Abstract
Context
Demonstrating the ability to mount a neutralizing antibody response to SARS-CoV-2 in the presence of diabetes is crucial to understand COVID-19 pathogenesis, reinfection potential, and vaccine development.
Objective
The aim of this study was to characterize the kinetics and durability of neutralizing antibody (Nab) response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the presence of hyperglycemia.
Methods
Using a lentiviral vector–based SARS-CoV-2 neutralization assay to measure Nabs, we characterized 150 patients randomly selected from a cohort of 509 patients with confirmed COVID-19 pneumonia. We analyzed Nab response according to the presence of diabetes or hyperglycemia, at the time of hospitalization and during the postdischarge follow-up: 1-, 3-, and 6-month outpatient visits.
Results
Among 150 randomly selected patients 40 (26.6%) had diabetes. Diabetes (hazard ratio [HR] 8.9, P < .001), glucose levels (HR 1.25 × 1.1 mmol/L, P < .001), and glucose variability (HR 1.17 × 0.6 mmol/L, P < .001) were independently associated with an increased risk of mortality. The neutralizing activity of SARS-CoV-2 antibodies in patients with diabetes was superimposable, as for kinetics and extent, to that of patients without diabetes. It was similar across glucose levels and correlated with the humoral response against the SARS-CoV-2 spike protein. Positivity for Nabs at the time of hospital admission conferred protection on mortality, both in the presence (HR 0.28, P = .046) or absence of diabetes (HR 0.26, P = .030). The longevity of the Nab response was not affected by diabetes.
Conclusion
Diabetes and hyperglycemia do not affect the kinetics and durability of the neutralizing antibody response to SARS-CoV-2. These findings provide the rational to include patients with diabetes in the early phase of the vaccination campaign against SARS-CoV-2.
Keywords: neutralizing antibodies, COVID-19, diabetes, survival rate, humoral response, SARS-CoV-2
Humoral immunity, in particular neutralizing antibodies (Nabs), is of central importance to protect the body against acutely cytopathic viruses (1). Understanding the kinetic and durability of protection from antibody-mediated immune response to SARS-CoV-2 is crucial to understand the pathogenesis of COVID-19, reinfection potential, and vaccine efficacy and development (2). This is even more relevant in populations at the greatest risk of mortality for serious COVID-19 disease, which include individuals with existing health conditions and older adults (3-5). Among health conditions, diabetes has been associated with an excess risk of severe/critical illness and mortality since the first reports (3, 6-9). In fact, patients with diabetes and COVID-19 pneumonia showed a 2-fold increased risk of admission to an intensive care unit and a 3-fold increased risk of in-hospital mortality (10). Little is known about the durability and nature of the humoral immune response to SARS-CoV-2 in individuals with diabetes (11, 12) and cases of COVID-19 recurrences have been indeed described in subjects with diabetes (13-17). More generally, whether hyperglycemia modulates the antibody response to a virus and its durability, it is still a matter of discussion (18, 19). Decreased immunological response to the hepatitis B vaccine was consistently reported in individuals with diabetes (20), while less consistent responses were noted for varicella zoster and influenza vaccines (21, 22). Some studies reported normal plasma immunoglobulin levels in the presence of diabetes, while reduced levels of immunoglobulin (Ig)G and IgM have been reported in others (23, 24). In addition, glycation of circulating immunoglobulins has been described (25). The binding ability of glycated antibodies to their respective antigens could be impaired, thereby compromising the immune response (26). Finally, in animal models, the IgM-producing B-1 lymphocyte function was reported to be impaired in the presence of hyperglycemia (27). Demonstrating the ability to mount and maintain an efficient antibody response in the presence of hyperglycemia is crucial for the vaccination campaigns to prevent SARS-CoV2 infection in patients with diabetes. We recently reported that the antibody response against multiple SARS-CoV-2 antigens in patients with diabetes is superimposable, as for timing, titers, and classes, to that of nondiabetic patients and is not influenced by glucose levels (28). Moreover, positivity for IgG against the SARS-CoV-2 spike Receptor Binding Domain (RBD) was predictive of survival, both in the presence and absence of diabetes (29). Here, we used a lentiviral vector–based SARS-CoV-2 neutralization assay to measure Nabs and analyzed them according to hyperglycemia (known diabetes or newly diagnosed diabetes) and antibody responses to the SARS-CoV-2 spike and RBD, as well as to other beta-coronaviruses (HCoV-OC43 and HCoV-HKU1). Our study cohort consisted of 150 individuals randomly selected among those with confirmed COVID-19 pneumonia admitted to the emergency or clinical departments at the San Raffaele Hospital in Milan between February 25 and April 19 2020.
Material and Methods
Study Population and Data Sources
The study population (n = 150) was randomly selected among the 509 patients with confirmed COVID-19 pneumonia (see also (28)), admitted between February 25 and April 19 2020 to the Emergency or Clinical departments of the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Hospital (30, 31) and with a serum sample stored in our institutional biobank. This cohort is part of the COVID-19 institutional clinical–biological cohort (COVID-BioB; ClinicalTrials.gov Identifier: NCT04318366). Confirmed COVID-19 pneumonia was defined as a SARS-CoV-2 positive reverse transcription polymerase chain reaction from a nasal/throat and radiological findings suggestive of COVID-19 pneumonia. Data were collected from medical chart reviews or directly by patient interview and entered in a dedicated electronic case report form. Data were cross-checked blind and verified by data managers and clinicians for accuracy. Routine blood tests included complete blood count with differential, renal and liver function tests, C-reactive protein, lactate dehydrogenase, serum ferritin, D-dimer, and interleukin 6.
Definition of diabetes
Study participants were defined as having diabetes if they had a documented diagnosis before the hospital admission for COVID-19 pneumonia (comorbid diabetes: fasting plasma glucose [FPG] ≥7.0 mmol/L or HbA1c ≥6.5% [48 mmol/mol], or prescription for diabetes medications) or if patients without a previous diagnosis of diabetes had a mean FPG ≥7.0 mmol/L during the hospitalization for COVID-19 pneumonia (newly diagnosed diabetes). We computed mean FPG and glucose variability (SD) from all fasting laboratory glucose values measured during hospitalization.
Anti-SARS-CoV-2 Antibodies Determination and Neutralization assay
Specific antibodies to different SARS-CoV2 antigens were tested by a luciferase immunoprecipitation system assay, as previously described (29). As viral antigens, we cloned several recombinant monomeric or multimeric SARS-CoV-2 proteins tagged with a Nanoluciferase reporter (Promega): the whole spike glycoprotein S1 + S2, S2, spike glycoprotein RBD, nucleocapsid protein. In addition, we produced spike S2 proteins of the HCoV-OC43 and HCoV-HKU1 beta coronaviruses and the hemagglutinin HA1 protein of the 2009 H1N1 pandemic flu virus. For antibody titrations, the sera that bound recombinant antigens above the linear range of the assay were serially diluted (1:10, 1:100, and 1:1000) in Tris-Buffered Saline and Tween 20 (TBST), retested until binding fell into the linear range, and the calculated arbitrary units (AU) corrected by multiplying for the corresponding dilution factor. Thresholds for antibody positivity were established upon a QQ plot analysis by selecting AU values at which the distribution of calculated arbitrary units deviated from normality. For ubiquitously present antibody responses like those against the 2009 pandemic flu HA and the HCoV-OC43 and HCoV-HKU1 S2 spike proteins, subjects were binned into terciles.
A lentiviral vector–based SARS-CoV-2 neutralization assay with VERO E6 cells was used to evaluate Nab responses. Neutralization titers were defined as the serum dilution at which relative luminescence units were reduced by 50% compared with virus control wells. Inhibitory dilution (ID) 50 was calculated with a linear interpolation method. The threshold for neutralizing antibody positivity was 1/40 dilution.
Statistical Analysis
Continuous variables are reported as median with interquartile range (IQR) in parenthesis, while categorical variables are reported as frequency or percent. Categorical variables were compared using the chi-squared or Fisher’s exact test; continuous variables were compared using the Wilcoxon rank sum or Kruskal–Wallis test. Imputation for missing data was not performed. The time to event was calculated from the date of symptom onset to the date of death, or of last follow-up visit, whichever occurred first. Survival was estimated according to Kaplan–Meier. To evaluate the association between antibodies positivity and time to death we used univariate and multivariate Cox proportional hazards models. The effect was reported as hazard ratio (HR) with the corresponding 95% CI, estimated using the Wald approximation. All survival analysis and association were stratified according to time since the onset of symptoms to blood sampling (weeks 1, 2, 3, ≥4). Multivariate analyses were performed including variables significant at the level of P < .05 in the univariate analysis. Two-tailed P values are reported, with P < .05 indicating statistical significance. All confidence intervals are 2-sided and not adjusted for multiple testing. Statistical analyses were performed with the SPSS 24 (SPSS Inc./IBM) and the R software version 3.4.0 (R Core Team (2017).
Results
Study Participants
Among the selected 150 cases with confirmed COVID-19 pneumonia, 134 patients (89.3%) were hospitalized and 24 (16%) were admitted to the ICU. As of November 25, 2020, median follow-up time after symptoms onset was 202 (95% CI 58-60) days. Twenty-nine patients died during follow-up (19.3%). Newly diagnosed diabetes and comorbid diabetes accounted respectively for 12% (n = 18) and 14.7% (n = 22) of the patients. The characteristics of study participants according to diabetes status are reported elsewhere (Table 1 and Table 2 (32)). We assessed the associations between baseline variables and diabetes using logistic regression. Older age (odds ratio [OR] 1.034 [95% IC 1.01-1.06]; P = .017), hypertension (OR 4.268 [1.93-9.44]; P < .001) and number of existing comorbidities (OR 3.393 [2.193-5.252]; P < .001) were all associated with diabetes. As for the treatment of diabetes, 45% of the patients were untreated (newly diagnosed diabetes), 2.5% were treated with lifestyle modifications, 37.5% with noninsulin oral or injectable antidiabetes medications, 12.5% with insulin, and 2.5% with insulin and oral diabetes medications. The median time from symptoms onset to hospital admission was 9 (5-13) and 8 (3.2-11) days for patients without and with diabetes, respectively (P = .144). Patients with diabetes did not report different symptoms at the time of admission than patients without diabetes, with the exception of a reduced prevalence of headache and chest pain (Table 2 (32)). The median days between symptoms onset and blood sampling for measuring the antibody response to SARS-CoV-2 was 10.5 (7-16) and 11 (6-17.7) in patients without or with diabetes, respectively, P = .818. At that time the presence of diabetes was associated with worst kidney function (serum creatinine: 102.1 [80.2–154.8] vs 83.4 [67.2-110.5] µmol/L, P = 0.025) and with an increase in the coagulatory cascade activation marker (D-dimer: 11.34 [4.97-19.9] vs 5.64 [1.67-11.31] nmol/L, P < .057) (see also Table 2 (32)).
Neutralization Titers and Antibody Responses to SARS-CoV-2 Stratified by Diabetes or FPG
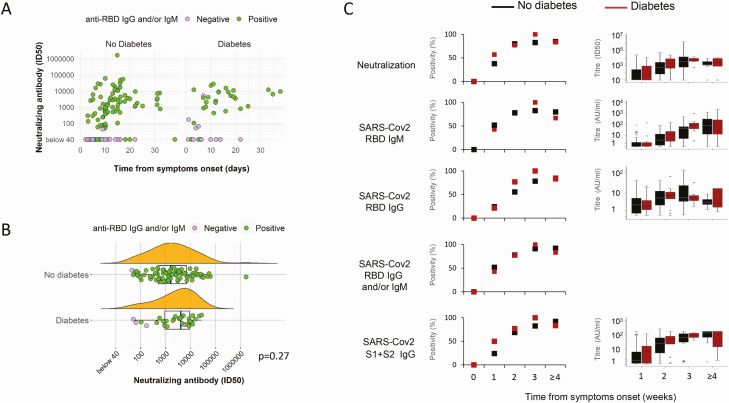
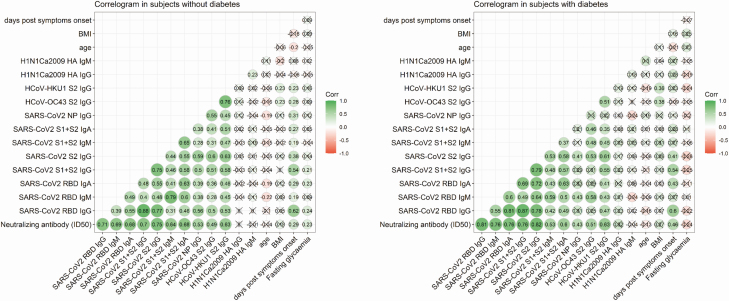
Determining the neutralizing capacity of SARS-CoV-2 antibodies is critical to understand putative protective effects of the immune response. In patients with COVID-19 pneumonia, Nabs were measured in all 150 subjects on the first sample available after hospital admission. Nab positivity was evident in 70% (ID50 1586 [482–7018]) and 75% (ID50 3996 [682-9059]) of subjects without and with diabetes, respectively (P = .684) (Fig. 1 and Table 3 (32)). Nab positivity and neutralization titers increased throughout the observation period, from week 1 to week 3, followed by stabilization or marginal decline at week 4 and beyond (Fig. 1). As expected (28), anti-RBD IgG, anti-S1 + S2 IgG, and the antinucleocapsid protein IgG responses were also not affected by diabetes (Fig. 1C and data not shown). A correlogram was made to visualize whether the neutralization titer correlated differently in subjects with or without diabetes with spike binding titers and other humoral responses. As reported in Figure 2, in patients with diabetes the cluster of correlation among the tested antibody titers is superimposable to that of nondiabetic patients, showing the highest correlation coefficients between neutralization activity and humoral response against the spike protein. The same analyses were made after stratification of subjects by FPG, independently from diabetes status (Fig. 1 and Table 3 (32)). Marginal differences between FPG strata were evident, including a higher neutralizing titer in patients with median FPG ≥7 mmol/L than in normoglycemic patients (Fig. 1 (32)). Taken together these results demonstrate that development of Nabs against SARS-CoV-2 is not impaired by diabetes or hyperglycemia.
Figure 1.
Neutralizing antibodies prevalence and titers based on diabetes status. Correlation between neutralizing antibody titers and days since symptoms onset (A) and neutralizing titers of Nab positive sample (B) in patients with COVID-19 pneumonia. Nabs were measured in 150 subjects at the first sampling available after hospital admission (median days from symptoms onset: 10.5 [7-16] and 11 [6-17.7] in patients without [n = 110] or with diabetes [n = 40], respectively). Results are shown as the reverse serum dilution giving an ID50 measured in each sample (circles), and below that we report a probability density estimate and a boxplot showing median, IQR with whiskers extending to 1.96 times the median, and outlier omission. Solid circles are positive (green) or negative (magenta) cases for SARS-CoV2 RBD IgG and/or IgM. Nabs, anti-SARS-CoV-2 RBD IgG and IgM, and S1 + S2 IgG were also stratified by duration of symptoms (weeks 1, 2, 3, ≥4) at the time of sampling and diabetes status (C). For each time point, results are percentage of positivity (left) and median of titers (right) (sample size: week 1: no diabetes n = 29, diabetes n = 14; week 2: no diabetes n = 45, diabetes n = 13; week 3: no diabetes n = 23, diabetes n = 7; week ≥4: no diabetes n = 13, diabetes n = 6). *P < .05 and **P < .01, χ 2 test or Mann–Whitney U test, diabetes vs no diabetes.
Figure 2.
Correlation matrix of different antibody responses in subject with or without diabetes. Correlograms show coefficients for various antibody responses with corresponding values matched to colors in the legend. Correlations are presented as Pearson coefficients after Log1p normalization. Crosses indicate correlation with P > .05.
Nab Response to SARS-CoV-2 and Survival in Subjects with and without Diabetes
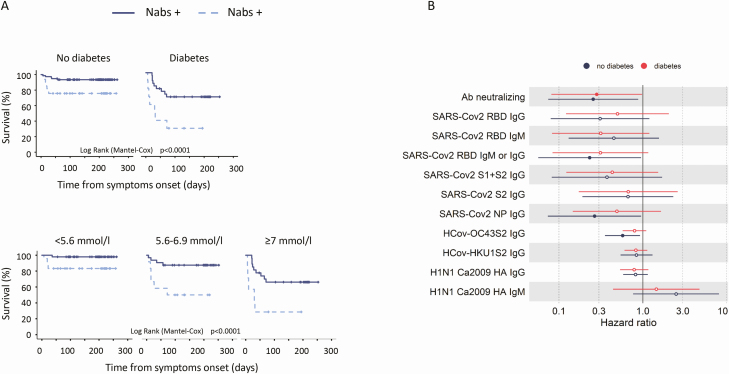
Results of Cox regression survival analysis, according to diabetes status and FPG are reported elsewhere (Table 4 (32)). As expected (28), multivariate analysis showed that diabetes status (HR 8.9, 95% CI 3.11-25.3, P < .001), FPG (HR 1.25 × 1.1 mmol/L, 95% CI 1.11-1.4, P < .001), and glucose variability (HR 1.17 × 0.6 mmol/L, 95% CI 1.07-1.25, P < .001) were independently associated with an increased risk of mortality. Kaplan–Meier survival analysis showed that the presence of Nabs was associated with improved patient survival after stratification for diabetes or FBG (Fig. 3A). Concordantly, the association between Nab positivity and risk of mortality was confirmed by Cox regression analysis stratified for symptoms duration at the time of sampling and adjusted for sex, age, and presence of diabetes (HR 0.28, 95% CI 0.126-0.623, P = .002) or FBG (HR 0.41, 95% CI 0.18.–0.92, P = .031). The protective effect of Nabs was confirmed even when the analysis was performed separately in subjects with (HR 0.28, 95% CI 0.08-0.98, P = .046) or without diabetes (HR 0.26, 95% CI 0.07-0.88, P = .030) (Fig. 3B). Overall, the effect on survival of the presence of SARS-CoV-2 spike binding antibodies or other humoral responses was similar in subject with or without diabetes (Fig. 3B).
Figure 3.
Neutralizing antibodies to SARS-CoV-2 and survival in patients with COVID-19, with or without diabetes. Kaplan–Meier patient survival estimates for 150 patients with COVID-19 pneumonia (A). Survival rate was estimated for the presence of neutralizing antibodies after stratification for diabetes or fasting plasma glucose. The log rank test was used to test differences in the estimated survival rate between Nab+ and Nab- individuals. Crosses indicate censored patients (censoring for lack of follow-up data). The forest plots (B) show the hazard ratios for death for each antibody tested. Cox regression analysis was adjusted for sex and age and stratified for the duration of symptoms at the time of blood sampling. Dots represent the HR; solid dots indicate P < .05.
Longevity of the Antibody Responses in Subject with and without Diabetes
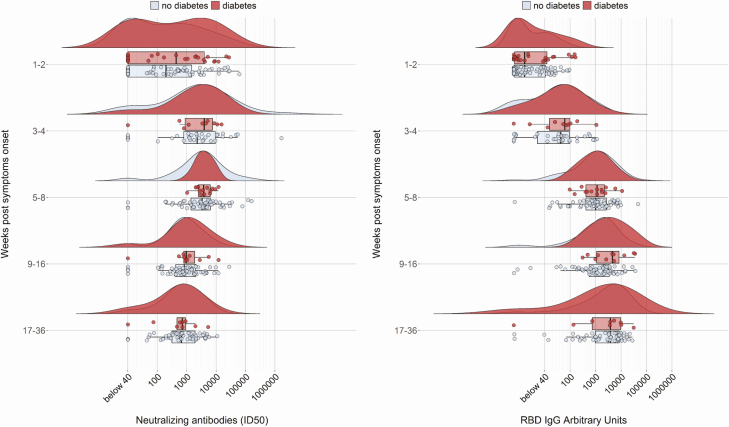
A postdischarge follow-up was planned, including outpatient visits at 1, 3, and 6 months (Table 1). Nabs continued rising until the first follow-up visit and decreased thereafter, but robust neutralizing activity was still present at the last follow-up visit (Fig. 4). Among spike binding antibodies, RBD IgM decreased after the initial peak starting from the first follow-up visit, while RBD and S1 + S2 IgG continued rising until the second visit and remained high thereafter. No difference in any of the antibodies tested at all times was evident between patients with or without diabetes (Table 1 and Fig. 4).
Table 1.
Longevity of the Nabs and SARS-CoV-2 spike binding antibody responsea
| Diabetes | Days from symptom | n | Neutralizing Antibody titers | SARS-Cov2 RBD IgG | SARS-Cov2 RBD IgM | SARS-Cov2 S1 + S2 IgG | |
|---|---|---|---|---|---|---|---|
| Sample at admission | No | 10.5 (7-16) | 110 | 539 (10-3358) | 4.89 (0.16-29) | 4.79 (1.68-12.15) | 29.35 (2.2-95.55) |
| Yes | 11 (6-17.75) | 40 | 1550 (20-7998) | 10.4 (0.3-87.4) | 4.96 (1.63-9.8) | 47.54 (4.58-96.39) | |
| P = .247 | P = .294 | P = .769 | P = .478 | ||||
| First visit | No | 42 (37-51) | 63 | 3593 (1753-6419) | 1261 (739-4097) | 4.89 (2.44-8.97) | 7739 (4153-16 673) |
| Yes | 43 (31-53.2) | 12 | 3771 (2328-6042) | 1756 (634-9739) | 3.05 (1.55-8.13) | 8285 (4827-20 439) | |
| P = .977 | P = .654 | P = .312 | P = .718 | ||||
| Second visit | No | 99 (95-109) | 61 | 819 (442-2022) | 2328 (850-4988) | 1.74 (0.74-5.12) | 5757 (2422-11 163) |
| Yes | 102 (93-114) | 11 | 968 (817-1980) | 3647 (980-5262) | 1.37 (0.88-4.39) | 9440 (3119-17 847 | |
| P = .536 | P = .973 | P = .244 | P = .352 | ||||
| Third visit | No | 209 (203-219) | 35 | 660 (387-1706) | 5565 (2423-9711) | — | 5176 (1932-8782) |
| Yes | 209 (192-215) | 5 | 588 (270-710) | 5292 (1956-10 019) | — | 7809 (1905-9282) | |
| P = .449 | P = .905 | P = .837 |
Neutralizing antibody titers are expressed as the reverse of the serum dilution giving a ID50.
aMedian titer was calculated on all available samples.
Figure 4.
Durability of neutralizing antibodies to SARS-CoV-2 in subject with or without diabetes. Kinetics of neutralizing antibodies titers and SARS-CoV-2 IgG expansion in COVID-19 sera stratified by the duration of symptoms at serum sampling (admission n = 150, 1st visit, n = 75, 2nd visit, n = 72, 3rd visit n = 40; see also Table 1). For each sample are shown the measured ID50 or arbitrary units (circles), the probability density estimate (with the half violin plot upscaled to maximum width for better visualization), box plot displaying median, IQR, and whiskers extending to 1.96 times the IQR.
Discussion
We recently demonstrated that diabetes or high blood sugar does not impair the IgG, IgM, and IgA response against multiple antigens of SARS-CoV-2 in a cohort of 509 patients with documented diagnosis of COVID-19 pneumonia prospectively followed at the IRCCS Hospital San Raffaele (28). In this study, we further investigated the humoral response by analyzing the kinetics and durability of the neutralizing antibody response to SARS-CoV-2 in the presence of diabetes. A random subsample of the original cohort was studied. The results have generated additional valuable knowledge about the humoral response against SARS-CoV2 in patients with hyperglycemia. First, in patients with diabetes the neutralizing activity of SARS-CoV-2 antibodies is superimposable, as for kinetics and extent, to that of nondiabetic patients and is not influenced by glucose levels. Second, in patients with COVID-19 pneumonia the neutralizing activity strongly correlates with the humoral response against the viral spike protein and is predictive of the survival rate, both in the presence or the absence of diabetes. Third, the longevity of the antibody response is not affected by diabetes.
These results were partly expected, but not taken for granted. Our previous data (28) regarding the development of antibodies against the spike protein in subjects with diabetes or hyperglycemia suggested that the neutralizing response would be effective in both populations. In fact, SARS-CoV-2 docking on ACE2 is blocked when neutralizing antibodies recognize the RBD on the S1 subunit of the spike protein (33, 34). Concordantly, our data confirm a significant correlation between the neutralizing activity and the antibody response against RBD or S1 + S2 spike protein in subjects with or without diabetes. An even stronger correlation was evident with the antibody response against the S2 subunit, which mediates membrane fusion for viral entry (35). In spite of this, the presence of valid neutralizing activity was not obvious. Antibody glycation has been observed in subjects with diabetes (25) and glycation can affect the function by weakening antigen binding (36). Moreover, although antibodies are generally beneficial and protective, sometimes virus-specific antibodies can promote pathology, a phenomenon defined as antibody-dependent enhancement (ADE). ADE can occur when non-neutralizing antibodies or antibodies at subneutralizing levels bind to viral antigens without blocking or clearing infection (37). Fcγ receptors, surface receptors on immune cells that recognize the Fc portion of IgG, were identified as the key mediators of ADE (38). Hyperglycemia and diabetes are known to be able to induce Fcγ receptors expression on phagocytic cells (39) and to sensitize macrophages to cytokine stimulations (40). Therefore, hypothetically in patients with diabetes the antibody response could lead to a worse clinical outcome (37). The 70% reduction in mortality in subjects with diabetes associated with the presence of a neutralizing antibody disproves this hypothesis. Accordingly, the question through what other mechanisms may diabetes and hyperglycemia contribute to increased mortality in COVID-19 pneumonia remains open. It is well known that venous thromboembolism, arterial thrombosis, and thrombotic microangiopathy substantially contribute to increased morbidity and mortality in patients with COVID-19 (41). As hypercoagulopathy was associated with diabetes and represented an independent predictor of mortality in our original cohort (28), we can speculate that the hypercoagulable state and endothelial dysfunction associated with diabetes (42), among others, could substantially contribute to increased morbidity and mortality in patients with COVID-19. Further research is needed to confirm this hypothesis.
Of great interest are the results on durability of responses after SARS-CoV-2 in the presence of diabetes. The durability of the immune response to SARS-CoV-2 is critical for understanding community outbreaks and serologic testing data, and to predict the longevity of vaccine protection. There is cumulative evidence showing that after SARS-CoV-2 infection the kinetics of the humoral response follows a classical pattern, and late responses are characterized by low or sometimes undetectable levels of IgM and modest IgA, but, at least up to 6 months, a mostly a robust IgG response associated with neutralizing activity (2, 43-46). Similar results have recently been reported after vaccination, even if with shorter follow-up (47). Whether the presence of comorbidity like diabetes affects the durability of the response is still unknown.
A major strength of our study is that deeply characterized a cohort of subjects with COVID-19 pneumonia for the Nab and Ig responses against multiple antigens of SARS-CoV-2. However, our study has some limitations. First, our cohort is limited to hospitalized patients and results could be different in SARs-CoV-2 infection with few symptoms. Second, due to feasibility considerations, we limited the analysis of Nabs to 150 out of 509 subjects of the original cohort. The selected subcohort appears superimposable to the original cohort as for age (64 ± 14 vs 63 ± 14), sex (male 69.3% vs 65.3%), diabetes prevalence (26.7% vs 27.2%), and clinical outcome (admission to intensive care unit 16% vs 15.4%, death 19.3% vs 18.4%). Despite this, we cannot exclude selection bias. Third, as the study included subjects with characteristics of type 2 diabetes, we should be cautious in generalizing our findings to persons with other type of diabetes. Moreover, the definition of newly diagnosed diabetes does not exclude stress-induced hyperglycemia that may have resolved during follow-up. A total of 18 subjects were classified as newly diagnosed diabetes. Among these, 7 died, and a valid glucose measure during follow-up was not obtained for 1. Of the remaining 10 subjects, 3 showed normalization of glucose concentration (30%), while 7 were confirmed as having diabetes (n = 3, 30%) or impaired fasting glycemia (IFG) (n = 4, 40%). The numbers are too small to differentiate the 2 conditions for the clinical outcome, or the kinetics and durability of the neutralizing antibody response to SARS-CoV-2. Fourth, even if we detected efficient and durable antibodies against SARS-CoV-2 after infection in patients with diabetes, we know little about the cellular response in these patients.
In conclusion, much evidence indicates that subjects with diabetes carry an adjusted OR of 3 to 4 for hospitalization, illness severity, and mortality compared with those without diabetes. It is clear from our study that diabetes or high blood sugar does not impair the efficiency of the humoral immune response against SARS-CoV-2. Concordantly, preliminary and still limited data showed similar vaccine efficacy across subgroups defined by the presence of coexisting diabetes (48). These findings provide the rationale to include patients with diabetes in the early phase of the vaccination campaign against SARS-CoV-2, also considering their increased risk of mortality from COVID-19 pneumonia.
Acknowledgments
Financial Support: This work was funded by IRCCS Ospedale San Raffaele (Program Project COVID-19 OSR-UniSR) and by Ministero della Salute (Italian Ministry of Health, Grant number: COVID-2020-12371617).
Author Contributions: L.P., G.S., and V.L. contributed to the conception of the study, wrote the manuscript, researched data, and contributed to the discussion. M.S., E.B., C.B., and I.M. contributed to the acquisition and analysis of antibody data and revised the manuscript. C.T. and F.C. contributed to the acquisition of samples, managed the biobanking activities and critically revised the manuscript, recruited patients, and managed sample biobanking. S.D., A.C., D.N., M.F.P., and M.B. contributed to the acquisition, analysis, and interpretation of neutralizing data and critically revised the manuscript. M.Sc. contributed to the design of the study and critically reviewed/edited the manuscript. L.P. is the guarantor of this work and, as such, had full access to all the data presented in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The final manuscript has been read and approved by all named authors.
Glossary
Abbreviations
- ADE
antibody-dependent enhancement
- FPG
fasting plasma glucose
- HR
hazard ratio
- ID
Inhibitory dilution
- Ig
immunoglobulin
- IQR
interquartile range
- IRCCS
Istituto di Ricovero e Cura a Carattere Scientifico
- Nab
neutralizing antibody
- OR
odds ratio
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Additional Information
Disclosures. The authors have no conflicts of interest to disclose in relation to the topic of this manuscript. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27(3):384-392. [DOI] [PubMed] [Google Scholar]
- 2. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roeker LE, Knorr DA, Pessin MS, et al. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Derosa L, Melenotte C, Griscelli F, et al. The immuno-oncological challenge of COVID-19. Nature Cancer. 2020;1(10):946-964. [DOI] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133(9):1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao R, Sun Y, Zhang Y, et al. Distinguishable immunologic characteristics of COVID-19 patients with comorbid type 2 diabetes compared with nondiabetic individuals. Mediators Inflamm. 2020;2020:6914878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lafaie L, Célarier T, Goethals L, et al. Recurrence or relapse of COVID-19 in older patients: a description of three cases. J Am Geriatr Soc. 2020;68(10):2179-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ravioli S, Ochsner H, Lindner G. Reactivation of COVID-19 pneumonia: a report of two cases. J Infect. 2020;81(2):e72-e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gousseff M, Penot P, Gallay L, et al. ; in behalf of the COCOREC study group . Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dou C, Xie X, Peng Z, et al. A case presentation for positive SARS-CoV-2 RNA recurrence in a patient with a history of type 2 diabetes that had recovered from severe COVID-19. Diabetes Res Clin Pract. 2020;166:108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng W, Guang TW, Yang M, et al. Positive results for patients with COVID-19 discharged form hospital in Chongqing, China. BMC Infect Dis. 2020;20(1):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhai X, Qian G, Wang Y, et al. Elevated B cell activation is associated with type 2 diabetes development in obese subjects. Cell Physiol Biochem. 2016;38(3):1257-1266. [DOI] [PubMed] [Google Scholar]
- 19. Kumar V. How could we forget immunometabolism in SARS-CoV2 infection or COVID-19? Int Rev Immunol. 2020:1-36. [DOI] [PubMed] [Google Scholar]
- 20. Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine among persons with diabetes: a systematic review of the literature. Diabetes Care. 2012;35(12):2690-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dos Santos G, Tahrat H, Bekkat-Berkani R. Immunogenicity, safety, and effectiveness of seasonal influenza vaccination in patients with diabetes mellitus: A systematic review. Hum Vaccin Immunother. 2018;14(8):1853-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verstraeten T, Fletcher MA, Suaya JA, et al. Diabetes mellitus as a vaccine-effect modifier: a review. Expert Rev Vaccines. 2020;19(5):445-453. [DOI] [PubMed] [Google Scholar]
- 23. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259-265. [DOI] [PubMed] [Google Scholar]
- 24. Guo X, Meng G, Liu F, et al. Serum levels of immunoglobulins in an adult population and their relationship with type 2 diabetes. Diabetes Res Clin Pract. 2016;115:76-82. [DOI] [PubMed] [Google Scholar]
- 25. Lapolla A, Tonani R, Fedele D, et al. Non-enzymatic glycation of IgG: an in vivo study. Horm Metab Res. 2002;34(5):260-264. [DOI] [PubMed] [Google Scholar]
- 26. Pampati PK, Suravajjala S, Dain JA. Monitoring nonenzymatic glycation of human immunoglobulin G by methylglyoxal and glyoxal: a spectroscopic study. Anal Biochem. 2011;408(1):59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jennbacken K, Ståhlman S, Grahnemo L, Wiklund O, Fogelstrand L. Glucose impairs B-1 cell function in diabetes. Clin Exp Immunol. 2013;174(1):129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lampasona V, Secchi M, Scavini M, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63(12):2548-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Secchi M, Bazzigaluppi E, Brigatti C, et al. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J Clin Invest. 2020;130(12):6366-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;22(2):91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dispinseri S, Lampasona V, Secchi M, et al. Data from: Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. Deposited 21 December 2020. https://figshare.com/articles/journal_contribution/%20Robust_neutralizing_antibodies_to_SARS-CoV-2_develop_%20and_persist_in_subjects_with_diabetes_and_COVID-19_pneumonia/%2013473405 [DOI] [PMC free article] [PubMed]
- 33. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mo J, Jin R, Yan Q, Sokolowska I, Lewis MJ, Hu P. Quantitative analysis of glycation and its impact on antigen binding. MAbs. 2018;10(3):406-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20(10):633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Devaraj S, Chen X, Adams-Huet B, Jialal I. Increased expression of Fc-γ receptors on monocytes in patients with nascent metabolic syndrome. J Clin Endocrinol Metab. 2013;98(9):E1510-E1515. [DOI] [PubMed] [Google Scholar]
- 40. Pavlou S, Lindsay J, Ingram R, Xu H, Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gu SX, Tyagi T, Jain K, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2020;1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwiatkowski J, Halupczok-Żyła J, Bolanowski M, Kuliszkiewicz-Janus M. The pathogenesis and available prevention options in patients with diabetic thrombophilia. Adv Clin Exp Med. 2018;27(10):1447-1452. [DOI] [PubMed] [Google Scholar]
- 43. Figueiredo-Campos P, Blankenhaus B, Mota C, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020;50(12):2025-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Widge AT, Rouphael NG, Jackson LA, et al. ; mRNA-1273 Study Group . Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.