Abstract
Most patients with coronavirus disease 2019 (COVID-19) experience asymptomatic disease or mild symptoms, but some have critical symptoms requiring intensive care. It is important to determine how patients with asymptomatic or mild COVID-19 react to severe acute respiratory syndrome coronavirus 2 infection and suppress virus spread. Innate immunity is important for evasion from the first virus attack, and it may play an important role in the pathogenesis in these patients. We measured serum cytokine levels in 95 patients with COVID-19 during the infection’s acute phase and report that significantly higher interleukin 12 and 2 levels were induced in patients with asymptomatic or mild disease than in those with moderate or severe disease, indicating the key roles of these cytokines in the pathogenesis of asymptomatic or mild COVID-19.
Keywords: IL-12, IL-2, innate immunity, COVID-19, asymptomatic infection
Interleukin 12 and 2 levels were significantly higher in patients with asymptomatic or mild coronavirus disease 2019 (COVID-19) (acute phase) than in those with moderate or severe disease, indicating the key roles of these cytokines in asymptomatic or mild COVID-19.
The ongoing pandemic of coronavirus disease 2019 (COVID-19) has had significant effects on many aspects of our daily lives, including socioeconomic and political issues. According to the World Health Organization COVID-19 dashboard, as of 25 October 2020, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had infected >42 million people worldwide and resulted in >1.1 million deaths. The clinical spectrum of COVID-19 ranges from an asymptomatic status to fatal infections.
It has been shown that several components of the immune response are dysregulated in patients with COVID-19 who have severe symptoms. Monocytes and macrophages were significantly dysregulated in patients with severe disease (reviewed in [1]). Dendritic cells and natural killer (NK) cells were depleted in patients with COVID-19 compared with healthy controls [2], and it has been determined that those cells are dysregulated in the setting of SARS-CoV-2 infection.
The immune response features of asymptomatic and mild COVID-19 are little known. It is important to determine how patients with asymptomatic or mild COVID-19 react to SARS-CoV-2 infection and suppress the spread of the virus. Because innate immunity is important for the host’s evasion from the first virus attack, it may play a role in the pathogenesis of asymptomatic or mild COVID-19. We therefore focused on the acute phase of SARS-CoV-2 infection to gain insights into differences among the cytokines induced by the innate immune response in patients with COVID-19 of differing severities, because different cytokines may affect the features of the disease.
MATERIALS AND METHODS
We investigated a total of 95 patients with COVID-19 (52 male and 43 female; median age [range], 50 [15–98] years) of different disease severities (16 asymptomatic, 49 mild, 11 moderate, and 19 severe) during the early phase of infection (ie, <10 days after symptom onset or after an asymptomatic patient’s notifiable contact with a positive case patient). Two of the 11 patients with moderate COVID-19 (18.2%) had mild disease when their blood samples were collected, but both eventually had moderate disease. Of the patients with severe COVID-19, 10 (52.6%) had mild and 3 (15.8%) had moderate disease when their blood samples were collected, disease that eventually became severe. The classification of disease severity was based on interim guidance on the management of COVID-19 from the World Health Organization [3].
COVID-19 diagnoses were based on polymerase chain reaction detection of the SARS-CoV-2 genome in nasopharyngeal swab samples. Symptomatic COVID-19 cases in patients without evidence of pneumonia or hypoxia were classified as mild. Cases in patients with clinical signs of pneumonia were classified as moderate (oxygen saturation as measured by pulse oximetry, ≥90% with room air) or severe (respirations >30/min, severe respiratory distress, or oxygen saturation <90% with room air). All patients were treated at Hyogo Prefectural Kakogawa Medical Center, Hyogo, Japan, from July to September 2020 and were consecutively recruited to this study. We included 20 healthy controls (13 male and 7 female; median age [range], 57 [27–64] years), who were SARS-CoV-2 immunoglobulin G–negative medical staff from the same facility [4].
Serum samples were subjected to the measurements of cytokines, chemokines, and growth factors with the Bio-Plex Pro Human Cytokine Screening 48-plex panel (Bio-Rad), following the manufacturer’s instructions, and results were read using the Bio-Plex 200 system. Experiments were done twice independently, both showing similar results. This study was approved by the ethical committee of Kobe University Graduate School of Medicine (approval code B200200), and written informed consent was obtained from all participants.
GraphPad Prism software (version 8.4.3) was used for statistical analysis and figures preparation. The Kolmogorov-Smirnov test was used to test the normality of data distribution. Nonnormally distributed data were analyzed using the Kruskal-Wallis test, and normally distributed data using 1-way analysis of variance. For all analyses, multiple comparisons test with Dunn method was used to compare serum cytokine levels between 2 groups. Results were considered statistically significant at P < .05.
RESULTS
Interleukin 12 and 2 Levels
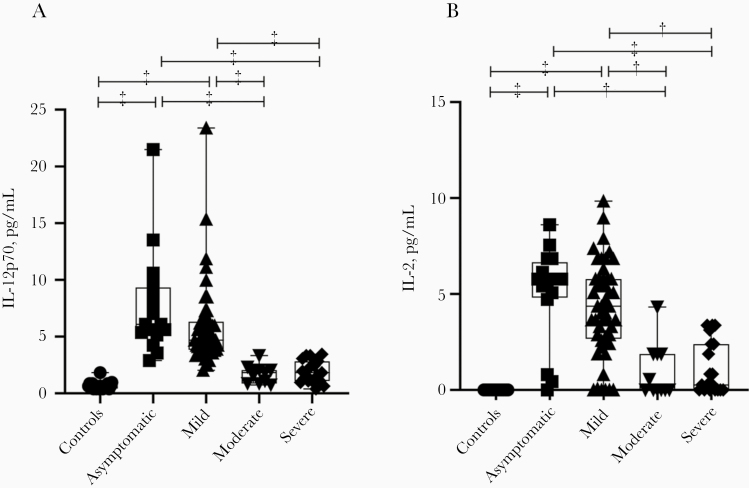
Levels of cytokines, chemokines, and growth factors were measured in the 115 blood samples from patients with COVID-19 and healthy controls. The most important finding was that interleukin 12 (IL-12) levels were significantly higher in both the asymptomatic and mild disease groups than in the moderate and severe groups (Figure 1A), but these levels were comparable between the moderate and severe disease groups and healthy controls. Similarly to the IL-12 findings, serum levels of interleukin 2 (IL-2) were significantly higher in patients with asymptomatic or mild COVID-19 than in those with moderate or severe COVID-19 (Figure 1B).
Figure 1.
Serum interleukin 12p70 (IL-12p70) (A) and interleukin 2 (IL-2) (B) levels in patients with asymptomatic or symptomatic (mild, moderate, or severe) coronavirus disease 2019 and healthy controls. Box plots show medians (middle lines) with first and third quartiles (boxes), and whiskers show maximum and minimum values. †P < .005; ‡P < .001.
Interleukin 18 and 6 Levels
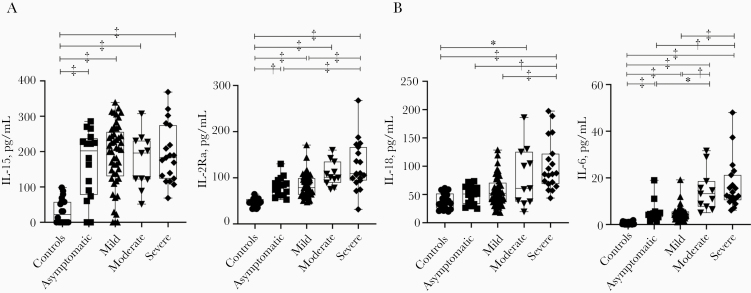
Our evaluation of other cytokines in patients with COVID-19 of differing severity revealed that the following cytokines were significantly higher in patients with COVID-19 than in healthy controls but did not differ by severity group: interleukin 15, 2Ra, 1Ra, 7, 10, 13, 1a, and 16, interferon (IFN) γ, monocyte chemoattractant protein 1, platelet-derived growth factor BB, and tumor necrosis factor α (Figure 2A and Supplementary Figure 1).
Figure 2.
Serum interleukin levels induced in patients with asymptomatic or symptomatic (mild, moderate, of severe) coronavirus disease 2019 (COVID-19) and healthy controls. A, Serum levels of interleukin 15 (IL-15) (left) and interleukin 2Ra (IL-2Ra) (right) were increased in patients with COVID-19, but they were not correlated with disease severity. B, Serum levels of interleukin 18 (IL-18) (left) and interleukin 6 (IL-6) (right) were increased in patients with COVID-19 and were correlated with disease severity. Box plots show medians (middle line) with first and third quartiles (boxes), and whiskers show maximum and minimum values. *P < .05; †P < .005; ‡P < .001.
We observed a different pattern in serum interleukin 18 (IL-18) levels. As shown in Figure 2B, these levels were significantly higher in the patients with symptomatic COVID-19 than in asymptomatic patients and healthy controls. They also increased in accordance with disease severity, although they were comparable in the asymptomatic and mild disease groups. Similarly, our present findings demonstrated that interleukin 6 (IL-6) levels increasing in accord with severity level of COVID-19 in the acute phase.
Discussion
Most patients with COVID-19 are asymptomatic or experience mild symptoms, but some experience critical symptoms requiring intensive care. A dysregulated immune response is a feature of severe COVID-19, but the immune response features of asymptomatic and mild COVID-19 are little known. In the current study, we analyzed cytokines induced by the innate immune response in patients with COVID-19 of differing severity, as different cytokines may affect the features of the disease.
In our measurement of 48 cytokines, chemokines, and growth factors, several interesting results were obtained. In early phase of SARS-CoV-2 infection, IL-12 levels were observed to be significantly higher in the asymptomatic and mild disease groups than in the moderate and severe disease groups. Similarly, Xu et al [5] reported that serum IL-12 levels were comparable between patients with mild (classified as moderate in our study) and those with severe COVID-19 . However, they did not include asymptomatic patients or those without pneumonia (whom we classified in the mild infection group).
IL-12 is secreted by dendritic cells and macrophages in response to microbial stimuli—including virus infection—and it acts on the IL-12 receptor, expressed mainly by activated T and NK cells [6]. IL-12, together with IL-15, IL-18, and type I IFN, enhances the cytotoxic activity of NK cells and induces secretion of IFN-γ. IFN-γ secreted by NK cells activates macrophages to destroy phagocytosed microbes. IL-12 is also known as a key inducer of T-helper 1 cell differentiation [7].
The current study is the first to show that during the acute phase of SARS-CoV-2 infection, a significantly higher level of IL-12 was induced in patients with asymptomatic or mild COVID-19 than in both those with moderate or severe symptoms and healthy controls. Interestingly, several studies showed that the numbers of peripheral NK cells were significantly lower in patients with severe COVID-19 than in healthy individuals [2, 8] or patients with mild COVID-19 [2]. Taken together, it is possible that induction of IL-12 is required to maintain NK cell numbers in the early phase of SARS-CoV-2 infection, and that this induction may play a role in evasion from virus spreading, as seen in asymptomatic patients and those with mild symptoms.
Similarly, serum IL-2 levels were found to be significantly higher in patients with asymptomatic and or mild COVID-19 than in those with moderate or severe symptoms. Long et al [9] showed that IL-2 levels were significantly higher in symptomatic than in asymptomatic COVID-19. Although their result seemed to differ from ours, this may reflect differences between studies in the time of sample collection after disease onset or notice of infection.
IL-2 is produced by CD4+ and CD8+ T cells, some B cells, and dendritic cells; its major function is to promote the proliferation of both CD4+ and CD8+ T cells [10]. Importantly, IL-2 is also known as a growth factor for NK cells; it promotes the production of NK-derived cytokines (tumor necrosis factor α, IFN-γ, and granulocyte-macrophage colony-stimulating factor [GM-CSF]) and has a synergistic effect with IL-18 to enhance the cytotoxicity and expansion of NK cells [10, 11]. Based on these findings together, it seems reasonable that a higher induction of IL-2 was observed in our asymptomatic and mild COVID-19 groups compared with the moderate and severe COVID-19 groups in the acute phase, and several studies have consistently observed higher NK cell counts in patients with mild COVID-19 than in those with severe disease [2, 8]. However, further study is required to understand the correlation between the induction of IL-2 and NK cell numbers in patients with COVID-19.
Younger persons infected with SARS-CoV-2 are more likely to have asymptomatic or mild infection than older persons [12]. The demographic characteristics of patients in the different severity groups are shown in Supplementary Table 1. Comparing median ages, patients with asymptomatic or mild COVID-19 were also significantly younger than those with severe disease (Supplementary Table 1 and Supplementary Figure 2A). However, we could not see any correlation between age of the subjects and either serum IL-12 or IL-2 levels in this study (Supplementary Figure 2B and 2C). This analysis would support a possible protective role of early induction of IL-12 and IL-2 in the severity of COVID-19.
The current investigation is the first to show that, during the acute phase of infection, significantly higher levels of IL-12 and IL-2 were induced in patients with asymptomatic or mild COVID-19 than in those who with moderate or severe symptoms, indicating the important roles of these 2 cytokines in the protection from severe COVID-19. Regarding IL-18 levels, our study showed that these were significantly higher in patients with severe COVID-19 than in those with milder symptoms. IL-18, known as an IFN-γ–inducing factor, is involved in the activation of NK cells, T-helper 1 and 2 cells, and macrophages. Its precursor, which is cleaved by caspase 1 to become biologically active IL-18, is constitutively expressed in monocytes, macrophages, dendritic cells, and endothelial cells [13].
IL-18 has been shown to have a very important role in acute respiratory distress syndrome (ARDS) [13], a feature of severe COVID-19. In case of ARDS caused by avian influenza virus (H5N1 and H7N9), PB1-F2 protein activates NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) in a prolonged manner, which then activates caspase 1, resulting in excess IL-18 production and a very detrimental IFN-γ–biased cytokine storm [13]. We observed high expression of IL-18 in our patients with severe COVID-19, and this finding together with the common occurrence of ARDS in patients with severe COVID-19 seem correlated, through an underlying mechanism that must be explored further.
Consistent with the other studies related to the cytokine expressions in patients with COVID-19 [14], our present findings demonstrated that IL-6 levels increased in accord with the severity of COVID-19 in the acute phase. Meta-analysis by Leisman et al [15] showed that serum IL-6 levels were significantly lower in severe or critical COVID-19 than in other critical diseases (sepsis, cytokine release syndrome, and ARDS unrelated to COVID-19), suggesting that factors other than cytokine storm—including endovasculitis, direct viral injury and lymphodepletion, and virus-induced immunosuppression—might be responsible for organ dysfunction in COVID-19. It was recently reported that the endothelial trans-signaling of IL-6 induces the production of plasminogen activator inhibitor 1 in vascular endothelial cells, further explaining the endotheliopathy and coagulopathy that commonly occur in patients with severe COVID-19 [16]. Recent studies and our present results indicate that the induction of IL-6 may play a role in the severity of COVID-19.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Kazuro Sugimura MD, PhD (executive vice president, Kobe University), for his full support to promote this study. We express our sincere gratitude for the cooperation and participation of the staffs of Hyogo Prefectural Kakogawa Medical Center.
Financial support. This work was supported by the Hyogo prefectural government.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McKechnie JL, Blish CA. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe 2020; 27:863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020; 17:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Clinical management of COVID-19. Interim guidance. 27 May 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed 30 July 2020.
- 4. Nagano T, Arii J, Nishimura M, et al. Diligent medical activities of a publicly designated medical institution for infectious diseases pave the way for overcoming COVID-19: a positive message to people working at the cutting edge. Clin Infect Dis doi:10.1093/cid/ciaa694. Published 31 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu ZS, Shu T, Kang L, et al. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal COVID-19 patients. Signal Transduct Target Ther 2020; 5:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Presky DH, Yang H, Minetti LJ, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci U S A 1996; 93:14002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abbas AK, Lichtman AH, Pillai S.. Cellular and molecular immunology. 7th ed. Philadelphia, PA: Elsevier/Saunders, 2012. [Google Scholar]
- 8. Maucourant C, Filipovic I, Ponzetta A, et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci Immunol 2020; 5:eabd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
- 10. Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004; 28:109–23. [DOI] [PubMed] [Google Scholar]
- 11. Son YI, Dallal RM, Mailliard RB, Egawa S, Jonak ZL, Lotze MT. Interleukin-18 (IL-18) synergizes with IL-2 to enhance cytotoxicity, interferon-gamma production, and expansion of natural killer cells. Cancer Res 2001; 61:884–8. [PubMed] [Google Scholar]
- 12. Liu Y, Mao B, Liang S, et al. Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J 2020; 55:2001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev 2018; 281:138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368:473–4. [DOI] [PubMed] [Google Scholar]
- 15. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020; 8:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang S, Tanaka T, Inoue H, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A 2020; 117:22351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.