Abstract
Background
Ethnicity can influence susceptibility to infection, as COVID-19 has shown. Few countries have systematically investigated ethnic variations in infection.
Methods
We linked the Scotland 2001 Census, including ethnic group, to national databases of hospitalizations/deaths and serological diagnoses of bloodborne viruses for 2001–2013. We calculated age-adjusted rate ratios (RRs) in 12 ethnic groups for all infections combined, 15 infection categories, and human immunodeficiency virus (HIV), hepatitis B (HBV) and hepatitis C (HCV) viruses.
Results
We analysed over 1.65 million infection-related hospitalisations/deaths. Compared with White Scottish, RRs for all infections combined were 0.8 or lower for Other White British, Other White and Chinese males and females, and 1.2–1.4 for Pakistani and African males and females. Adjustment for socioeconomic status or birthplace had little effect. RRs for specific infection categories followed similar patterns with striking exceptions. For HIV, RRs were 136 in African females and 14 in males; for HBV, 125 in Chinese females and 59 in males, 55 in African females and 24 in males; and for HCV, 2.3–3.1 in Pakistanis and Africans.
Conclusions
Ethnic differences were found in overall rates and many infection categories, suggesting multiple causative pathways. We recommend census linkage as a powerful method for studying the disproportionate impact of COVID-19.
Keywords: epidemiology, ethnicity, infectious disease
Introduction
Ethnicity has been defined as ‘the social group a person belongs to and either identifies with or is defined by others as a result of a mix of cultural and other factors including language, diet, religion, ancestry and physical features’.1 Important differences between ethnic groups have been found in many countries for all-cause mortality 2,3 and numerous diseases.4,5 Given the multi-dimensional nature of ethnicity and the numerous types and causes of infections, their inter-relationships are likely to be extremely complex. The importation of infections by migrants from high prevalence regions is well-recognized.6,7 Socioeconomic circumstances, diet, smoking, sexual behaviour and attitudes to immunization are some of many other factors related to infection risk that could vary by ethnic group.8 The causal complexity has recently been highlighted by the disproportionate health impact of COVID-19 on ethnic minorities in England and the USA.9,10 However, only the USA 11,12 and New Zealand 13,14 have taken a systematic approach to exploring the relationships between infection and race/ethnicity more generally.
The Scottish Health and Ethnicity Linkage Study (SHELS) created a national census-linked retrospective cohort of 4.62 million people, permitting comparisons between ethnic groups for many health conditions and indicators.15 The findings have informed Scottish race equality policy.16 SHELS found a >2-fold difference in hospitalization rates for lower respiratory tract infections between the lowest (Chinese) and highest (Pakistani) among both males and females.17 In the present study, we aimed to explore the relationships between infection and ethnic group in Scotland as comprehensively as our dataset would permit. We compared ethnic groups for(i) combined hospitalization and death rates for all infections and a wide range of infection categories and (ii) rates of serologically confirmed diagnoses of human immunodeficiency virus (HIV), hepatitis B Virus (HBV) and hepatitis C Virus (HCV).
Methods
Study population
An overview of the SHELS cohort has been published15 and more details of the methods are provided in the supplementary material. About 4.62 million individuals in the Scotland Census 2001 were linked by probability matching to the Community Health Index (CHI), a register of patients using the National Health Service in Scotland (NHS Scotland) which generates a unique CHI number for each individual. As the CHI number is included in many NHS databases, we could then link the Census to NHS Scotland hospital discharge and mortality records, and to databases for people with serologically confirmed infection with HIV, HBV or HCV.
Ethnic groups
As agreed by the Scottish Parliament, respondents to the 2001 Census could self-select their ethnic group from 14 pre-defined categories, largely reflecting the pattern of immigration to Scotland over the previous 50 years.18 This legally required field had a 95.7% completion rate, increased to 100% by imputation.19 For the cohort’s breakdown by ethnic group, see Supplementary Table S1. For the hospitalization and death analyses, we combined the Bangladeshi and other South Asian groups and removed the ‘Other ethnic groups’ category due to its extreme heterogeneity. The remaining 12 were: White Scottish, Other White British, White Irish, Other White (mainly from Europe, North America and Australasia), any mixed background (parents from more than one ethnic group), Chinese, Indian, Pakistani, Other South Asian (from any other South Asian country), Caribbean, African and Black Scottish/Other Black. For the HIV, HBV and HCV analyses, small numbers restricted analysis to between five and seven ethnic groups.
International Classification of Disease 10th Revision codes
We prepared a comprehensive list of International Classification of Disease 10th Revision (ICD-10) codes for infection diagnoses, largely using that developed by the US Centers for Disease Control and Prevention 20 and refined by Baker and colleagues (Supplementary material S2).13 As Baker et al. used the ICD-10-AM (Australian Modification), we translated relevant AM codes into those used in the UK.
Outcome measures
We extracted all hospitalization and death records dated from 1 May 2001 to 30 April 2013 for all persons in the linked database where the record included a relevant ICD-10 code in any diagnostic position. Deaths were combined with hospitalizations as the number of deaths was too small to enable separate analyses. All infections of sufficient severity to contribute to a hospital admission or death were thus included. All valid cases were combined for the ‘All Infections’ analyses. Following the method of Baker et al.,13 we initially grouped cases into 39 categories of infection (Supplementary Table S8). Based on our pre-specified data analysis plan (Supplementary materials 2 and 3), we restricted our analysis to the 15 categories averaging at least 1000 cases of hospitalization/death annually, judging this number sufficient to compare most ethnic groups. For the HIV, HBV and HCV analyses, we used all linked reports of a first diagnosis from 1 May 2001 to 30 April 2013.
Data analysis
We used person-years as the denominator, censored for death or transfer of registration from NHS Scotland to elsewhere in the UK, and categorized by sex and ethnic group. We used Poisson regression with robust variance to calculate age-adjusted rates of hospitalization or death per 100 000 person-years. We calculated age-adjusted rate ratios (RRs) with 95% confidence intervals (CI) using the White Scottish male or female population as the reference group (RR = 1.0). For ‘All Infections’, the analysis was further adjusted using a proxy measure of socioeconomic status (SES), combining three indicators which were consistently associated in the same direction with the outcomes across ethnic groups and by sex: the Scottish Index for Multiple Deprivation (SIMD; an area-based measure), household tenure and a combined measure of highest educational level.21,22 Country of birth was categorized as either being born in the UK or the Republic of Ireland (UK/I) or born elsewhere.
Data were analysed using SAS V9.4 (SAS Institute Inc., Cary, North Carolina, USA).
In our Tables, we colour-coded RRs as blue within ±0.1 of 1.0, green < 0.9 and red > 1.1. The darker the shade of green or red, the greater is the combination of the difference from the reference value and the precision based on the 95% CI, as shown in the Table keys.
Permissions, data security and reporting procedure
The NHS Scotland Research Ethics Committee A and the Privacy Advisory Committee of NHS National Services Scotland granted full ethical and other approvals for the linkage, data security and analyses. Only researchers with appropriate clearance and training could access the data. All analyses and outputs were approved by the Disclosure Committee of National Records of Scotland. Following their disclosure guidance, the numbers of cases were presented rounded to the nearest five to safeguard anonymity, but analysis and RR estimates were based on real numbers. In reporting, we complied with the STROBE/RECORD checklists.23
Results
All infections
A total of 1 652 865 linked cases recorded during 48.8 million person-years of observation met our criteria and were included in the analysis of all infections (Supplementary Table S3). Table 1 shows the rates for all infection-related episodes during 2001–13 by ethnic group, and RRs for each group in comparison with the White Scottish, adjusted in turn for age; age and whether born in the UK/I; and age and SES. RRs were 0.8 or lower for Other White British, Other Whites and Chinese males and females and were reasonably precisely estimated, for example, Chinese females, 0.64 (95% CI 0.59–0.70). They were 1.30 (1.23–1.38) for Pakistani males and 1.20 (1.13–1.26) among Pakistani females and 1.24 (1.02–1.51) in African females. The RRs for African and Black Scottish males were around 1.2, but imprecisely estimated. The RRs for the remaining ethnic groups were all close to 1.0. Adjustment for whether born in the UK/I or SES resulted in a small increase in the RR (<0.2) for some groups, but minimal change for most. (Supplementary Tables S3–S5 for all RRs and 95%CIs).
Table 1.
Age-adjusted rates and adjusted rate ratios for all infection-related hospitalizations and deaths among males and females in the 2001 Scotland Census-linked cohort, April 2001–March 2013. White Scottish = 1·0
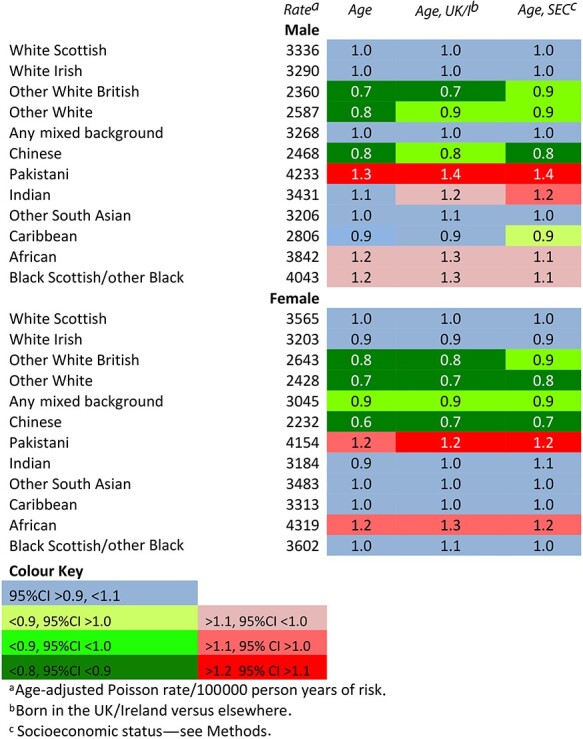
|
Infection categories
Tables 2 and 3 summarize the RRs among males and females for the 15 selected infection categories. The ethnic group patterns were similarly distinctive for males and females. The White Scottish and White Irish groups had very similar rates. For Other White British, Other Whites and Chinese groups, RRs were typically below 0.8 in most categories. In contrast, among Pakistani males and females, RRs were above 1.1 (with 95% CIs above 1.0) for 13 and 11 categories, respectively and above 1.2 (with 95% CIs above 1.1) for five. For the other ethnic groups, there was a more inconsistent picture. (Supplementary Tables S10–S24 for all RRs and 95% CIs).
Table 2.
Age-adjusted rate ratios for males in 12 ethnic groups for 15 infection categories in the Scotland 2001 Census-linked cohort, all cases of hospitalization or death, April 2001 to March 2013. White Scottish = 1·0
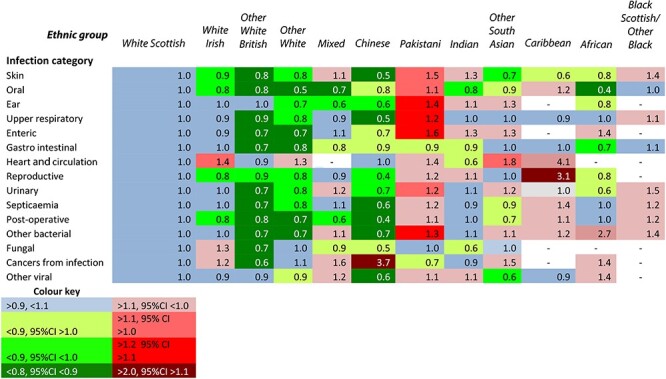
|
Table 3.
Age-adjusted rate ratios for females in 12 ethnic groups and 15 infection categories for cases of hospitalization or death in the Scotland 2001 Census-linked cohort, April 2001 to March 2013. White Scottish = 1·0
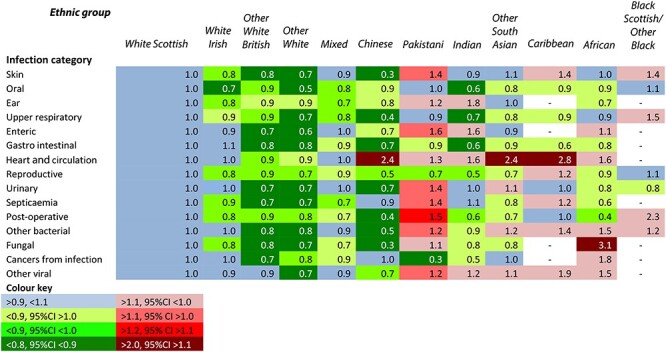
|
RRs were much higher for certain infection categories in one or more ethnic groups. Chinese males had an RR of 3.7 for cancers from infection. Infections of the heart and circulation appeared more frequent among Chinese (RR = 2.3), Other South Asian (2.3) and Caribbean females (2.8) and males (4.08). The incidence of reproductive tract infections among Caribbean males (3.1), fungal infections among African females (3.1), and other viral infections among Caribbean females (1.9) was also high.
HIV, HBV and HCV
Table 4 shows the analyses for HIV, HBV and HCV serodiagnoses. There were huge differences between ethnic groups, with different patterns for each virus. For HIV, RRs were 14 for African males and 136 for African females, with absolute rates per 100 000 person-years of 61 and 113, respectively. For HBV, all groups except the Other White British had markedly higher RRs, most notably the Chinese (males 59, females 125), Africans (males 24 and females 55) and Pakistanis (males 11, females 18). For HCV, White Irish males and females had markedly lower RRs, whereas Pakistani males and females and African and Mixed females had higher RRs, although the 95% CI included 1.0. Adjustment for being born in the UK/I reduced the RRs substantially for HIV among African males and females and for HBV among all the groups except the Other White British but had little effect for HCV. (Supplementary Tables S6–S8 for all RRs and 95%CIs and Supplementary Table S9 for a confirmatory analysis of HBV rates for persons born in the UK/I). Adjustment for SES had little effect on the RR for any group except for HCV among White Irish males and females, for whom it increased by about 0.2.
Table 4.
Age-adjusted rates and adjusted rate ratios for newly diagnosed cases of HIV, HBV and HCV infection among males and females in the 2001 Scotland census-linked cohort, April 2001–March 2013. White Scottish = 1.0
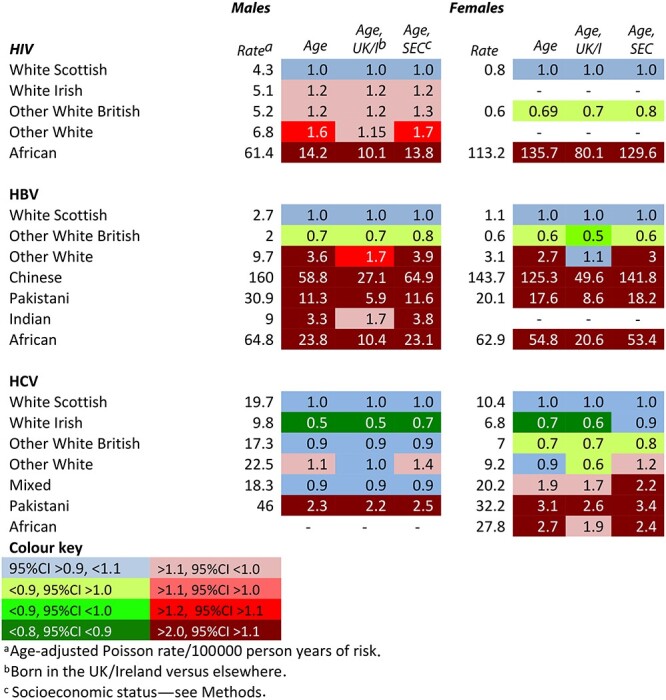
|
Discussion
Main findings of this study
Compared with the White Scottish group, overall hospitalization/death rates with a diagnosis of infection were lower by at least 10% for both males and females in the Other White British, Other White and Chinese groups, and higher by at least 10% in the Pakistani group. Our analysis also revealed widely varying rates in specific categories of infection between ethnic groups, in some categories by >10-fold. Compared to the White Scottish, rates of serologically confirmed HIV infection were >100 times higher among African females and >10 times higher in African males; rates of HBV infection were >50 times higher among Chinese males and females and also much higher among Africans and Pakistanis. Differences in HCV were less marked, in part due to relatively high rates among the White Scottish.24
What is already known on this topic
In a US nationwide sample of over 40 million hospitalizations with a first diagnosis of infection during 1996–2006, overall age-adjusted rates were 31% and 44% higher in Hispanics and Blacks, respectively than in the non-Hispanic White majority, but 35% lower among people of Asian/Pacific Island ethnicity.11 However, in 26% of cases, ethnicity was not recorded. In a study from Arizona with near complete ethnicity data, a more nuanced picture emerged, with both North American Indians and Blacks having 22% higher rates of hospitalization for all infections combined than Whites, but lower rates in several categories such as abdominal and post-operative infections.25 Another US study found Blacks and Hispanics had higher seroprevalence rates than Whites for most of six infectious diseases including hepatitis A virus, HBV and H pylori.12 In Alaska, the hospitalization rate due to infection was 3.7 times higher for American Indian/Alaskan Natives than Whites.26 In New Zealand, rates of hospitalization with a primary diagnosis of any infection during 1989–2008 were around twice as high among both Māori and Pacific Islanders compared with White Europeans.13 A smaller cohort study of infants in New Zealand reported similar findings.27 Indigenous Australians were found to have a rate of septicaemia 14 times that of non-Indigenous Australians, strongly associated with severe socioeconomic disadvantage.28
In Europe, there has been recognition of much higher prevalences of TB, HBV, HCV and HIV among migrants 7,8 but seldom have data been available accurately to compare rates between representative samples of different ethnic groups.29,30 In Amsterdam, several ethnic minority groups had 3–13 times higher rates of HBV markers than the native Dutch, but lower rates for HCV.31 In Scotland, compared with the White Scottish, the estimated prevalence of chronic HBV infection was >100 times higher among East Asians (China and four other countries combined) and >10 times higher among South Asians (India, Pakistan and seven other countries).32 A detailed analysis of COVID-19 deaths in England and Wales during 2 March to 28 July 2020 found much higher age-adjusted death rates in Black and South Asian groups, mainly explained by socioeconomic factors.33 Similarly higher age-adjusted COVID-19 death rates among non-White groups have been found in the USA.12
What this study adds
To our knowledge, this is the first study of the relationships between ethnicity and infection using a census-linked cohort with complete ethnicity reporting, linked to high quality national databases of clinical and serological records. It compares ethnic groups for a wider range of infections than in any previous study, revealing substantial differences in many categories but most strikingly for HIV and HBV.
Although a descriptive study of ethnic differences in health cannot prove causation, it can generate hypotheses and inform policy and practice. Davey Smith and colleagues considered a range of possible causes of apparent health differences between ethnic groups: artefactual; migration; culture, beliefs and behaviours; biology; socioeconomic factors; racism; health service use and access.10 The diagnostic coding in our study was based on clinical or laboratory evidence and unlikely to be influenced by the patient’s ethnic group. The observed differences are thus likely to be real within the bounds of statistical confidence. Originating from a country of higher prevalence is an obvious explanation for the high rates of HIV we found among Africans, and of HBV among Chinese, Africans and Pakistanis, supported by the much lower RRs when adjusted for being born in the UK/I. It is also a plausible explanation for higher rates of fungal infections among African females, heart and circulatory infections among Chinese, Other South Asian and Caribbean females and reproductive system infections in Caribbean males.34 Many of the cases of ‘cancer from infection’ among Chinese males were due to nasopharyngeal carcinoma, associated with the Epstein–Barr Virus (EBV) and unusually common in parts of China.35,36 Cultural and behavioural differences could contribute to the lower rates among the Chinese compared with the Pakistanis in some of the infection categories examined. For example, most of the cases of typhoid reported in the UK occur in people who have recently visited relatives in Pakistan, India or Bangladesh.37 Possibly lower rates of pneumococcal or influenza immunization among Pakistanis could increase the risk of lower respiratory tract infections.17 Certain genetically inherited conditions such as sickle cell disease and thalassaemia increase the risk of a range of serious infections and are much more prevalent in some ethnic groups than others.38,39 However, to determine whether any of the differences we have observed has a genetic basis would require extensive further research.
Unfavourable socioeconomic circumstances can perpetuate living conditions for Indigenous and other ethnic minorities in which a range of infections can prosper.12,14,24,25 When they result from discriminatory policies, laws and practices, they meet the definition of structural or systemic racism1, recently identified as a likely contributor to ethnic differences in the COVID-19 pandemic in England and Wales.40 However, in our study, ethnic minorities often had lower rates of infections than the White Scottish majority, and adjustment for SES made little difference to overall hospitalization/death rates. This perhaps reflects the relatively favourable SES of many people in ethnic minorities in Scotland (Supplementary Table S1a) and a relatively inclusive policy environment.41,42 The lack of evidence in our hospitalization/death data to suggest differential health service access or use by ethnic groups is supported by a broader study of hospitalization for all causes.43 Language difficulties or cultural differences may inhibit attendance at screening services, as an earlier SHELS analysis of breast screening has suggested.44 As effective treatment or immunization exists, there is a strong case for promoting screening services among ethnic groups with a high prevalence of TB, HIV and HBV.7,8 Given the stigma and fear that still surround these conditions, involving community representatives in their design and implementation is desirable. A finding from the SHELS data that African men were twice as likely as White Scottish men to present with late HIV infection has prompted the Scottish Government to call for efforts to encourage more African men to seek HIV testing.16
Limitations of this study
To ensure large enough numbers for analysis, some of the ethnic groups we used are very heterogeneous, e.g. Other White, African or Other South Asian. Nevertheless, we had sufficient numbers to study only 15 of the 39 pre-determined categories of infection. If disaggregation into more narrowly defined ethnic groups and infection categories had been possible, other important differences might have been revealed. As we selected cases with an infection recorded in any diagnostic position on the clinical or death record, the contribution of the infection to the hospitalization or death will vary, but this is unlikely to be biased by ethnic group. Due to small numbers, our adjustments of the hospitalization/death data for SES and country of birth were limited to all infections aggregated.
Conclusions
This census linkage study reveals complex and sometimes unexpected differences between ethnic groups in the incidence or prevalence of a wide range of infections. The many possible causal explanations suggest a wide range of different interventions may be appropriate. As recently recommended by a Scottish expert group, census linkage offers an effective method for studying the disproportionate impact of COVID-19 on ethnic minorities.45
Supplementary Material
Acknowledgements
This work was supported by the Chief Scientist Office [CZH/4/878]; Health Protection Scotland; and NHS Health Scotland [no grant numbers]. Information Services Division (ISD) of NHS National Services Scotland and National Records of Scotland provided in-house technical support. Katikireddi was supported by a NHS Research Scotland Senior Clinical Fellowship [SCAF/15/02]; the Medical Research Council [MC-UU-2017/13 and MC-UU-12017/15] and the Chief Scientist Office [SPHSU13 and SPHSU15]. Sheikh is supported by BREATHE - The Health Data Research Hub for Respiratory Health [MC_PC_19004]. BREATHE is funded through the UK Research and Innovation Industrial Strategy Challenge Fund and delivered through Health Data Research UK.
L D Gruer, Honorary Professor
G I Cézard, Research Fellow
L A Wallace, Principal Healthcare Scientist
S J Hutchinson, Professor
A F Douglas, Research Manager
D Buchanan, Head of Section
S V Katikireddi, Clinical Senior Research Fellow
A D Millard, Research Fellow
D J Goldberg, Professor
A Sheikh, Professor
R S Bhopal, Professor
Contributor Information
L D Gruer, Usher Institute, University of Edinburgh, Edinburgh EH8 9AG, UK.
G I Cézard, Population and Health Research Group, School of Geography and Sustainable development, University of St Andrews, St Andrews KY16 9AL, UK.
L A Wallace, Health Protection Scotland, NHS National Services Scotland, Glasgow G2 6QE, UK.
S J Hutchinson, School of Health and Life Sciences, Glasgow Caledonian University, Glasgow G4 0BA, UK.
A F Douglas, Usher Institute, University of Edinburgh, Edinburgh EH8 9AG, UK.
D Buchanan, Information Services Division, NHS National Services Scotland, Edinburgh EH12 9EB, UK.
S V Katikireddi, MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, Glasgow G2 3AX, UK.
A D Millard, MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, Glasgow G2 3AX, UK.
D J Goldberg, Health Protection Scotland, NHS National Services Scotland, Glasgow G2 6QE, UK.
A Sheikh, Usher Institute, University of Edinburgh, Edinburgh EH8 9AG, UK.
R S Bhopal, Usher Institute, University of Edinburgh, Edinburgh EH8 9AG, UK.
Conflict of interests
None declared.
Authors’ contributions
RSB was the principal investigator. GL and HS chaired the mortality and hospitalization and bloodborne virus subgroups, respectively. All authors contributed to and agreed the design of the study. DA managed the study. CG drafted the data analysis plan, discussed it with all authors and carried out the statistical analysis. HS, WL and GD interpreted the bloodborne virus analysis. All the other authors contributed to the interpretation of the other findings. GL conducted the literature search and drafted and finalized the paper. All authors commented on all the drafts and approved the final version.
Other contributions
T Varley reviewed all the ICD-10 codes for infections. AH and TK gave administrative help. CF as co-applicant helped to set up the study; CP was a co-investigator and had the idea of linking the census data to the health data and performed most of the linkage of the census to the CHI; AS advised on the use of census data; CS advised on and assisted with the bloodborne virus databases; DC advised on and assisted with linkage to the health databases; JP advised on data analysis, particularly the social and economic variables.
Data sharing
Researchers who wish to access the data should apply to National Records of Scotland (https://www.nrscotland.gov.uk/) and ISD (http://www.isdscotland.org/). They are maintained in a secure environment and governed by ethical and other restrictions on access.
References
- 1. Johnson M, Bhopal R, Ingleby J et al. A glossary for the first world congress on migration, ethnicity, race and health. Public Health 2019;172:85–8. [DOI] [PubMed] [Google Scholar]
- 2. Aldridge RW, Nellums LB, Bartlett S et al. Global patterns of mortality in international migrants: a systematic review and meta-analysis. Lancet 2018;392:2553–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhopal RS, Gruer L, Cezard G et al. Mortality, ethnicity, and country of birth on a national scale, 2001–2013: a retrospective cohort (Scottish health and ethnicity linkage study). PLoS Med 2018;15:e1002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikram UZ, Mackenbach JP, Harding S et al. All-cause and cause-specific mortality of different migrant populations in Europe. Eur J Epidemiol 2016;31:655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gruer L, Stanaway FF, Krasnik A, Zeegers Paget D. 1st World Congress on migration, ethnicity, race and health. Eds. Eur J Public Health 2018;28(Suppl 1):1–205. [Google Scholar]
- 6. European Centre for Disease Prevention and Control . Public Health Guidance on HIV, Hepatitis B and C Testing in the EU/EEA – An Integrated Approach. Stockholm: ECDC, 2018. [Google Scholar]
- 7. European Centre for Disease Prevention and Control . Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants within the EU/EEA. Stockholm: ECDC, 2018. [Google Scholar]
- 8. Davey Smith G, Chaturvedi N, Harding S et al. Ethnic inequalities in health: a review of UK epidemiological evidence. Crit Public Health 2000;10:375–408. [Google Scholar]
- 9. Public Health England . Disparities in the Risk and Outcomes of COVID-19. London: PHE, 2020. [Google Scholar]
- 10. APM Research Lab Staff . The Color of Coronavirus: COVID-19 deaths by race and ethnicity in the US. https://www.apmresearchlab.org/covid/deaths-by-race (10 December 2020, date last accessed).
- 11. Christensen K, Holman R, Steiner C et al. Infectious disease hospitalizations in the United States. Clin Infect Dis 2009;49:1025–35. [DOI] [PubMed] [Google Scholar]
- 12. McQuillan G, Kruszon-Moran D, Kottiri B et al. Racial and ethnic differences in the Seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988-1994. Am J Public Health 2004;94:1952–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker M, Barnard L, Kvalsvig A et al. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 2012;379:1112–9. [DOI] [PubMed] [Google Scholar]
- 14. Hobbs M, Morton S, Atatoa-Carr P et al. Ethnic disparities in infectious disease hospitalisations in the first year of life in New Zealand. J Paediatr Child Health 2017;53:223–31. [DOI] [PubMed] [Google Scholar]
- 15. Bhopal R, Fischbacher C, Povey C et al. Cohort profile: Scottish health and ethnicity linkage study of 4.65 million people exploring ethnic variations in disease in Scotland. Int J Epidemiol 2011;40:1168–75. [DOI] [PubMed] [Google Scholar]
- 16. Scottish Government . A fairer Scotland for all: race equality action plan, 2017-2021. Edinburgh: 2017. https://www.gov.scot/publications/fairer-scotland-race-equality-action-plan-2017-2021-highlight-report/. [Google Scholar]
- 17. Simpson C, Steiner M, Cézard G et al. Ethnic variations in morbidity and mortality from lower respiratory tract infections: a retrospective cohort study. J Roy Soc Med 2015;108:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scottish Government and General Register Office of Scotland . Scotland’s New Official Ethnicity Classification. Edinburgh, 2008. https://www2.gov.scot/resource/doc/233640/0063967.pdf. [Google Scholar]
- 19. National Records of Scotland . 2001. Census Variables, Appendix D Report P15. https://www.nrscotland.gov.uk/statistics-and-data/census/2001-census/a-review/data-quality/2001-census-variables/results-and-conclusions/appendix-d
- 20. Pinner RW, Teutsch SM, Simonsen L et al. Trends in infectious diseases mortality in the United States. JAMA 1996;275:189–93. [PubMed] [Google Scholar]
- 21. Scottish Government . Scottish Index of Multiple Deprivation, Edinburgh, Scottish Government, 2016. https://www.gov.scot/Topics/Statistics/SIMD.
- 22. Fischbacher CM, Cezard G, Bhopal RS et al. Measures of socioeconomic position are not consistently associated with ethnic differences in cardiovascular disease in Scotland: methods from the Scottish health and ethnicity linkage study (SHELS). Int J Epidemiol 2014;43:129–39. [DOI] [PubMed] [Google Scholar]
- 23. Benchimol E, Smeeth L, Guttmann A et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roy K, Hutchinson S, Wadd S et al. Hepatitis C virus infection among injecting drug users in Scotland: a review of prevalence and incidence data and the methods used to generate them. Epidemiol Infect 2007;135:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Callinan L, Holman R, Esposito D, McDonald M. Racial/ethnic disparities in infectious disease hospitalizations in Arizona. J Health DisparitRes Pract 2013;6:49–71. [Google Scholar]
- 26. Gounder P, Holman R, Seeman S et al. Infectious disease hospitalizations among American Indian/Alaska native and non-American Indian/Alaska native persons in Alaska, 2010-201. Public Health Rep 2017;132:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hobbs M, Atatoa Carr P, Fa’alili-Fidow J et al. How differing methods of ascribing ethnicity and socio-economic status affect risk estimates for hospitalisation with infectious disease. Epidemiol Infect 2018;1–9. doi: 10.1017/S0950268818002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Einsiedel L, Fernandes L, Joseph S et al. Non-communicable diseases, infection and survival in a retrospective cohort of indigenous and non-indigenous adults in Central Australia. BMJ Open 2013;3:e003070. doi: 10.1136/bmjopen-2013-003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alvarez-del Arco D, Monge S, Azcoaga A et al. HIV testing and counselling for migrant populations living in high-income countries: a systematic review. Eur J Public Health 2012;23:1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu J, Wörmann T, Popp J et al. Changing epidemiology of hepatitis B and migration - a comparison of six northern and North-Western European countries. Eur J Public Health 2012;23:642–7. [DOI] [PubMed] [Google Scholar]
- 31. Zuure F, Bil J, Visser M et al. Hepatitis B and C screening needs among different ethnic groups: a population-based study in Amsterdam, the Netherlands. JHEP Reports 2019. doi: 10.1016/j.jhepr.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schnier C, Wallace L, Templeton K et al. Use of laboratory-based surveillance data to estimate the number of people chronically infected with hepatitis B living in Scotland. Epidemiol Infect 2014;142:2121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Office for National Statistics . Updating ethnic contrasts in deaths involving the coronavirus (COVID-19), England and Wales: deaths occurring 2 March to 28 July 2020. United Kingdom Office of National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/updatingethniccontrastsindeathsinvolvingthecoronaviruscovid19englandandwales/deathsoccurring2marchto28july2020.
- 34. WHO Expert Consultation Group . Rheumatic Fever and Rheumatic Heart Disease. Geneva: World Health Organisation, 2004. [Google Scholar]
- 35. Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer 2014;33:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu M, Yuan J-M. Epidemiology of nasopharyngeal carcinoma. Cancer Biol Ther 2002;12:421–9. [DOI] [PubMed] [Google Scholar]
- 37. NHS Website. Overview: typhoid fever. https://www.nhs.uk/conditions/typhoid-fever/ (6 January 2021, date last accessed).
- 38. Booth C, Inusa B, Obaro S. Infection in sickle cell disease: a review. Int J Infect Dis 2010;14:e2–e12. [DOI] [PubMed] [Google Scholar]
- 39. Vento S, Cainelli F, Cesario F. Infection and thalassaemia. Lancet Infect Dis 2006;6:226–33. [DOI] [PubMed] [Google Scholar]
- 40. Public Health England . Beyond the data: Understanding the impact of COVID-19 on BAME groups. London: Public Health England, 2020. [Google Scholar]
- 41. Bhopal RS. The quest for culturally sensitive health-care systems in Scotland: insights for a multi-ethnic Europe. J Public Health 2012;34:5–11. [DOI] [PubMed] [Google Scholar]
- 42. Scottish Government . Race Equality Framework for Scotland 2016 to 2030. Edinburgh, Scottish Government, 2016. https://www.gov.scot/publications/race-equality-framework-scotland-2016-2030/
- 43. Gruer L, Millard A, Williams L et al. All-cause hospitalisation differences by ethnic group: a data linkage cohort study of 4.62 million people in Scotland 2001-13. Public Health 2018;161:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bansal N, Bhopal R, Steiner M, Brewster D. Major ethnic group differences in breast cancer screening uptake in Scotland are not extinguished by adjustment for indices of geographical residence, area deprivation, long-term illness and education. Br J Cancer 2012;106:1361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Expert Reference Group on COVID-19 and Ethnicity . Initial Advice and Recommendations on Systemic Issues. Edinburgh, Scottish Government 2020. https://www.gov.scot/groups/expert-reference-group-on-covid-19-and-ethnicity/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


