Abstract
Purpose
Comorbidities making up metabolic syndrome (MetS), such as obesity, type 2 diabetes, and chronic cardiovascular disease can lead to increased risk of coronavirus disease-2019 (COVID-19) with a higher morbidity and mortality. SARS-CoV-2 antibodies are higher in severely or critically ill COVID-19 patients, but studies have not focused on levels in convalescent patients with MetS, which this study aimed to assess.
Methods
This retrospective study focused on adult convalescent outpatients with SARS-CoV-2 positive serology during the COVID-19 pandemic at NewYork Presbyterian/Weill Cornell. Data collected for descriptive and correlative analysis included SARS-COV-2 immunoglobin G (IgG) levels and history of MetS comorbidities from April 17, 2020 to May 20, 2020. Additional data, including SARS-CoV-2 IgG levels, body mass index (BMI), hemoglobin A1c (HbA1c) and lipid levels were collected and analyzed for a second cohort from May 21, 2020 to June 21, 2020. SARS-CoV-2 neutralizing antibodies were measured in a subset of the study cohort.
Results
SARS-CoV-2 IgG levels were significantly higher in convalescent individuals with MetS comorbidities. When adjusted for age, sex, race, and time duration from symptom onset to testing, increased SARS-CoV-2 IgG levels remained significantly associated with obesity (P < 0.0001). SARS-CoV-2 IgG levels were significantly higher in patients with HbA1c ≥6.5% compared to those with HbA1c <5.7% (P = 0.0197) and remained significant on multivariable analysis (P = 0.0104). A positive correlation was noted between BMI and antibody levels [95% confidence interval: 0.37 (0.20-0.52) P < 0.0001]. Neutralizing antibody titers were higher in COVID-19 individuals with BMI ≥ 30 (P = 0.0055).
Conclusion
Postconvalescent SARS-CoV-2 IgG and neutralizing antibodies are elevated in obese patients, and a positive correlation exists between BMI and antibody levels.
Keywords: obesity, diabetes, SARS-CoV-2, COVID-19, antibody
Although most individuals who contract severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) present with mild novel coronavirus disease (COVID-19) symptoms, some patients will develop severe pneumonia, acute respiratory distress syndrome, multiorgan failure, and/or death (1). As the COVID-19 pandemic progresses, epidemiological data indicate that obesity, type 2 diabetes, and chronic cardiovascular disease can impact its severity, leading to a poorer prognosis and poorer outcome (2-6). SARS-CoV-2 immunoglobin G (IgG) and neutralizing antibodies have been documented to be higher in severely or critically ill COVID-19 patients, during both the acute and convalescent phases (7,8), but no such studies have focused specifically on patients with metabolic disorders. Due to the growing prevalence of diabetes, obesity, and hypertension (HTN), it is crucial to understand the unique characteristics of COVID-19 infection in people with these comorbidities. Furthermore, the relevance of such inquiries are important because obesity and type 2 diabetes may adversely impact the immune system, as indicated by pre-COVID-19 era studies showing how these conditions lower vaccine efficacy and increase inflammatory states (9,10).
To better understand whether obesity and diabetes influence SARS-CoV-2 antibody production in nonhospitalized COVID-19 cases, we performed a retrospective cohort study focusing on adult, nonpregnant, convalescent COVID-19 patients who tested positive for SARS-CoV-2 IgG in the outpatient setting at NewYork-Presbyterian/Weill Cornell Medical Center.
Methods
Patients and data sources
This study was approved by the Weill Cornell Medicine Institutional Review Board (#20-03021671). Neutralizing antibody testing at the Wadsworth Center was performed under the approval of the NYSDOH Institutional Review Board (#20–021).
A retrospective cohort analysis was performed to compare SARS-CoV-2 IgG and neutralizing antibodies in convalescent COVID-19 patients who were seropositive in the outpatient setting at NewYork-Presbyterian/Weill Cornell Medical Center. All participants in this study were adult nonpregnant patients who were not hospitalized (previously or at the time of antibody testing) due to SARS-CoV-2 infection.
The first cohort of patients was tested for SARS-CoV-2 IgG between April 17, 2020 and May 20, 2020. Data collected from the electronic medical record (Epic, Verona, WI, USA) for this cohort included demographics, clinical characteristics, comorbidities/conditions, and estimated date of symptom onset. The comorbidities/conditions included obesity [body mass index (BMI) ≥30 kg/m2]; type 2 diabetes; HTN; hyperlipidemia (HLD); and chronic lung, cardiac, hepatic, and renal diseases. These conditions were included in the analysis if the condition was noted to be an active medical condition in the electronic medical record. SARS-CoV-2 IgG index values (IV) for each patient were collected from the Pylon 3D analyzer software (see following discussion), which serves as a proxy for antibody levels.
The second cohort of patients underwent SARS-CoV-2 IgG testing between May 21, 2020, and June 21, 2020. As with the first retrospective study, only adult nonpregnant patients were included. Exclusion criteria included a negative SARS-CoV-2 antibody result, hospitalization (previously or at the time of antibody testing) due to SARS-CoV-2 infection and the lack of concurrent hemoglobin A1c (HbA1c) or lipid panel [total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TG)] laboratory data.
Residual serum samples, when available, were stored at −80℃ for the neutralization assay comparison described next.
SARS-CoV-2 IgG assay
Serum IgG antibodies against SARS-CoV-2 was measured using the Pylon COVID-19 IgG assay on the Pylon 3D analyzer (ET HealthCare, Palo Alto, CA, USA) as previously described (11). Briefly, the assay was performed using a unitized test strip containing wells with predispensed reagents. The COVID-19 reagent contains biotinylated recombinant versions of the SARS-CoV-2 S-protein receptor binding domain and trace amount nucleocapsid protein as antigens that bind specific IgG. The specificity of this assay is 98.8% as previously determined from an analysis of 320 pre-COVID-19 samples. The assay was implemented clinically as a laboratory developed test under New York State Department of Health regulations. The IV had been previously described (11). In short, the normalized IV was determined by the instrument readout of the test sample divided by instrument readout at cut off. An IV ≥ 1 was designated as a positive result for this assay.
SARS-CoV-2 pseudovirus neutralization assay
Pseudovirus stocks
Stocks of 293T cells (American Tissue Culture Collection [ATCC]) were seeded 24 h prior to transfection in a 150 cm2 flask at 60% confluency. An 18-µg aliquot of the pHIV-1NL4-3 ΔEnv-NanoLuc plasmid (obtained from Paul Bieniasz, The Rockefeller University, NY, USA) was co-transfected with 6 µg of pSARS-CoV-2-SΔ19 (also from Paul Bieniasz) using the Effectene (Qiagen) reagent. At 16 h after transfection, the cells were carefully washed twice and the medium replaced. Two days later, the cell supernatant (30 mL) was centrifuged at 1600 revolutions per minute for 10 min. The supernatant was then layered on to a 20% sucrose cushion and centrifuged at 200 000 G for 1.5 h at 4°C. The pseudovirus pellet was resuspended in 30 mL of assay medium (Dulbecco’s Minimal Essential Medium supplemented with 10% fetal bovine serum, 20 IU/mL of Penicillin/Streptomycin (Gibco) and Glutamax (Gibco), and then aliquoted and frozen at −80°C.
Neutralization assay
One day before infection, 96-well black tissue culture plates were coated with 50 µg/mL of Poly-L-Lysine for at least 2 h, then seeded with 1× 104 293T/ACE2cl.22 cells/well (also from Paul Bieniasz). On the day of infection, serum or plasma samples (heat-inactivated earlier for 30 min at 56°C) were centrifuged using a microcentrifuge set at maximum speed for 10 min to remove particulate matter. The clarified samples were diluted 10-fold in assay medium and sterile filtered (Spin-X columns; Costar). They were then serially diluted in six 4-fold steps, mixed with pseudovirus, incubated at 37°C for 1 h and added to the target cells. The pseudovirus stocks had been previously titrated on the same cells. The amount added to the neutralization assay yielded a luciferase signal of 2× 106 relative light units (RLU) in the absence of serum or plasma. Each assay plate included virus control wells (cells and pseudovirus, no serum/plasma sample), viral input wells (pseudovirus only, no cells or sample) and cell-background wells (cells only, no pseudovirus or sample). Two days post infection, the medium was carefully removed from each well before addition of Lysis Buffer (Promega) at 50 µL/well. The plates were incubated for 15 min at room temperature on a shaker, NanoGlo substrate (Promega) was added (25 µL/well), and the plates were incubated for 5 min at room temperature. Luminescent counts per second (RLU) were detected using an Enspire reader (Perkin Elmer).
Neutralization assay data analysis
The average RLU values derived from duplicate viral input wells were subtracted from duplicate values obtained using serum/plasma samples. The background-corrected values were plotted as percentage inhibition. The 8 virus control wells were used to determine the 100% infection level for each plate.
Plaque reduction neutralization test
The plaque reduction neutralization test (PRNT) assay was performed at the Wadsworth Center at the New York State Department of Health (12). The PRNT assay detects viral specific antibodies based on their ability to neutralize their cognate viral infections in Vero E6 cells (C1008, ATCC CRL-1586). Briefly, 100 µL of 2-fold serially diluted test sera were mixed with 100 µL of 200 plaque forming units of SARS-CoV-2, isolate USA-WA1/2020 (BEI Resources, NR181 52281) and incubated at 37°C in 5% CO2 for 1 h. Vero E6 cells (C1008, ATCC CRL-1586) were inoculated with the virus:serum mixture (100 uL) and adsorption was allowed to proceed for 1 h at 37°C in in 5% CO2. A 0.6% agar overlay prepared in cell culture maintenance medium (Eagle’s Minimal Essential Medium, 2% heat-inactivated fetal bovine serum, 100 µg/mL Penicillin G, 100 U/mL Streptomycin) was added at the conclusion of adsorption A second agar overlay containing 0.2% Neutral red was added 2 days post infection. After incubation for an additional 1 day, the number of plaques in each sample were recorded. The titer was reported as the inverse of the highest dilutions of sera providing 90% (PRNT90) reduction in the number of viral plaques, relative to virus-only infection.
Hemoglobin A1c and lipid panel
HbA1c was performed on the TOSOH Automated Gycohemoglobin Analyzer HLC-723G8 (Tosoh Bioscience, Tokyo, Japan). This is a National Glycohemoglobin Standardization Program–certified method. Lipid panel (TC, HDL, LDL, and TG) was performed on the Siemens ADVIA Chemistry XPT System (Siemens Healthineers, Erlangen, Germany).
Statistical analysis
Bivariate associations were evaluated using Fisher’s exact test between 2 categorical variables: t-test or analysis of variance (ANOVA) between numerical variable and categorical variable. Correlations between 2 numerical variables were assessed by Pearson correlation coefficient or polyserial correlation. Univariable and multivariable linear regression were further used to assess the multivariable association between antibody levels and explanatory variables of interest. Confounding factors include age, sex, race, and duration from symptom onset to testing. Data are presented as mean ± SD or median with interquartile range for continuous variables and proportion for categorical variables. Ninety-five percent confidence interval (95%CI) of rates were calculated based on exact binomial distribution. P-values < 0.05 were considered significant. Analyses were performed using statistical software SAS Version 9.4 (SAS Institute, Cary, NC, USA) or GraphPad Prism Version 8.4.1 (GraphPad Software, La Jolla, CA, USA).
Results
Our initial retrospective study focused on outpatients during the period April 17, 2020 to May 20, 2020. Available clinical information, including metabolic syndrome (MetS) comorbidities type 2 diabetes, HTN, HLD, and excess body weight were collected from the electronic medical record (EMR) and included for analysis when available. In total, 1055 outpatients were SARS-CoV-2 antibody positive. Of these, 8.7% (92/1055) had type 2 diabetes, 10.7% (113/1055) had HTN, and 10.4% (110/1055) had HLD. Of the 424 patients that had a BMI (kg/m2) noted in the EMR, 25.9% (110/424) were obese and 27.4% (116/424) were overweight (defined as BMI ≥30 and BMI ≥25 and <30, respectively).
Patient characteristics are summarized in Tables 1 and 2.
Table 1.
Patient characteristics by diabetes status
| Characteristics | Total (n = 1055) | No type 2 diabetes (n = 963) | Type 2 diabetes (n = 92) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 40.89 (12.44) | 39.87 (11.95) | 51.64 (12.48) |
| Median (IQR) | 37.00 (31.00–49.00) | 36.00 (30.00–47.00) | 51.00 (42.00–61.00) |
| Sex, n (%) | |||
| Female | 705 (66.8) | 648 (67.3) | 57 (62.0) |
| Male | 350 (33.2) | 315 (32.7) | 35 (38.0) |
| Race, n (%) | |||
| Asian | 129 (12.8) | 120 (13.0) | 9 (10.0) |
| Black/African American | 91 (9.0) | 75 (8.2) | 16 (17.8) |
| Declined | 196 (19.4) | 184 (20.0) | 12 (13.3) |
| Other | 193 (19.1) | 174 (18.9) | 19 (21.1) |
| White | 401 (39.7) | 367 (39.9) | 34 (37.8) |
| BMI (kg/m 2 ), n (%) | |||
| <25 | 198 (46.7) | 193 (52.0) | 5 (9.4) |
| ≥25 and <30 | 116 (27.4) | 101 (27.2) | 15 (28.3) |
| ≥30 | 110 (25.9) | 77 (20.8) | 33 (62.3) |
| Median (IQR) | 25.44 (22.79–30.00) | 24.87 (22.50–29.05) | 31.53 (27.76–36.33) |
| Hypertension, n (%) | 113 (10.7) | 73 (7.6) | 40 (43.5) |
| Hyperlipidemia, n (%) | 110 (10.4) | 67 (7.0) | 43 (47.3) |
| Days from symptom onset to testing, median (IQR) | 39.67 (31.76–49.63) | 38.94 (31.70–48.94) | 43.76 (34.58–53.19) |
Abbreviations: BMI, body mass index; diabetes, type 2 diabetes; IQR, interquartile range.
Table 2.
Patient characteristics by BMI
| Characteristics | BMI (kg/m2) | |||
|---|---|---|---|---|
| Total (n = 424) | <25 (n = 198) | ≥25 and <30 (n = 116) | ≥30 (n = 110) | |
| Age | ||||
| Mean (SD) | 43.58 (13.07) | 40.05 (12.11) | 45.33 (12.82) | 48.11 (13.35) |
| Median (IQR) | 41.00 (33.00–53.00) | 36.00 (31.00–47.00) | 43.00 (34.00–55.50) | 48.00 (37.00–58.00) |
| Sex, n (%) | ||||
| Female | 302 (71.2) | 154 (77.8) | 73 (62.9) | 75 (68.2) |
| Male | 122 (28.8) | 44 (22.2) | 43 (37.1) | 35 (31.8) |
| Race, n (%) | ||||
| Asian | 46 (11.2) | 27 (14.1) | 15 (13.5) | 4 (3.7) |
| Black/African American | 44 (10.8) | 8 (4.2) | 11 (9.9) | 25 (23.4) |
| Declined | 64 (15.6) | 26 (13.6) | 25 (22.5) | 13 (12.1) |
| Other | 69 (16.9) | 33 (17.3) | 19 (17.1) | 17 (15.9) |
| White | 186 (45.5) | 97 (50.8) | 41 (36.9) | 48 (44.9) |
| Diabetes, n (%) | 53 (12.5) | 5 (2.5) | 15 (12.9) | 33 (30.0) |
| Median BMI (kg/m 2 ) (IQR) | 25.44 (22.79–30.00) | 22.61 (21.15–23.60) | 27.29 (26.04–28.39) | 34.12 (31.46–37.22) |
| Hypertension, n (%) | 52 (12.3) | 4 (2.0) | 13 (11.2) | 35 (31.8) |
| Hyperlipidemia, n (%) | 74 (17.5) | 16 (8.1) | 22 (19.0) | 36 (32.7) |
| Days from symptom onset to testing, median (IQR) | 41.57 (31.87–50.90) | 38.22 (30.75–48.79) | 45.64 (35.94–52.94) | 41.60 (32.68–53.55) |
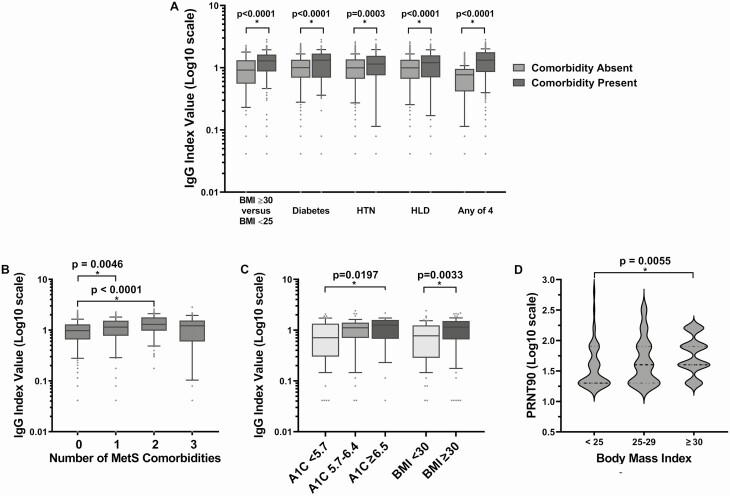
SARS-CoV-2 IgG levels were significantly higher in individuals with MetS comorbidities when compared to those without these comorbidities (Fig. 1; Table 3). When analyzed for number of MetS comorbidities, those individuals with 1 or 2 MetS comorbidities had increased levels of SARS-CoV-2 antibodies, but this significant increase did not remain in those individuals with 3 or more MetS comorbidities (Fig. 1B), likely due to a lower sample population.
Figure 1.
SARS-CoV-2 IgG levels are significantly higher in non-severe COVID-19 individuals with one or more MetS comorbidities (A and B), elevated HbA1c or BMI ≥ 30 (C), and SARS-CoV-2 neutralizing antibody titers are significantly higher in nonsevere COVID-19 individuals with increased BMI (D). (A) SARS-CoV-2 IgG levels are significantly higher in nonsevere (nonhospitalized) COVID-19 individuals with MetS comorbidities. P values obtained by the t-test (equal variances); BMI ≥ 30 remained statistically significant when adjusted for age, sex, and race on multivariable analysis (P < 0.0001). This first cohort data was obtained from the time period of April 17, 2020 to May 20, 2020. (B) SARS-CoV-2 IgG levels are significantly higher individuals with one or two MetS comorbidities. P values obtained by t-test (equal variances); data obtained from the first cohort during the time period of April 17, 2020 to May 20, 2020. (C) SARS-CoV-2 IgG levels are significantly higher in nonsevere (nonhospitalized) COVID-19 individuals with elevated HbA1c and BMI ≥ 30. P values obtained by the ANOVA or t-test (equal variances); HbA1c ≥ 6.5 and BMI ≥ 30 remained statistically significant when adjusted for age, sex, and race on multivariable analysis (P = 0.0104 and P < 0.0001, respectively). This second cohort data was obtained from the time period of May 21, 2020 to June 21, 2020. (D) SARS-CoV-2 neutralizing antibody titers are significantly higher in nonsevere (nonhospitalized) COVID-19 individuals increased BMI. P-values obtained by ANOVA; data obtained from the first cohort during the time period of April 17, 2020 to May 20, 2020.
Table 3.
Patient characteristics and metabolic comorbidities vs Log10-IgG Index values
| Characteristics | Log10 IgG Index value | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | P-valuea | |||
| Sex | Total | 1055 | 1.02 | 0.52 | 0.1294 | [T] |
| Female | 705 | 1 | 0.5 | |||
| Male | 350 | 1.06 | 0.56 | |||
| Race | Total | 814 | 1.02 | 0.53 | 0.0052 | [A] |
| Asian | 129 | 1.1 | 0.49 | |||
| Black/African American | 91 | 1.12 | 0.49 | |||
| Other | 193 | 1.05 | 0.55 | |||
| White | 401 | 0.95 | 0.54 | |||
| Obesity based on BMI (kg/m2) | Total | 424 | 1.05 | 0.56 | <0.0001 | [A] |
| BMI <25 | 198 | 0.92 | 0.53 | |||
| BMI ≥25 and <30 | 116 | 1.07 | 0.56 | |||
| BMI ≥30 | 110 | 1.27 | 0.56 | |||
| Diabetes | Total | 1055 | 1.02 | 0.52 | <0.0001 | [T] |
| Absent | 963 | 1 | 0.51 | |||
| Present | 92 | 1.23 | 0.62 | |||
| HTN | Total | 1055 | 1.02 | 0.52 | 0.031 | [T] |
| Absent | 942 | 1.01 | 0.51 | |||
| Present | 113 | 1.12 | 0.63 | |||
| HLD | Total | 1055 | 1.02 | 0.52 | 0.0172 | [T] |
| Absent | 945 | 1.01 | 0.51 | |||
| Present | 110 | 1.13 | 0.63 |
a P values obtained from the statistical tests: [A] = ANOVA; [T] = t-test (equal variances). P < 0.05 was considered statistically significant.
In contrast, antibody levels were not significantly different in patients with comorbidities not associated with MetS, such as autoimmune disorders or chronic lung, renal or hepatic disease, compared to individuals without these disorders. Individuals with type 1 diabetes were not included as part of the MetS comorbidity analyses as there were very few in our cohort (n = 3). Furthermore, the significance of MetS in those with type 1 diabetes is not completely understood, as its frequency with MetS has not been extensively researched (13). As MetS is generally associated with type 2 diabetes in the literature, this study focused on those individuals with type 2 diabetes.
SARS-CoV-2 IgG levels varied significantly by race (P = 0.0052; Table 3), with higher values found in black or African American individuals than other races. SARS-CoV-2 IgG levels also weakly positively correlated with age [Pearson coefficient 95%CI: 0.23 (0.17-0.29); P < 0.0001]. These observations perhaps reflect the increased prevalence of comorbidities, such as obesity and diabetes, among nonwhite and older populations (14,15). Multivariable analysis showed that when adjusted for age, sex, race, and time duration from symptom onset to testing, increased SARS-CoV-2 IgG levels remained significantly associated with obesity (P < 0.0001).
In light of these correlations, and the growing literature on hyperglycemia becoming a prognostic predictor of outcome in hospitalized patients with COVID-19 (16), we investigated the association of SARS-CoV-2 IgG levels with metabolic laboratory biomarkers, such as HbA1c and lipid. Unfortunately, due to the COVID-19 epidemic stay-at-home orders in New York City, these laboratory values were unavailable as patients were not undergoing their regular laboratory testing during the time period of the first study. Thus, a second retrospective study was undertaken, focusing on the association of SARS-CoV-2 IgG levels with HbA1c and lipid levels in outpatients during the period from May 21, 2020 to June 21, 2020 (ie, after outpatient medical practices reopened). This second study focused on nonpregnant, nonhospitalized patients with a positive SARS-CoV-2 serological test who also had provided samples for an HbA1c assay or a lipid panel (TC, HDL, LDL, and TG) on the same day as the SARS-CoV-2 antibody test.
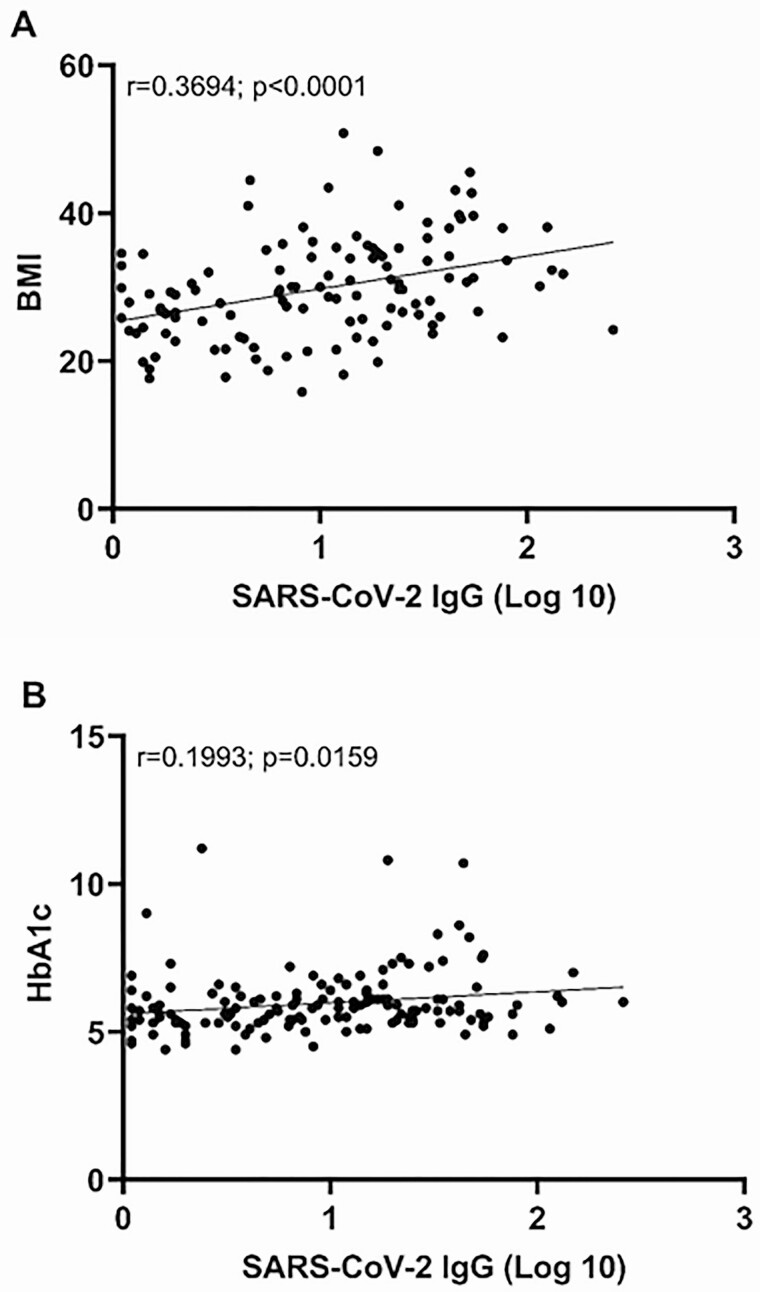
SARS-CoV-2 IgG levels were significantly higher in patients with HbA1c ≥ 6.5% compared to those with HbA1c < 5.7% (P = 0.0197; Fig. 1C). The difference remained significant when adjusted for age, sex, and race on multivariable analysis (P = 0.0104). A positive correlation was noted between the HbA1c and antibody levels [Pearson coefficient 95% CI: 0.20 (0.04-0.35) P = 0.0159] (Fig. 2B, Table 4). As BMI data were also available in the EMR for this second cohort, a repeat analysis confirmed the significantly higher SARS-CoV-2 IgG levels in obese patients (P = 0.0033; Fig. 1C). Furthermore, a positive correlation was noted between BMI and antibody levels [Pearson coefficient 95%CI: 0.37 (0.20–0.52) P < 0.0001] (Fig. 2A; Table 4). However, there was no significant association between SARS-CoV-2 IgG levels and any component of the lipid panel test (Table 4).
Figure 2.
Pearson correlation between the SARS-CoV-2 IgG index values and (A) BMI and (B) HbA1C.
Table 4.
Correlation between log10-IgG-Index values and clinical measures
| Variable | n | Mean | SD | Median | Min | Max | Pearson coefficient (95% CI) | P-valuea |
|---|---|---|---|---|---|---|---|---|
| IgG Index (log 10) | 146 | 0.95 | 0.58 | 0.97 | 0.04 | 2.41 | ||
| HbA1c | 146 | 5.96 | 1.08 | 5.70 | 4.40 | 11.20 | 0.20 (0.04-0.35) | 0.0157 |
| BMI | 119 | 29.75 | 6.99 | 29.34 | 15.82 | 50.79 | 0.37 (0.20-0.52) | 0.0001 |
| HDL | 113 | 190.10 | 40.12 | 186.00 | 96.00 | 293.00 | −0.10 (−0.28-0.08) | 0.2699 |
| LDL | 113 | 61.06 | 18.55 | 61.00 | 12.00 | 111.00 | −0.14 (−0.31-0.05) | 0.1467 |
| TC | 113 | 104.63 | 35.43 | 104.00 | 20.00 | 239.00 | −0.10 (−0.28-0.09) | 0.2980 |
| TG | 113 | 122.12 | 67.32 | 107.00 | 32.00 | 379.00 | 0.14 (−0.05-0.31) | 0.1506 |
a P value of Pearson correlation coefficient, testing Ho: Rho = 0. P < 0.05 was considered statistically significant.
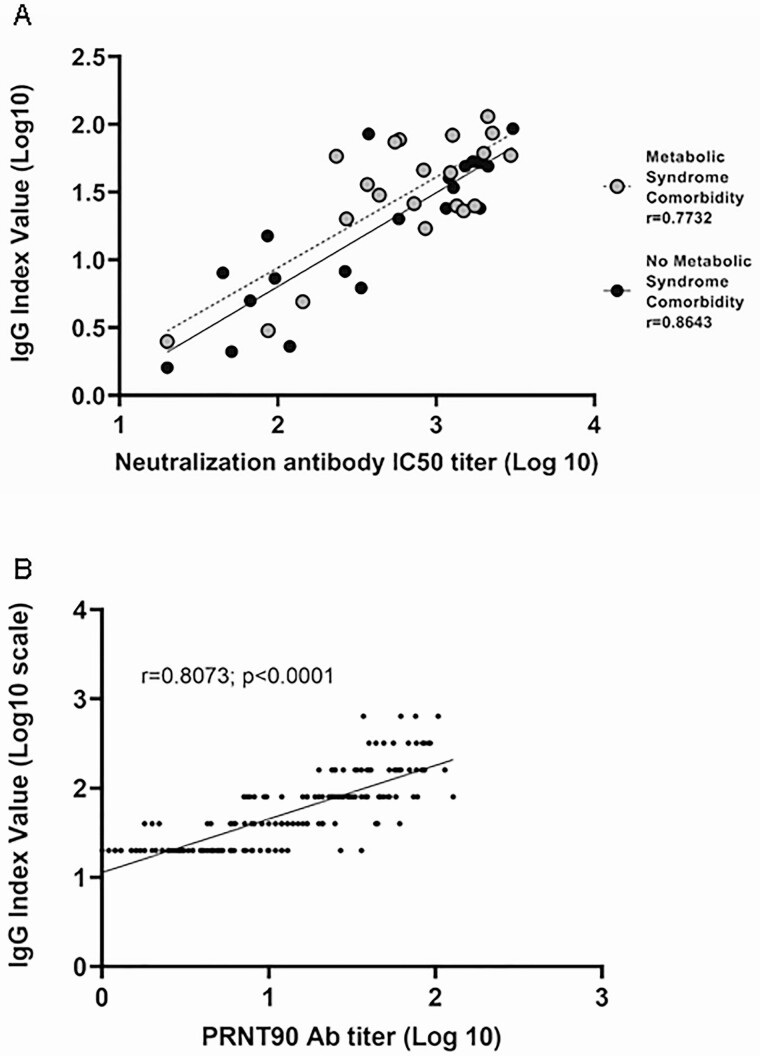
SARS-CoV-2 IgG values on the Pylon 3D analyzer were compared to the neutralizing antibody titers of 2 different neutralization assays. The first comparison was performed at Weill Cornell Medicine and entailed a comparison of 42 samples between the pseudo neutralization assay and the Pylon 3D analyzer’s SARS-CoV-2 IgG values. A strong correlation between the 2 assays (Fig. 3A, r = 0.7768) was noted. This strong correlation remained when stratified by MetS status where MetS patients (n = 21; r = 0.7732) and non-MetS patients (n = 21; r = 0.8643) were examined separately.
Figure 3.
SARS-CoV-2 IgG levels positively correlate with neutralization assay titers. (A) Pearson correlation between the SARS-CoV-2 IgG index values and in-house pseudo-neutralization assay titers. P-value for both cohorts was <0.0001. When the cohorts are combined, r = 0.7768; P value < 0.0001. (B) Pearson correlation between the SARS-CoV-2 IgG index values and PRNT90 neutralization assay titers (Wadsworth Center). r = 0.8073; P value < 0.0001.
In a separate correlation experiment, 188 samples from the time period April 17, 2020 to May 20, 2020 were sent to New York State Wadsworth Center. As seen with the pseudo neutralization assay at Weill Cornell, a strong correlation was found between the 2 assays (Fig. 3B, r = 0.8073). Furthermore, SARS-CoV-2 neutralizing antibody titers were significantly higher in nonsevere (nonhospitalized) COVID-19 individuals with BMI ≥ 30 compared to those with BMI < 25 (Fig. 1D; P = 0.0055).
Discussion
The physiological and immunological mechanisms underlying the association between COVID-19 severity and diabetes or obesity are poorly understood. Previous studies had focused on COVID-19 risks factors among the inpatient population (2,17-19). To our knowledge, this is the first analysis investigating SARS-CoV-2 antibody levels in nonhospitalized, convalescent individuals with MetS-related comorbidities.
The severe morbidity and mortality of COVID-19 among those with type 2 diabetes may be partially due to a dysfunctional immune system associated with uncontrolled diabetes (20,21). As individuals with type 2 diabetes and obesity are thought to be in a general state of metabolic inflammation, and the innate and adaptive arms of their immune systems may be in dysregulation (9,21), the increased levels in SARS-CoV-2 IgG and neutralizing antibodies in this population were unexpected. However, a study focusing on individuals with type 2 diabetes and obesity and their response to influenza vaccination reported similar findings to those here—namely, that vaccine-induced anti-influenza IgG levels were significantly higher in individuals with higher BMIs (22). That study reported no correlation between diabetes or HbA1c levels and the antibody response to vaccination, whereas our study noted significantly higher SARS-CoV-2 IgG levels in patients with HbA1c ≥ 6.5% compared to those with HbA1c < 5.7% (Fig. 1C). A positive correlation between HbA1c and antibody levels was also noted, albeit a weak one (r = 0.1993; P = 0.0159).
One must question whether the elevations in SARS-CoV-2 antibody levels may possibly reflect an overall increase in serum immunoglobulin levels in this cohort. Reported baseline dysfunction in the humoral immune response in individuals with MetS comorbidities have been mixed. For instance, one study noted decreased IgG and immunoglobin M levels independently related to the prevalence of adult type 2 diabetes (23). In contrast another cross-sectional study was unable to identify an association with overall serum immunoglobin levels in individuals with diabetes but found that dyslipidemia was associated with a lower serum IgG concentration (24). The relationship between serum levels of immunoglobulins and MetS were further explored in the TCLSIH cohort study (25) and demonstrated that decreased IgG and increased immunoglobin A were independently related to a higher prevalence of MetS. Further studies will be needed to explore any association between the metabolic syndrome comorbidities, immunoglobulin levels, and its role in the disease progression in COVID-19.
The possibility arose that the IgG produced by individuals with MetS comorbidities in this study are not fully functional antibodies. Antibodies to SARS-CoV-2 detected by the cyclic enhanced fluorescence assay method do not necessarily have antiviral activity and may not be representative of protective immunity. We therefore compared the SARS-CoV-2 IgG levels to 2 accepted neutralizing antibody assays with different methodologies. Strong correlations were found between SARS-CoV-2 IgG levels and the 2 neutralization assays, one of which is considered a gold standard at New York State’s Wadsworth Center (Fig. 3B). The correlation remained when only samples from MetS patients were examined (Fig. 3A; r = 0.7732).
Based on the correlation data between the neutralization assay titers and SARS-CoV-2 IgG levels, one could only presume that the correlation between BMI and neutralizing antibodies exist. This was confirmed upon further analysis where the PRNT90 neutralization antibody titers were found to be significantly elevated in those with a BMI ≥ 30 (Fig. 1D). Nonetheless, the underlying physiological explanation for elevated levels of both binding and neutralizing antibodies at the postconvalescent stage remains unclear. Future studies should investigate whether the correlations we have found extend to other parameters of humoral immunity such as, but not limited to, overall immunoglobulin levels.
This study has the standard limitations associated with retrospective studies, such as the lack of power and missing data. For instance, sufficient routine laboratory data were not available in the first part of the study, which necessitated a second cohort analysis. Our study also may not have captured possible confounding factors related specifically to COVID-19 infection. For example, the recall bias regarding symptom onset and its relation to serological testing could be a possible confounder that accounts for the increased IgG levels. Although not significant on multivariate analysis, there had been a significant difference in the duration from symptom onset to testing when the cohorts were stratified by BMI (Table 2). However, no statistically significant correlation was found between SARS-CoV-2 IgG levels and the period between symptom onset and testing (P = 0.0801).
Furthermore, it is not known if MetS patients with higher initial IgG levels will maintain them over time as antibody titers can decay markedly within 3 months of SARS-CoV-2 infection (26,27). Indeed, the initial antibody response to a trivalent influenza vaccine dropped significantly more in obese individuals than healthy weight individuals by 11 months (28). Prospective longitudinal serological studies that follow antibody levels over an extended period of time from early infection to convalescence may help our understanding of the emergence and duration of immunity in this population.
Given the early time period of the pandemic in this study, reverse transcription polymerase chain reaction testing resources were limited and were prioritized for those exhibiting severe symptoms. As such, the majority of these COVID-19 outpatients did not have a recorded SARS-CoV-2 reverse transcription polymerase chain reaction test in the EMR, and they also recovered without hospitalization. Comparing antibody responses in hospitalized and outpatient populations and in those with or without MetS may further explain why COVID-19 is particularly severe in the MetS population. Indeed, previous studies comparing patients with severe and nonsevere symptoms have described higher SARS-CoV-2 IgG antibody levels in those with severe symptoms (29-31). Our observations suggest that individuals with MetS comorbidities may also develop a more pronounced inflammatory response leading to higher antibody levels, but this inflammatory response was not as severe as those in the hospitalized patient population. As testing resources become more readily available, future studies could focus on whether SARS-CoV-2 viral loads in the MetS population also play a role in an elevated antibody response.
The apparent association between obesity and glycemic control in type 2 diabetes with COVID-19 outcomes warrants further investigation. Future studies may focus on determining whether these comorbidities are independent and whether severity and duration of obesity, type 2 diabetes, or poorer glycemic control play a role (6,16,32-34). The retrospective study by Maddaloni et al attempted to clarify the association between hospitalized type 2 diabetes and worse COVID-19 outcomes (35). The authors suggest that this association was driven by overall poor cardiometabolic health rather than type 2 diabetes itself, since patients presenting with more than 1 cardiometabolic condition had poorer outcomes.
Similarly, this current study found that nonhospitalized individuals with more than one MetS comorbidity produced significantly higher antibody levels compared to those with no or only 1 MetS comorbidity (Fig. 1). Furthermore, this study noted that there was less of a correlation between HbA1c and SARS-CoV-2 antibody levels than with BMI and antibody levels. (Fig. 2) Together, this raises the possibility that the poorer outcomes and more robust immune response is somehow associated with the co-occurring MetS comorbidities, as typically seen in those with obesity, as opposed to glycemic control.
Finally, MetS traditionally has been associated with type 2 diabetes; therefore, this study focused on individuals with type 2 diabetes. However, given the increased prevalence of obesity and overlap of MetS in patients with type 1 diabetes, future studies can further examine the relationship between MetS comorbidities and SARS-CoV-2 antibody levels in patients with type 1 diabetes (36). Altogether, such future studies may yield a better understanding of the possible link between MetS comorbidities and SARS-CoV-2 antibody levels, which could then facilitate a tailored approach to screening, monitoring, and vaccination practices.
Acknowledgments
The authors thank Gargi Debnath for additional technical assistance and David B. Sacks for his valuable expert advice and comments.
Financial Support: This research was partially funded by a COVID-19 research grant from Weill Cornell Medicine Translational Research Program of the Department of Pathology and Laboratory Medicine at Weill Cornell Medicine (grant number COVID3376; Zhen Zhao) and National Institutes of Health (R01 grant number AI36082 and AI 110657; John P. Moore). Testing at the Wadsworth Center was supported by internal funding at the New York State Department of Health.
Glossary
Abbreviations
- BMI
body mass index
- COVID-19
coronavirus disease 2019
- EMR
electronic medical record
- HbA1c
hemoglobin A1c
- HLD
hyperlipidemia
- HTN
hypertension
- IV
SARS-CoV-2 IgG index values IV
- MetS
metabolic syndrome
- PRNT
plaque reduction neutralization test
- SARS-CoV
severe acute respiratory syndrome coronavirus
- TC
total cholesterol
- TG
triglyceride
Additional Information
Disclosure Summary: ZZ received seed instruments and sponsored travel from ET Healthcare. The remainder of the authors declare that they have no conflict of interests with the present work.
Data Availability
The data sets analyzed during the current study are not publicly available but are available from the corresponding author as per Weill Cornell Medicine policies.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid 19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. ; Consortium atNC-R. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caussy C, Pattou F, Wallet F, et al. ; COVID Outcomes HCL Consortium and Lille COVID–Obesity Study Group . Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. [DOI] [PubMed] [Google Scholar]
- 8. Lynch KL, Whitman JD, Lacanienta NP, et al. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. Clin Infect Dis. Published online July 2020. doi:10.1093/cid/ciaa979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zmora N, Bashiardes S, Levy M, Elinav E. The role of the immune system in metabolic health and disease. Cell Metab. 2017;25(3):506-521. [DOI] [PubMed] [Google Scholar]
- 11. Yang HS, Racine-Brzostek SE, Lee WT, et al. SARS-CoV-2 antibody characterization in emergency department, hospitalized and convalescent patients by two semi-quantitative immunoassays. Clin Chim Acta. 2020;509:117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee WT, Girardin RC, Dupuis AP, et al. Neutralizing antibody responses in COVID-19 convalescent sera. J Infect Dis. 2021;223(1):47-55. [DOI] [PMC free article] [PubMed]
- 13. McGill M, Molyneaux L, Twigg SM, Yue DK. The metabolic syndrome in type 1 diabetes: does it exist and does it matter? J Diabetes Complications. 2008;22(1):18-23. [DOI] [PubMed] [Google Scholar]
- 14. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief No. 288. October 2017. [PubMed]
- 15. Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of diabetes by race and ethnicity in the United States, 2011-2016. JAMA. 2019;322:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sardu C, D’Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an Integrated Health Care System in California. JAMA. 2020;323(21):2195-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan WJ, Ni ZY, Hu Y, et al. ; Covid-19 CMTEGf. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pal R, Banerjee M. Are people with uncontrolled diabetes mellitus at high risk of reinfections with COVID-19? Prim Care Diabetes. 2021;15(1):18-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheridan PA, Paich HA, Handy J, et al. The antibody response to influenza vaccination is not impaired in type 2 diabetics. Vaccine. 2015;33(29):3306-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo X, Meng G, Liu F, et al. Serum levels of immunoglobulins in an adult population and their relationship with type 2 diabetes. Diabetes Res Clin Pract. 2016;115:76-82. [DOI] [PubMed] [Google Scholar]
- 24. Lin D, Bridgeman MB, Brunetti L. Evaluation of alterations in serum immunoglobulin concentrations in components of metabolic syndrome, obesity, diabetes, and dyslipidemia. BMC Cardiovasc Disord. 2019;19(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Fu J, Gu Y, et al. Relationship between serum levels of immunoglobulins and metabolic syndrome in an adult population: a population study from the TCLSIH cohort study. Nutr Metab Cardiovasc Dis. 2019;29(9):916-922. [DOI] [PubMed] [Google Scholar]
- 26. Liu A, Li Y, Peng J, Huang Y, Xu D. Antibody responses against SARS-CoV-2 in COVID-19 patients. J Med Virol. Published online June 2020. doi:10.1002/jmv.26241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu A, Wang W, Zhao X, et al. Disappearance of antibodies to SARS-CoV-2 in a Covid-19 patient after recovery. Clin Microbiol Infect. 2020;26(12):1703-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012;36(8):1072-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie J, Ding C, Li J, et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 2020;92(10):2004-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y, Zuiani A, Fischinger S, et al. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell. 2020;183(6):1496-1507.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li K, Huang B, Wu M, et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun. 2020;11(1):6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068-1077.e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh AK, Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: a narrative review. Diabetes Res Clin Pract. 2020;165:108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ademolu A. Whipple triad its limitations in diagnosis and management of hypoglycemia as a co-morbidity in covid-19 diabetics and diabetes mellitus in general: a review. Int J Diabetes Endocrinol. 2020;5:23-26. [Google Scholar]
- 35. Maddaloni E, D’Onofrio L, Alessandri F, et al. ; CoViDiab Study Group . Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II). Cardiovasc Diabetol. 2020;19(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chillarón JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63(2):181-187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets analyzed during the current study are not publicly available but are available from the corresponding author as per Weill Cornell Medicine policies.