of cases have been reported in Black and Hispanic/Latino children [3]. Recent publications of a similar multisystem inflammatory syndrome in adults (MIS-A) further suggests this might not be unique to children [4]. To date, there is a paucity of published data regarding the interval of time following acute SARS-CoV-2 infection for which the diagnosis of MIS-C should be considered. Herein, we describe a child diagnosed with MIS-C who presented 16 weeks after an initial documented COVID-19 illness.
CASE DESCRIPTION
A previously healthy 15-year-old Black female developed the acute onset of a pruritic, urticarial facial rash, which progressed over 3 days to include her neck, arms, and trunk with subsequent odynophagia, exudative pharyngitis, polyarthralgia with myalgia, edema of the hands and feet, and ultimately a temperature of 38.6°C (101.5°F). Her medical history is notable for symptomatic COVID-19 illness 16 weeks prior associated with fever, respiratory symptoms, and anosmia and confirmed at that time by COVID-19 reverse transcription-polymerase chain reaction (RT-PCR). She also has a history of migraines treated with Amitriptyline, exercise-induced asthma, and seasonal allergic rhinitis. She has no drug allergies. Family history is notable for maternal history of ulcerative colitis and maternal grandmother with adrenal cancer. She has been in a virtual school, is up to date on routine immunizations, and recently attended a “haunted house” with her friends 2 weeks prior; the remainder of a comprehensive and detailed exposure history was unrevealing.
One day prior to admission, she presented to the emergency department, where she was SARS-CoV-2 negative by RT-PCR (Biofire Defense, Salt Lake City, UT, USA), received a working diagnosis of infectious mononucleosis, and was treated with 60 mg of prednisone. She was subsequently admitted the following day after returning to care due to persistent symptoms, new fever, and signs of dehydration.
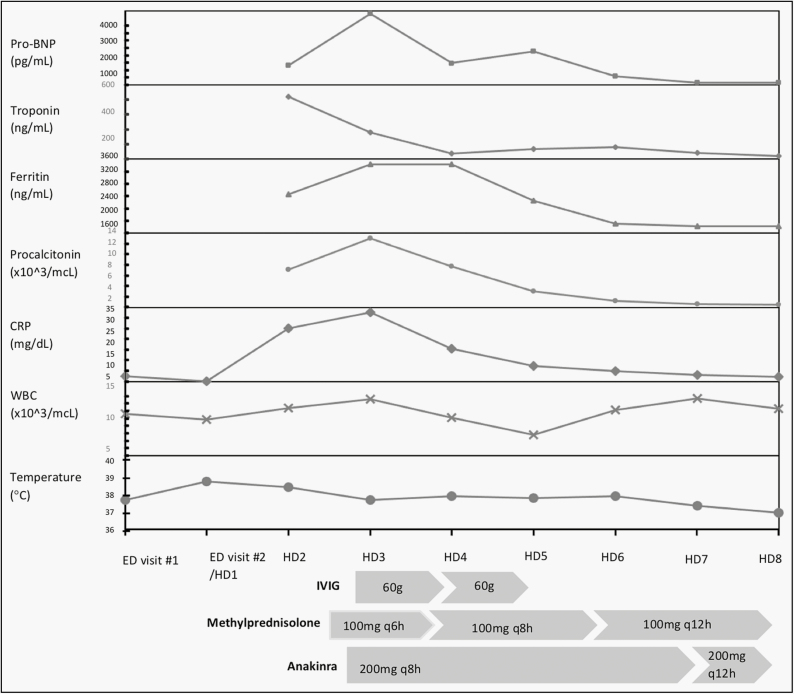
Upon admission, vital signs were notable for heart rate 122, blood pressure 125/77, respiratory rate 16, oxygen saturation 100%, temperature 37.7°C, and pertinent findings of the initial physical examination included the following: trismus, exudative pharyngitis with midline uvula and no oropharyngeal bulge, normal conjunctiva, tender right-sided 1 cm cervical lymphadenopathy overlying the sternocleidomastoid muscle, absence of hepatosplenomegaly, diffuse muscular tenderness to palpation, and symmetric arthralgia without effusion or warmth, mild facial swelling most prominently over the eyelids, mild non-pitting edema over the dorsum of the hands, fingers, and feet, and erythematous, blanching, pruritic wheals on her face, chest, and arms, in addition to a clustered collection of petechial lesions on her right wrist. A second SARS-CoV-2 RT-PCR (Biofire) was negative. Initial management was supportive and symptomatic without antibiotics. During the first 36 hours of her hospitalization, she developed chest pain, emesis, recurrence of fever, and marked elevation in her inflammatory markers. Notable labs and treatment are demonstrated in Figure 1 and Table 1. Her electrocardiogram demonstrated nonspecific T wave changes. Her echocardiogram showed mild diastolic dysfunction and dilation of the left main and left anterior descending coronary arteries with maximal Z-score measurement of 3.3.
Figure 1.
The graphical trend of pertinent lab values and temperature throughout hospitalization and the timing and dosing of anti-inflammatory treatment during the admission for multisystem inflammatory syndrome in children. Abbreviations: IVIG, intravenous immunoglobulin; Pro-BNP, pro-B-type natriuretic peptide; CRP, C-reactive protein; WBC, white blood cell; HD, hospital day; ED, Emergency Department.
Table 1.
The Numerical Values of Notable Laboratory Results on Admission, at Discharge, and Minimum/Maximum Values During Hospitalization
| Lab Test | Ref Range | Initial Value | Min/Max Value During Admission | Final Value |
|---|---|---|---|---|
| Troponin-HS (ng/mL) | 0-19 | 522.3 | 119.1/522.3 | 119.1 |
| Pro-BNP (pg/mL) | 5.0-125.0 | 1330 | 154.9/4820 | 154.9 |
| ALT (U/L) | 5-30 | 14 | 13/19 | 19 |
| AST (U/L) | 13-35 | 27 | 26/29 | 29 |
| Albumin (g/dL) | 3.6-5.2 | 3.8 | 2.5/3.8 | 3 |
| ESR (mm/hr) | 0-25 | 22 | 22/49 | 40 |
| CRP (mg/dL) | 0.000-0.500 | 2.43 | 1.49/32.64 | 1.49 |
| Procalcitonin (ng/mL) | 0.00-0.25 | 7.13 | 0.51/12.96 | 00.51 |
| Ferritin (ng/mL) | 7-140 | 2453 | 1414/3434 | 1422 |
| IL-1b (pg/mL) | ≤25 | <5 | — | — |
| IL-2 (pg/mL) | ≤3 | 3 | — | — |
| IL-4 (pg/mL) | ≤8 | 15 | — | — |
| IL-5 (pg/mL) | ≤1 | 1 | — | — |
| IL-6 (pg/mL) | ≤6 | 423 | — | — |
| IL-8 (pg/mL) | ≤21 | 3 | — | — |
| IL-10 (pg/mL) | ≤6 | 9 | — | — |
| IL-18 (pg/mL) | 89-540 | 23424 | — | — |
| IFN gamma (pg/mL) | ≤5 | 2 | — | — |
| TNF alpha (pg/mL) | ≤7 | 1 | — | — |
| GM-CSF (pg/mL) | ≤1 | 2 | — | — |
| Fibrinogen (mg/dL) | 212-433 | 678 | 411/684 | 411 |
| D-Dimer (µg/mL) | <0.5 | 16.26 | 0.77/16.26 | 0.77 |
| LDH (U/L) | 100-190 | 318 | 290/357 | 357 |
| WBC (×10^3/µL) | 4.5-13.5 | 10.6 | 7.8/12.7 | 10.4 |
| Lymphocytes (%) | 18.0-50.0 | 8.0 | 8.0/25.5 | 25.5 |
| Platelets (×10^3/µL) | 150-1450 | 249 | 217/286 | 217 |
Abbreviations: Pro-BNP, pro-B-type natriuretic peptide; CRP, C-reactive protein; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ESR, erythrocyte sedimentation rate; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; LDH, lactate dehydrogenase.
Treatment for MIS-C was initiated, as shown in Figure 1, with intravenous immunoglobulin (IVIG) 2 gm/kg, methylprednisolone 2 gm/kg every 6 hours, anakinra 4 mg/kg every 8 hours, and high-dose aspirin 80 mg/kg/day divided every 6 hours. She tolerated high-dose anakinra and steroids and responded to targeted immunomodulatory treatment to downregulate interleukin (IL)-1 pathway with improved physical exam findings, normalization of inflammatory markers, and resolved coronary artery dilation and diastolic dysfunction. She had subjective improvement within 12 hours and objective improvement in inflammatory biomarkers within 24 hours following the initiation of treatment.
SARS-CoV-2 qualitative serology was immunoglobulin M (IgM) negative and IgG positive (LabCorp). Her cytokine profile (Cincinnati Children’s Hospital Medical Center) was notable for elevated IL-4, IL-6, IL-10, granulocyte-macrophage colony-stimulating factor, IL-18, and S100A8/S100A9 Heterodimer assay. An evaluation for possible other inflammatory processes and infectious etiological triggers completed prior to IVIG administration was unrevealing: throat cultures with normal flora, a negative blood culture, negative monospot, no acute Epstein-Barr virus (EBV) or cytomegalovirus (CMV) infection by serology, negative parvovirus B19 IgM, fourth generation HIV serology negative, antistreptolysin O (ASO) and anti-DNAse B normal for age, and negative respiratory pathogen PCR panel (Biofire).
DISCUSSION
The initial constellation of symptoms, and a single febrile episode, in the context of a C-reactive protein of 2.43 mg/dL (normal < 0.5), and a long interval period since her prior COVID-19 illness made us doubt a diagnosis of MIS-C on initial presentation. MIS-C has been described as presenting within 2 to 6 weeks, typically within 21 to 25 days, of an illness compatible with acute COVID-19 [5]. To our knowledge, the longest time span, for children, specifically documented between initial exposure with a positive PCR and MIS-C diagnosis was 5 weeks in an 8-week-old infant exposed at 2 weeks of life who developed MIS-C symptoms at 7 weeks old [6]. The recent CDC definition for MIS-A requires infection within the preceding 12 weeks. Yet, the longest reported duration between a positive PCR and MIS-A diagnosis is 41 days in a 33-year-old male [4]. A systematic review of MIS-C describes initial asymptomatic or mild COVID-19 infections followed 2 to 6 weeks later by a MIS-C diagnosis [7]. Our multidisciplinary team and institutional experience with MIS-C previously had been consistent with a similar risk period [8, 9]. This expected time interval made us question this diagnosis earlier in the case.
The initial differential primarily focused on causes of acute infections, mononucleosis-like illnesses, and systemic autoimmune disease. The brief exposure to steroids initially, on earlier presentation prior to admission, likely masked her fever, but the progression of myocarditis continued. She had no known risk factors to suggest rickettsial infection or acute retroviral syndrome. The marked increase in her inflammatory markers, myocarditis, and dilated coronary arteries brought MIS-C to the top of the differential; no identifiable alternative diagnoses, positive SARS-CoV-2 IgG antibodies, and a rapid improvement with treatment confirmed MIS-C.
CDC Morbidity and Mortality Weekly Reports (MMWR) have provided a comprehensive description of MIS-C cases reporting clinical characteristics with positive PCR testing and serologies but have lacked information about the timing of positive tests and symptoms onset. A CDC latent class analysis (LCA) measurement model categorized MIS-C cases into 3 main classes [10]. This patient’s labs and clinical presentation would be most compatible with LCA 1 given her prominent cardiovascular involvement with episodes of emesis as the gastrointestinal manifestation; mucocutaneous, musculoskeletal, and neurological finders were also present. Neurological features have been reported to occur in a pooled mean proportion of 38.7% of patients with MIS-C and would include the myalgia, weakness, and mild headache seen in this patient [11]. About 57% of MIS-C cases describe dermatological findings involving a wide range of cutaneous manifestations to include polymorphic, maculopapular, morbilliform, erythroderma, urticarial, reticular, petechial, and purpuric with varying distribution on the body [12]. Her presentation with an urticarial, pruritic rash of the face initially before any other symptoms appears to be uncommon with only one of the cases reporting similar findings.
The underlying immunopathogenesis for the acute inflammatory process in MIS-C is yet to be fully elucidated. Autoantibodies, activation of IL-1 pathway, altered interferon response with delayed viral clearance, and antibody-dependent enhancement (ADE) have been postulated as possible causes [9, 13, 14]. Interestingly, she had a high level of S100, a known biomarker for an activated innate immune response. With 2 negative PCR tests and IgM seronegative at the time of admission, a secondary SARS-CoV-2 infection would be unlikely to have occurred in this case. At this time, there have been over 2 million COVID-19 cases in children in the United States alone, with cases continuing to rise, characterizing that the period of risk for MIS-C is critical to anticipatory guidance and management decisions going forward.
CONCLUSIONS
This case highlights the difficulties of diagnosing MIS-C as the pandemic progresses and there are no longer isolated waves within a local region but rather continuous high-level community spread. Additionally, it challenges the assumption of a 2 to 6 week presentation time for MIS-C after acute COVID-19. Our case demonstrates a 113-day interval between acute COVID-19 illness and MIS-C diagnosis; future case reports should continue to enumerate this interval so the expected lag period between acute infection and inflammatory syndrome can be better defined. Two major questions warrant rapid exploration to assist in managing and counseling patients going forward:
What is the true lag time between SARS-CoV-2 PCR (+) primary infection and the risk for developing MIS-C?
Could reexposure to SARS-CoV-2 following prior infection or possibly a different microbial exposure trigger MIS-C?
We call attention to the period of risk for MIS-C extending up to 16 weeks after a documented SARS-CoV-2 infection. The mechanisms responsible for MIS-C as well as the risk period warrant future studies and should be a priority for further research.
Notes
Acknowledgments. We thank the Uniformed Services University of the Health Sciences for supporting this publication as well as the entire multidisciplinary pediatric and inpatient team at the Walter Reed National Military Medical Center who helped care for this patient.
Disclaimer. The views expressed in this case report are those of the authors and do not reflect the official policy of the Department of U.S. Army, U.S. Navy, U.S. Air Force, Uniformed Services University, Department of Defense, or U.S. Government. The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute an endorsement or implied endorsement on the part of the author, Department of Defense, or any other component agency.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Emergency preparedness and response: multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Accessed December 29, 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 3. Centers for Disease Control and Prevention. Multisystem inflammatory syndrome (MIS-C). Accessed December 29, 2020. https://www.cdc.gov/mis-c/index.html
- 4. Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez OM, Bridges ND, Goldmuntz E, Pascual V. The immune roadmap for understanding multi-system inflammatory syndrome in children: opportunities and challenges. Nat Med 2020; 26:1819–24. [DOI] [PubMed] [Google Scholar]
- 6. Orlanski-Meyer E, Yogev D, Auerbach A, et al. Multisystem Inflammatory Syndrome in Children Associated With Severe Acute Respiratory Syndrome Coronavirus-2 in an 8-Week-Old Infant. J Pediatr Infect Dis Soc 2020; doi: 10.1093/jpids/piaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine 2020; 26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cirks BT, Geracht JC, Jones OY, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case report on managing the hyperinflammation. Mil Med 2021; 186:e270–e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMurray JC, May JW, Cunningham MW, Jones OY. Multisystem inflammatory syndrome in children (MIS-C), a post-viral myocarditis and systemic vasculitis—a critical review of its pathogenesis and treatment. Front Pediatr 2020; 8(871). doi: 10.3389/fped.2020.626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children – United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020; 69(32):1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020; 20(11):e276–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol 2020; doi: 10.1016/j.clindermatol.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 2020; 20:453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183:968–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]