Abstract
The scale-up of a chiral bicyclic homopiperazine of pharmaceutical interest was investigated. The outcome and safety profile of a key batch ring-expansion step via Schmidt rearrangement was improved using continuous-flow chemistry. The selectivity of nitrogen insertion for the ring expansion was improved via an alternative photochemical oxaziridine rearrangement under mild conditions, which when converted to continuous-flow in a simple and efficient flow reactor allowed the first photochemical scale-up of a homopiperazine.
Keywords: homopiperazine, continuous-flow, Schmidt, oxaziridine, photochemical, microreactor
Introduction
Seven-membered heterocycles are important structures in pharmaceutical and industrial chemistry.1 Those with two nitrogen atoms in a 1,4-relationship are of particular pharmaceutical interest and include the top-selling benzodiazepine class of central nervous system active drugs. As part of our cancer research program into lysyl oxidase (LOX) inhibitors, we required a scalable route to chiral 3,6-diazabicyclo[3.2.2]nonanes (bridged homopiperazines) 1 (Figure 1).2 The configuration of the dimethyl-substituted bridge of this stereoisomer imparted superior stability to our inhibitors against in vivo metabolism compared with a range of bridged homopiperazine isomers. This conformationally restricted heterocycle, which could be accessed in either enantiomeric form, represents a versatile building block of general interest for drug discovery.
Figure 1.
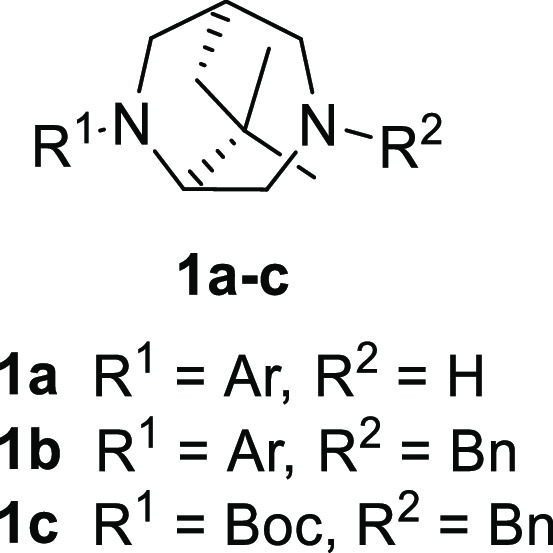
3,6-Diazabicyclo[3.2.2]nonanes (bridged homopiperazines).
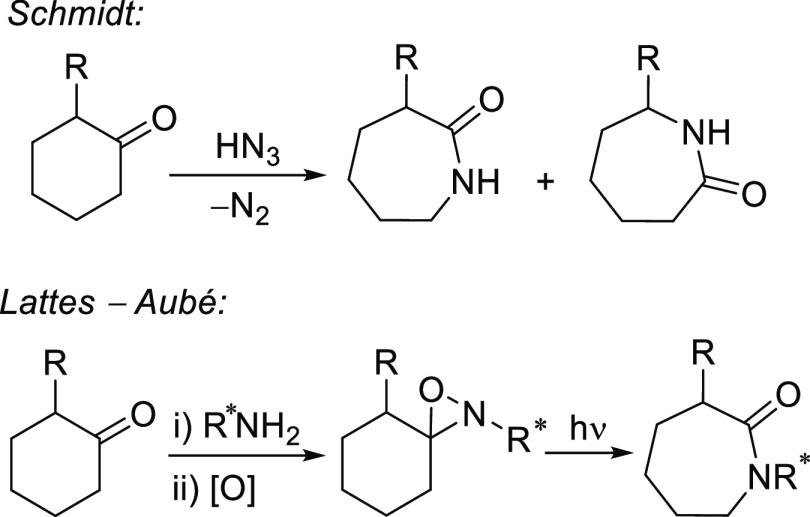
Despite the important biological activities of fused diazepines, the homopiperazine ring system is somewhat under-represented in drugs,3 in part because of the absence of general or scalable methods for its synthesis. Ring expansion by nitrogen insertion of more readily accessible six-membered rings is a commonly used strategy.4,5 The manufacture of ε-caprolactam, the key precursor for Nylon-6, involves a ring expansion via Beckmann rearrangement of cyclohexanone oxime and highlights an important industrial application of this approach.6 The related Schmidt rearrangement converts cyclic ketones to ring-expanded lactams using hydrazoic acid (HN3) generated in situ from sodium azide and a strong acid (Scheme 1).7−9 It is atom-economical and performs this transformation in a single step without the isolation of the intermediate oxime that is usually required in the Beckmann rearrangement. Hydrazoic acid is a toxic, volatile gas with explosive properties, and as such, its generation on scale in batch reactors must be carefully controlled.
Scheme 1. Ring Expansion via Schmidt and Oxaziridine Rearrangements.
Recent advances in flow chemistry, in particular microreactor technology, have demonstrated the safe utilization of hazardous reagents such as HN3 within small-volume enclosed reactors such that only small quantities are present at any one time.10−12 Recently reported was a Schmidt protocol using methanesulfonic acid–1,2-dimethoxyethane (MsOH–DME)13 that proved to be efficient at rearrangements of cyclohexanone and a range of acetophenones.14 Rearrangements of aryl carboxylic acids using superacidic TMSN3/triflic acid conditions,15 microwave-assisted intramolecular Schmidt rearrangements,16 and Beckmann rearrangements using trifluoroacetic acid17 and visible light18 have also recently been demonstrated in continuous-flow microreactors, providing safer protocols and precedent for the scalable use of these chemistries.
For unsymmetrical ketones (Scheme 1), nitrogen insertion reactions can lead to a mixture of lactam products that is dependent on the migration propensity of the carbonyl substituents. Additionally, bridged bicyclic ketones can give rise to mixtures of lactams from competitive methylene and bridgehead methine migration.19 The reaction outcomes are heavily influenced by the substrate: whereas Beckmann rearrangements of the oximes derived from bicyclo[2.2.2]octanone and the related 2-azabicyclo[2.2.2]octanones give exclusively bridgehead migration products,20,21 Schmidt reactions of bicyclo[2.2.2]octanone give solely the methylene migration product,22 and Schmidt reactions of 2-azabicyclo[2.2.2]octanones give rise to mixtures.23 There is a single report of the Schmidt reaction of 2-(4-methoxyphenyl)-2-azabicyclo[2.2.2]octan-5-one, which bears an unsubstituted ethano bridge, giving rise to a mixture of 3,6-and 2,6-diazabicyclo[3.2.2]nonanones in modest yields.24 The ratios of the migration products are affected by the presence of ring and bridge substituents25 and influenced by reaction conditions, including the acid used and the acid concentration, as demonstrated in acyclic systems.26
A promising yet underutilized strategy for controlling the regioselectivity of nitrogen insertion was reported by Lattes27,28 and developed by Aubé29−32 and proceeds via the photochemical rearrangement of oxaziridines (Scheme 1). The oxaziridine intermediate could be formed selectively via a combination of diaxial and chiral amine-controlled imine oxidation and upon photochemical activation by ultraviolet light would rearrange under stereoelectronic control to one lactam with high selectivity.33 For α-substituted ketones, a product distribution complementary to that from the Schmidt reaction was possible.27
Photochemistry can be successfully implemented for large-scale manufacture, as exemplified by the industrial-scale Toray process, although its widespread acceptance as a practical method has been hampered by a lack of reliably scalable processes.34 Continuous-flow photochemical reactors have been developed to address this problem, though their design has tended toward the use of higher-energy, more intense light sources within increasingly complex reactors.35 A practical reactor developed by GlaxoSmithKline and the Booker-Milburn group utilizing a fluoropolymer (FEP)-wrapped high-power UV source was used to demonstrate a range of cycloaddition reactions.36 This system was successfully utilized for the scale-up of a chiral bicyclic lactam using photochemical oxaziridine rearrangement chemistry, with high rearrangement selectivity at moderate reaction conversions.37 More recently, higher-throughput reactors based on kilowatt UV light sources such as the Firefly parallel tube flow reactor38 and continuous Taylor vortex reactor39 have been developed and have achieved impressive productivities of multiple kilograms per day.
Reaction activation by light can be an environmentally benign process. As the majority of photochemical reactions occur only within a short distance from the solution interface exposed to radiation (see the Supporting Information), efforts toward more efficient and sustainable energy use have seen the continued development of photoreactors with micrometer-sized channels.34,40−42 These are highly effective at utilizing incoming radiation and allow the use of more efficient low-power lamps, light-emitting diodes, or natural light sources, which in turn reduce reactor complexity and offer advantages in safety. Achieving the productivity of macro-flow systems then requires only extending the reaction time or numbering-up multiple low-cost devices.
Herein we describe our work toward the scale-up of chiral bicyclic homopiperazines 1. Continuous-flow chemistry was used to improve the safety and outcome of the Schmidt rearrangement over the batch method. The important progress described here is the first use of a photochemical oxaziridine rearrangement to achieve the scale-up of a homopiperazine. An efficient and inexpensive microreactor was designed to achieve scalable photochemistry using continuous-flow.
Results and Discussion
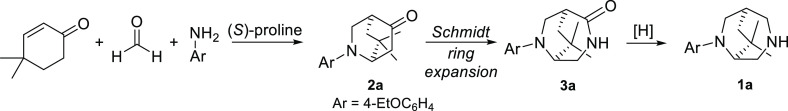
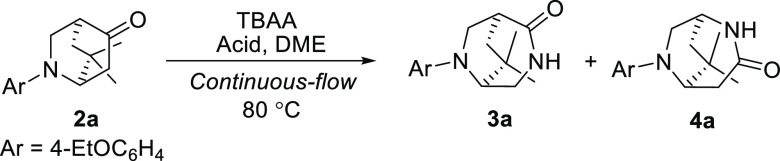
Our initial approach to the synthesis of homopiperazine 1a was based on the Schmidt reaction of 2-azabicyclo[2.2.2]octanone 2a (Scheme 2), which was readily available in high enantiomeric purity on a multigram scale using (S)-proline-catalyzed aza-Diels–Alder batch chemistry.43 Subsequent amide reduction of 3a would furnish the required homopiperazine 1a which could undergo further modification to homopiperazines 1b and 1c.
Scheme 2. Initial Synthetic Route to Bicyclic Homopiperazine 1a.
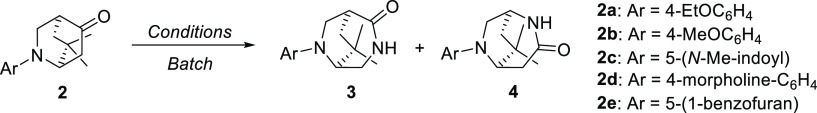
Initial experiments were carried out on a 0.5–2.5 g (1–9 mmol) scale of ketones 2 in batch mode. Upon treatment of 2a (Ar = 4-EtOC6H4) with sodium azide in concentrated sulfuric acid, lactam products from methylene migration (3a) and bridgehead methine migration (4a) were obtained, albeit in low overall yield (Table 1, entry 1). Similarly modest yields were obtained from a range of N-aryl-substituted ketones 2b–e, but in each case selectivity for the desired lactam 3 was moderate or non-existent (Table 1, entry 2–5). The use of polyphosphoric acid (115% H3PO4 grade) as the solvent and proton source gave similar results (entry 7). When concentrated acids were used, strong magnetic stirring was required to ensure efficient mixing. Our attempts to improve the reaction outcome by employing Lewis acidic conditions (TiCl4; entry 6) or using trifluoroacetic acid (entry 8) were unsuccessful. Accurate control of the reaction time was challenging, as quenching the reaction required a lengthy (2 h) neutralization of the concentrated acid solvent. Extending the reaction time from 2.5 to 4 h led to a complex reaction mixture and poor recovery of organic material. Increasing the reaction scale (4-fold in 2b; entry 9) while attempting to maintain a 2.5 h reaction duration gave a higher conversion to lactam products, but the selectivity for the desired lactam 3 was diminished, likely affected during the 3 h required to fully quench the reaction.
Table 1. Ring Expansion via Schmidt and Beckmann Rearrangement in Batch.
| yield (%) |
|||||
|---|---|---|---|---|---|
| entry | substrate | conditionsa | 3 | 4 | 3:4 |
| 1 | 2a | NaN3, H2SO4, 0 °C | 26 | 15 | 1.7:1 |
| 2 | 2b | NaN3, H2SO4, 0 °C | 10 | 8 | 1.3:1 |
| 3 | 2c | NaN3, H2SO4, 0 °C | 20 | 12 | 1.6:1 |
| 4 | 2d | NaN3, H2SO4, 0 °C | 27 | 17 | 1.6:1 |
| 5 | 2e | NaN3, H2SO4, 0 °C | 15 | 17 | 0.9:1 |
| 6 | 2e | NaN3, TiCl4, CH2Cl2, 0 °Cb | –c | –c | – |
| 7 | 2a | NaN3, PPA, 0 °Cd | 19 | 11 | 1.7:1 |
| 8 | 2a | NaN3, TFA, AcOH, rte | –c | –c | – |
| 9 | 2b | NaN3, H2SO4, 0 °Cf | 24 | 31 | 0.8:1 |
| 10 | 2a | NH2OH·HCl, MeOH/CH2Cl2 (5:3), rt, 3 h, then SOCl2, THF, 0 °Cg | 0 | 39 | 0:1 |
| 11 | 2a | NH2OH·HCl, Et3N, MeOH/CH2Cl2 (5:3), 0 °C, 3 h, then SOCl2, THF, 0 °Cg | 12 | 19 | 0.6:1 |
2.3 equiv of NaN3, 2.5 h, unless otherwise stated. Scale: 1–9 mmol of 2.
2.5 equiv of TiCl4.
No reaction after 16 h.
PPA = polyphosphoric acid.
1.5 equiv of trifluoroacetic acid.
Scale: 36 mmol of 2b.
1.3 equiv of NH2OH·HCl.
The related Beckman rearrangement usually gives a product distribution determined by the stereochemistry of the intermediate oxime.44,45 Treatment of 2a with hydroxylamine-O-sulfonic acid in formic or acetic acid23 led only to decomposition products. Stepwise formation of the intermediate oxime with hydroxylamine under thermodynamic conditions (Table 1, entry 10) (stereoisomer ratio of 9.1:1 by 1H NMR) followed by rearrangement yielded exclusively lactam 4a. Conditions were optimized for formation of the kinetic oxime (entry 11) (stereoisomer ratio of 0.32:1 by 1H NMR), but the rearrangement preference for lactam 4a could not be overcome.
We were at this time approaching our maximum scale limit for the generation of hydrazoic acid in our laboratory. The additional hazards presented by the large volumes of concentrated acid necessary for the Schmidt rearrangement and the impractical nature of the lengthy neutralization procedure prompted us to investigate scaling the Schmidt reaction in continuous-flow. We envisaged that the microreactor system using MsOH–DME13 in combination with tetrabutylammonium azide (TBAA) as a soluble azide source, as recently demonstrated by Jia,14 could provide a practical way to overcome these issues.
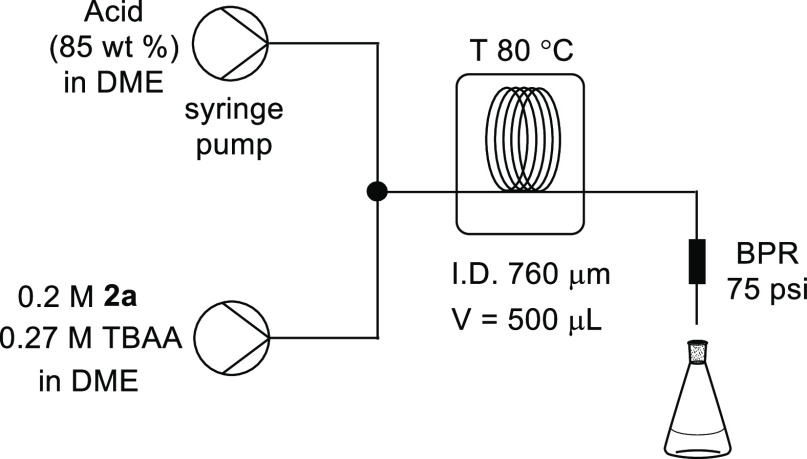
With a similar microreactor system (Figure 2) under the optimized conditions (0.20 M ketone, 0.27 M TBAA, 67% MsOH; tR = 5 min, 80 °C), bicyclic ketone 2a gave only trace rearrangement products. Increasing the average residence time to 6.7 min (Table 2, entry 1) improved the reaction conversion, but a modest preference for the undesired lactam 4a was observed. The acid strength, in particular the use of dilute sulfuric acid, has been shown to have an important influence on the Schmidt reaction of alkyl cyclopropyl ketones, reversing the product distribution seen with the concentrated acid.26 No additional difficulties were encountered using sulfuric acid in the microreactor, though to facilitate its handling the concentrated acid required dilution to 85 wt % with DME to reduce its viscosity. The relative flow rates of the ketone/azide and acid solutions were then varied to achieve the acid concentrations stated (Table 2, entries 3–6) while a constant average residence time (8.6 min) was maintained.46
Figure 2.
Continuous-flow Schmidt microreactor setup.
Table 2. Schmidt Rearrangement in Continuous-Flowa.
| yield (%) |
||||||
|---|---|---|---|---|---|---|
| entry | c2a (M) | acid, wt % | tR (min) | 3a | 4a | 3a:4a |
| 1 | 0.20 | MsOH, 67 | 6.7 | 8 | 10 | 0.8:1 |
| 2 | 0.20 | H2SO4, 66 | 6.7 | 16 | 17 | 1:1.1 |
| 3 | 0.20 | H2SO4, 48 | 8.6 | 14 | 12 | 1.2:1 |
| 4 | 0.20 | H2SO4, 59 | 8.6 | 28 | 26 | 1.1:1 |
| 5 | 0.20 | H2SO4, 69 | 8.6 | 29 | 26 | 1.1:1 |
| 6 | 0.20 | H2SO4, 77 | 8.6 | 40 | 39 | 1:1 |
| 7 | 0.31 | H2SO4, 77 | 8.6 | 41 | 37 | 1.1:1 |
TBAA = tetrabutylammonium azide (1.35 equiv). c2a = concentration of 2a. tR = average retention time.
We would like to highlight a number of safety precautions taken here. During operation, the micromixer and reactor were submerged in water baths containing 5 wt % NaHCO3 in order that any leak in the system could be quickly identified by the evolution of gas. Excess HN3 in the outflow stream was neutralized upon collection into a vessel containing 2 M NaOH/ice (1:1), and operation of the microreactor was carried out behind a blast shield. Furthermore, our reaction-scale limit was set such that in the event of total release of all azide as hydrazoic acid and fume cupboard failure, the concentration within the laboratory could not reach predefined limits.
Although using sulfuric acid had little effect on the product ratio at the elevated temperatures in the microreactor, increasing the acid concentration significantly improved the reaction conversions and overall yields. However, in order to achieve the increased acidity in this system, the relative flow rate of ketone/azide to acid solution had to be reduced significantly, resulting in a productivity of only 0.04 mmol h–1 for 3a (entry 6). Increasing the ketone/azide concentration to the upper solubility limit of the ketone in DME (0.31 M; entry 7) allowed only a marginal improvement in productivity to 0.06 mmol h–1.
Although utilizing the continuous-flow format allowed us to improve the efficiency of the Schmidt reaction over our batch process and, importantly, provided an effective way of mitigating hazards associated with the generation of large amounts of HN3, we were nevertheless dissatisfied with the low productivity and the equivalent of waste lactam that needed to be discarded through poor regioselectivity for the nitrogen insertion. While further optimization of the productivity of the system may be possible, reaction regioselectivity would likely remain an issue.
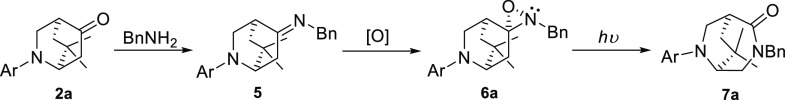
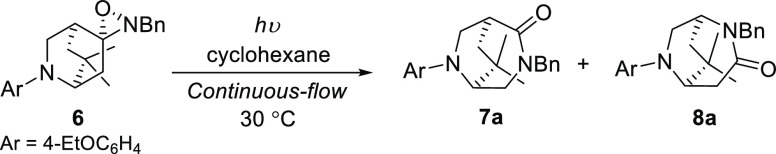
We were attracted by the oxaziridine rearrangement chemistry of Lattes and Aubé as a potential solution. The rearrangement of oxaziridines to amides proceeds under stereoelectronic control, with generally good regioselectivity for the amide where the carbon substituent anti to the nitrogen lone pair migrates.33 Chiral amines have previously been used with prochiral substrates in order to exert facial control of the imine oxidation. For ketone 2a, we hypothesized that condensation with a simple amine under equilibrating conditions would provide predominantly imine 5 (Scheme 3) because of the steric influence of the bridgehead. Benzylamine was therefore chosen as the amine on the basis of its low cost, ready availability, and ease of removal by hydrogenolysis. Because of the conformational restriction imposed by the bridge, oxidation could then occur from the less hindered (exo) face to give desired oxaziridine diastereoisomer 6a.31 Photochemical rearrangement would then provide benzyl-protected lactam 7a.33
Scheme 3. Proposed Oxaziridine Rearrangement to 7a.
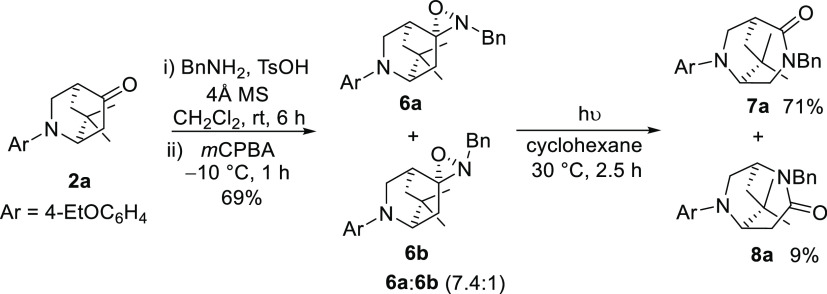
Pleasingly, gram-scale (4–18 mmol) batch condensation of ketone 2a with benzylamine in the presence of catalytic 4-toluenesulfonic acid resulted in complete imine formation within 6 h at room temperature (Scheme 4). After filtration, addition of several oxidants, including peracetic acid and Oxone, gave complex reaction mixtures. m-Chloroperbenzoic acid proved to be more selective, providing diastereomeric oxaziridines 6a and 6b in ratios of 4:1 at room temperature to 9:1 at −10 °C. Oxaziridine 6b arises from oxidation of the minor imine stereoisomer likely formed by isomerization during the imine workup and/or oxidation steps. Its formation could be minimized by prompt filtration of the imine solution under an atmosphere of dry nitrogen gas and by performing the oxidation below room temperature.
Scheme 4. Oxaziridine Rearrangement in Batch.
The oxaziridines formed were crystalline solids that were conformationally stable at room temperature and isolated by filtration through a short silica plug. Attempts to separate them by crystallization or chromatography on a preparative scale were unsuccessful. Pleasingly, upon ultraviolet irradiation of the diastereomeric mixture of 6 (6a:6b = 7.4:1) in degassed cyclohexane in a small-scale batch reactor, rearrangement gave ring-expanded lactams 7a and 8a in high overall yield with the oxaziridine isomer ratio translating well to the lactam ratio (7a:8a, 7.9:1). Cyclohexane, diethyl ether, and acetonitrile were tested as solvents for the rearrangement. Although each gave the desired rearrangement products, in acetonitrile minor side reactions could be observed (5–10%) leading to partial recovery of ketone 2a. The low boiling point of diethyl ether led to some solvent loss inside the batch reactor, and it was rejected as a suitable solvent for this reason. Cyclohexane gave the cleanest conversion to lactam products and was taken forward as the preferred solvent.
The imine formation and oxidation steps were optimized to provide oxaziridine 6a, and our attention then turned toward scale-up of the photochemical step. We expected that a continuous-flow microreactor system could provide a simple and inexpensive solution. A low-pressure lamp efficient at conversion of input power to UV C radiation could be used, without the need for the specialized UV-transparent (quartz) immersion-well glassware and forced coolant as required by higher-pressure lamps, which emit strongly in the infrared. Furthermore, opportunity for exposure to the operator of intense UV radiation would be minimized.
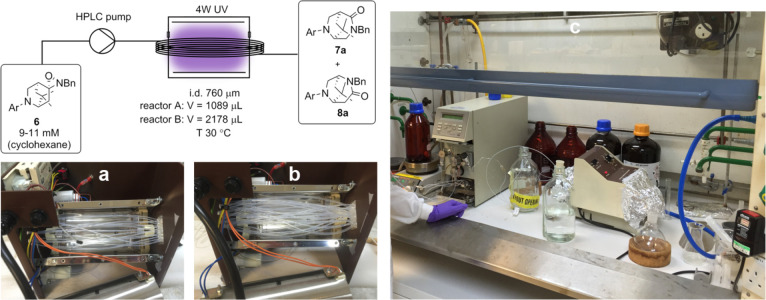
A parallel tube flow microreactor (Figure 3) was constructed as follows. The setup consisted of an HPLC pump, a 15 m length of UV-transparent perfuoroalkoxy (PFA) tubing (760 μm, i.d.), and a collection vessel arranged in series. The PFA tubing was threaded several times through the batch photochemical microreactor, comprising a 4 W UV C bulb inside a cylindrical reflector, such that serial sections of tubing could be exposed to UV radiation (reactor A, 16-pass, V = 1089 μL, 2.40 m irradiated length; full details are provided in the Supporting Information).
Figure 3.
Continuous-flow photochemical microreactor setup. (a, b) View inside reactor with the cylindrical metal reflector removed: (a) reactor A, 16-pass and (b) reactor B, 32-pass. (c) Full setup inside a 1.8 m fume cupboard.
Cyclohexane solutions of oxaziridine 6 (6:1 to 9:1 by 1H NMR) were thoroughly degassed with argon, pumped through the photochemical reactor, and collected at the outlet. Initial experiments were conducted at close to the upper solubility limit of 6 in cyclohexane (11 mM, 4 g L–1). High reaction conversions were observed at a flow rate of 0.30 mL min–1 (Table 3, entry 1). However, in an identical run with these parameters, the less soluble lactam products began to precipitate inside the reactor tubing, reducing the reaction conversion. Lowering the oxaziridine concentration to 9 mM (3.3 g L–1) was sufficient to completely overcome this issue. The flow rate could then be increased to an optimum value of 0.75 mL min–1 while maintaining the reaction conversion at above 95% (entry 2).
Table 3. Oxaziridine Rearrangement in Continuous-Flow.
| yield (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | reactor | c (mM)a | flow rate (mL min–1) | tR (min)b | t (h)c | 7a | 8a | 7a:8a | productivity (mmol h–1)d | STY (mmol h–1 mL–1)e |
| 1 | A | 11 | 0.30 | 3.6 | 17 | 71 | 12 | 5.9:1 | 0.14 | 0.12 |
| 2 | A | 9 | 0.75 | 1.5 | 26 | 82 | 11 | 7.5:1 | 0.32 | 0.29 |
| 3 | A | 9 | 0.75 | 1.5 | 42 | 84 | 11 | 7.6:1 | 0.33 | 0.30 |
| 4 | B | 9 | 1.20 | 1.8 | 8 | 85 | 10 | 8.5:1 | 0.53 | 0.24 |
c = concentration.
tR = average retention time.
t = time.
Productivity = concentration × flow rate × yield × 60.
STY is the space-time yield, given by STY = productivity/reactor volume.
Under these conditions, no maintenance of the flow reactor was required, and it could be operated continually for an extended period (42 h) without decay of the reaction conversion (Table 3, entry 3). Furthermore, the cyclohexane solvent used could be distilled and recycled through the reactor without loss of reaction performance.
In order to determine the maximum capability of this system, a modified reactor was tested. The length of tubing exposed to the UV lamp was doubled (reactor B, 32-pass, V = 2178 μL, 4.80 m irradiation length). With this design, the flow rate was increased until a drop in reaction conversion was observed. At a flow rate of 1.20 mL min–1, a productivity of 0.53 mmol h–1 (space-time yield = 0.24 mmol h–1 mL–1) for 7a was possible (Table 3, entry 4). To benchmark the efficiency of this system against other flow reactors, our system employing a low-power (4 W) lamp compares favorably to the FEP-wrapped reactor design used for photochemical rearrangements to chiral lactams, which demonstrated a productivity of 24.9 mmol h–1 (space-time yield = 0.19 mmol h–1 mL–1) employing a medium-power (450 W) lamp.47 Although the present flow reactor was used only to demonstrate the gram-scale synthesis of 7a (total amount synthesized: 17 g, 45 mmol), scaling to greater throughput should be straightforward by either extending the reaction time, numbering-up multiple flow reactors of this design, or translating the reaction to a larger-volume reactor.
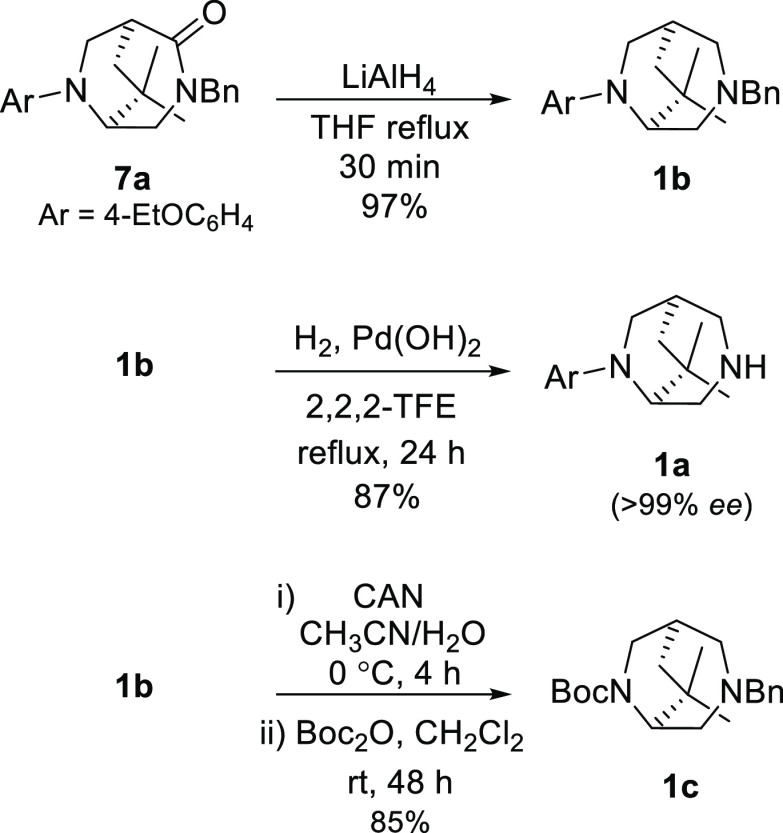
Homopiperazinone 7a was isolated by flash chromatography, and further chemistry was carried out on the gram scale in batch mode (Scheme 5). Reduction with LiAlH4 in THF gave versatile homopiperazine intermediate 1b bearing orthogonal protecting groups. Differential deprotection was then possible. Catalytic hydrogenolysis to homopiperazine 1a was achieved over palladium hydroxide using 2,2,2-trifluoroethanol (2,2,2-TFE) as the solvent,48 which avoided the formation of N-alkylated byproducts observed when the reaction was performed in ethanol. The optical purity of 1a was determined by derivatization and analysis on a chiral stationary phase (>99% ee). The ethoxyphenyl group of 1b could be removed efficiently by oxidation with cerium ammonium nitrate. During the reaction, complete monodeprotection to the NH-homopiperazine was observed, but isolation of the basic homopiperazine from the reaction mixture proved to be challenging. In-situ tert-butoxycarbonyl protection assisted isolation to provide homopiperazine 1c.
Scheme 5. Reduction and Deprotection to Homopiperazines 1a–c.
Conclusion
A key ring-expansion step for the scale-up of a chiral bicyclic homopiperazine via Schmidt rearrangement and Lattes–Aubé chemistry was investigated. Adopting continuous-flow allowed access to preferential conditions for the Schmidt rearrangement and an improved safety profile over the batch process, although the productivity and selectivity for the required homopiperazine isomer remained moderate. Oxaziridine rearrangement chemistry gave greater selectivity for the ring-expansion step under milder reaction conditions. Conversion of the photochemical rearrangement step to continuous-flow in an efficient flow reactor utilizing a low-power source allowed the multigram scale-up of the otherwise difficult to access homopiperazines 1a–c. Future advances in UV-LED or solar energy capture technology will likely further improve the potential for more environmentally benign methods for photochemical synthesis and encourage the development of efficient and scalable routes to other pharmaceutically useful nitrogen-containing heterocycles.
Experimental Section
(1R,4S)-2-(4-Ethoxyphenyl)-7,7-dimethyl-2-azabicyclo[2.2.2]octan-5-one (2a)
This compound was prepared following a modified literature procedure.43 To a solution of p-phenetidine (10 mL, 84.5 mmol), 4,4-dimethyl-2-cylohexen-1-one (11.12 mL, 77.5 mmol), and l-proline (2.43 g, 21.1 mmol) in DMSO (200 mL) was added dropwise 37% aqueous formaldehyde (5.25 mL, 70.4 mmol). The reaction mixture was stirred at room temperature for 16 h and then diluted with EtOAc (250 mL) and water (250 mL). The layers were separated, and the aqueous phase was extracted with EtOAc (2 × 150 mL). The combined organic phases were washed with water (5 × 150 mL) and brine (100 mL), dried (MgSO4), and concentrated under vacuum. Recrystallization (methanol/water) afforded 2a (13.66 g, 71%) as a light-brown solid. [α]D12 = −85.9 (c = 1.7, CHCl3). 1H NMR (500 MHz, CDCl3) δ 6.90–6.76 (m, 2H), 6.69–6.50 (m, 2H), 3.97 (q, J = 7.0 Hz, 2H), 3.75 (t, J = 2.7 Hz, 1H), 3.53–3.40 (m, 2H), 2.68 (dd, J = 18.9, 2.1 Hz, 1H), 2.62 (p, J = 2.8 Hz, 1H), 2.47 (dd, J = 18.9, 3.2 Hz, 1H), 1.77 (d, J = 3.0 Hz, 2H), 1.38 (t, J = 7.0 Hz, 3H), 1.09 (s, 3H), 1.08 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 214.0, 150.5, 143.1, 116.1 (2C), 111.9 (2C), 64.3, 58.2, 47.8, 45.9, 41.2, 38.8, 36.0, 30.1, 28.8, 15.2. HRMS calcd for C17H24NO2 [M + H]+ 274.1807, found 274.1801.
Schmidt Rearrangement of 2a in Continuous-Flow (Table 2, Entry 6)
A solution of H2SO4 (85% w/w) in 1,2-dimethoxyethane (flow rate: 3.00 mL hr–1) and a binary solution of 2a (0.20 M) and tetrabutylammonium azide (0.27 M) in 1,2-dimethoxyethane (0.50 mL hr–1) were fed via syringe pump through the microreactor coil (760 μm i.d., 500 μL volume) immersed in a water bath at 80 °C. After steady-state conditions were reached (17 min), the outflow was collected for 3.5 h. The material in the collection vessel was brought to pH 9 with 2 M NaOH and then extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were washed with brine (30 mL), dried (MgSO4), and concentrated under vacuum. Column chromatography (ethyl acetate/petroleum ether) afforded (1S,5S)-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonan-2-one (3a) (40 mg, 40%) and (1S,5R)-6-(4-ethoxyphenyl)-9,9-dimethyl-2,6-diazabicyclo[3.2.2]nonan-3-one (4a) (39 mg, 39%).
(1S,5S)-6-(4-Ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonan-2-one (3a)
1H NMR (500 MHz, CDCl3) δ 6.88–6.82 (m, 2H), 6.68–6.61 (m, 2H), 5.48 (s, 1H), 3.97 (q, J = 7.0 Hz, 2H), 3.67 (s, 1H), 3.55 (dt, J = 10.8, 1.7 Hz, 1H), 3.49 (dt, J = 12.6, 2.8 Hz, 1H), 3.44 (ddd, J = 12.6, 3.1, 1.8 Hz, 1H), 3.38 (dd, J = 10.8, 4.9 Hz, 1H), 2.93–2.87 (m, 1H), 1.95 (dt, J = 14.3, 1.8 Hz, 1H), 1.65 (dd, J = 14.3, 5.9 Hz, 1H), 1.38 (t, J = 7.0 Hz, 3H), 1.19 (s, 3H), 1.10 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 177.9, 150.9, 142.9, 116.1 (2C), 112.3 (2C), 64.3, 60.1, 46.5, 43.6, 41.4, 37.2, 35.5, 32.0, 28.6, 15.2. HRMS calcd for C17H25N2O2 [M + H]+ 289.1916, found 289.1911.
(1S,5R)-6-(4-Ethoxyphenyl)-9,9-dimethyl-2,6-diazabicyclo[3.2.2]nonan-3-one (4a)
1H NMR (500 MHz, CDCl3) δ 7.19 (t, J = 9.6 Hz, 1H), 6.91–6.82 (m, 2H), 6.68–6.51 (m, 2H), 3.97 (q, J = 7.0 Hz, 2H), 3.65–3.54 (m, 1H), 3.53–3.43 (m, 3H), 2.90–2.72 (m, 2H), 1.92 (d, J = 14.2 Hz, 1H), 1.69 (dd, J = 14.3, 4.9 Hz, 1H), 1.37 (t, J = 7.0 Hz, 3H), 1.16 (s, 3H), 1.06 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 174.7, 150.8, 143.1, 116.1 (2C), 112.3 (2C), 64.3, 57.4, 51.4, 47.2, 41.8, 36.9, 36.2, 32.0, 29.4, 15.2. HRMS calcd for C17H24N2O2Na [M + Na]+ 311.1735, found 311.1721.
(1S,2R,4R)-2′-Benzyl-5-(4-ethoxyphenyl)-8,8-dimethyl-5-azaspiro[bicyclo[2.2.2]octane-2,3′-[1,2]oxaziridine] (6)
To a mixture of 2a (5.00 g, 18.29 mmol) and 4 Å molecular sieves (10 g) in anhydrous CH2Cl2 (35 mL) was added dropwise benzylamine (2.00 mL, 18.29 mmol) followed by TsOH·H2O (7 mg, 0.03 mmol), and the mixture was allowed to stand for 6 h at room temperature. The reaction mixture was filtered promptly through glass wool to remove the molecular sieves using anhydrous CH2Cl2 (25 mL) under a stream of dry nitrogen gas. The solution was then cooled to −10 °C, and a solution of mCPBA (≤77 wt %, 4.92 g, 21.95 mmol) in anhydrous CH2Cl2 (40 mL) was added dropwise under rapid stirring. After the addition, the reaction mixture was maintained at −10 °C for 1 h and then allowed to warm to room temperature. The reaction mixture was cooled to 0 °C and then quenched by the addition of saturated Na2S2O3/saturated NaHCO3 (1:1, 10 mL) followed by water (40 mL). The phases were separated, and the organic phase was extracted with CH2Cl2 (2 × 50 mL). The combined organic phases were washed successively with 10% aqueous NaHCO3 (50 mL) and brine (30 mL), dried (MgSO4), and concentrated under vacuum. Column chromatography (ethyl acetate/cyclohexane) afforded 6 (4.79 g, 69%) as a mixture of diastereoisomers (7.4:1).
(1S,2R,2′s,4R)-2′-Benzyl-5-(4-ethoxyphenyl)-8,8-dimethyl-5-azaspiro[bicyclo[2.2.2] octane-2,3′-[1,2]oxaziridine] (6a, Major Isomer)
1H NMR (500 MHz, CDCl3) δ 7.43 (d, J = 7.3 Hz, 2H), 7.37 (t, J = 7.3 Hz, 2H), 7.31 (t, J = 7.3 Hz, 1H), 6.85 (d, J = 9.1 Hz, 2H), 6.60 (d, J = 9.1 Hz, 2H), 3.98 (q, J = 6.9 Hz, 2H), 3.90 (app q, J = 15.1 Hz, 2H), 3.58 (t, J = 2.5 Hz, 1H), 3.51 (dt, J = 10.9, 2.5 Hz, 1H), 3.26 (dt, J = 9.8, 2.2 Hz, 1H), 2.61 (dd, J = 15.5, 2.2 Hz, 1H), 2.26 (dd, J = 15.5, 3.5 Hz, 1H), 1.67 (t, J = 2.5 Hz, 1H), 1.60–1.57 (m, 2H), 1.39 (t, J = 6.9 Hz, 3H), 0.98 (s, 3H), 0.89 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 150.0, 143.4, 136.4, 128.8 (2C), 128.7 (2C), 127.8, 116.2 (2C), 111.5 (2C), 86.2, 64.4, 59.4, 55.9, 46.2, 38.5, 37.1, 34.9, 30.1, 29.0, 27.9, 15.2. HRMS calculated for C24H31N2O2 [M + H]+ 379.2386, found 379.2370.
Photochemical Rearrangement in Continuous-Flow (Table 3, Entry 3)
A solution of 6 (6.11 g, 16.10 mmol) in cyclohexane (1.875 L, c = 3.26 g L–1) was pumped through PFA tubing (15.00 m total length, 760 μm i.d., 6800 μL volume), including a section irradiated with a 4 W UV C bulb (λmax = 254 nm) (2.40 m length, 1089 μL volume, 760 μm i.d.), for 41.5 h via an HPLC pump (flow rate 0.75 mL min–1, average residence time 87 s) and collected at the outlet. The solvent was removed under vacuum and recycled. Column chromatography (ethyl acetate/cyclohexane) gave (1S,5S)-3-benzyl-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonan-2-one (7a) (5.11 g, 84%) and (1S,5R)-2-benzyl-6-(4-ethoxyphenyl)-9,9-dimethyl-2,6-diazabicyclo[3.2.2]nonan-3-one (8a) (660 mg, 11%).
(1S,5S)-3-Benzyl-6-(4-ethoxyphenyl)-9,9-dimethyl-3,6-diazabicyclo[3.2.2]nonan-2-one (7a)
1H NMR (500 MHz, CDCl3) δ 7.32–7.24 (m, 5H), 6.86 (d, J = 8.8 Hz, 2H), 6.65 (d, J = 9.1 Hz, 2H), 4.82 (d, J = 14.5 Hz, 1H), 4.26 (d, J = 14.5 Hz, 1H), 3.99 (q, J = 6.9 Hz, 2H), 3.62 (t, J = 3.2 Hz, 1H), 3.58 (d, J = 10.7 Hz, 1H), 3.41 (dd, J = 5.4, 10.7 Hz, 1H), 3.37 (d, J = 3.5 Hz, 2H), 3.17–3.15 (m, 1H), 1.95 (d, J = 14.2 Hz, 1H), 1.68 (dd, J = 5.7, 14.2 Hz, 1H), 1.39 (t, J = 6.9 Hz, 3H), 1.07 (s, 3H), 0.94 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 174.9, 150.7, 142.8, 136.9, 128.6 (2C), 128.3 (2C), 127.5, 115.9 (2C), 112.3 (2C), 64.1, 60.6, 50.6, 49.6, 46.6, 42.1, 37.2, 35.1, 31.6, 27.9, 15.8. HRMS calcd for C24H31N2O2 [M + H]+ 379.2386, found 379.2364.
(1S,5R)-2-Benzyl-6-(4-ethoxyphenyl)-9,9-dimethyl-2,6-diazabicyclo[3.2.2]nonan-3-one (8a)
1H NMR (500 MHz, CDCl3) δ 7.36–7.30 (m, 5H), 6.85 (d, J = 9.1 Hz, 2H), 6.63 (d, J = 9.1 Hz, 2H), 4.80 (d, J = 14.8 Hz, 1H), 4.53 (d, J = 14.8 Hz, 1H), 3.99 (q, J = 7.3 Hz, 2H), 3.66–3.64 (m, 1H), 3.51 (t, J = 3.8 Hz, 1H), 3.43–3.39 (m, 1H), 3.31 (d, J = 11.4 Hz, 1H), 2.93 (dq, J = 4.1, 12.9 Hz, 2H), 1.70 (dt, J = 2.2, 14.2 Hz, 1H), 1.55 (dd, J = 4.7, 14.5 Hz, 1H), 1.39 (t, J = 6.9 Hz, 3H), 1.11 (s, 3H), 1.07 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 171.3, 150.9, 142.9, 137.5, 128.7 (2C), 128.4 (2C), 127.6, 115.9 (2C), 112.9 (2C), 64.1, 58.4, 52.6, 51.5, 51.0, 39.8, 37.6, 35.4, 31.5, 28.6, 15.1. HRMS calcd for C24H31N2O2 [M + H]+ 379.2386, found 379.2368.
Acknowledgments
We thank Prof. Ian Collins (Institute of Cancer Research, U.K.) and Matthew Roberts (CRUK Manchester Institute, U.K.) for their assistance in the preparation of this manuscript.
Glossary
Abbreviations
- LOX
lysyl oxidase
- tR
average residence time
- DME
1,2-dimethoxyethane
- MsOH
methanesulfonic acid
- TBAA
tetrabutylammonium azide
- TsOH·H2O
p-toluenesulfonic acid monohydrate
- Bn
benzyl
- PFA
perfluoroalkoxy
- UV
ultraviolet
- STY
space-time yield
- THF
tetrahydrofuran
- Boc
tert-butoxycarbonyl
- CAN
cerium ammonium nitrate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.oprd.0c00361.
Experimental procedures, including setup and operation of the Schmidt microreactor and the photochemical flow reactor, and characterization data for compounds 1a–c, 2a, and 3a–8a (PDF)
This work was supported by the CRUK Manchester Institute Drug Discovery Unit (C5759/A12328), Cancer Research UK funding to The Institute of Cancer Research (C309/A11566, C309/A8274, and C107/A10433), and the Wellcome Trust (1003X, 103021/Z/13/Z, and 100282/Z/12/Z).
The authors declare the following competing financial interest(s): M.B., M.A., L.M.H.L., D.A.S., N.M., D.N.-D., A.Z., and C.S. have filed a patent application that includes the therapeutic use of bridged homopiperazine inhibitors. All authors M.B., M.A., H.A., L.M.H.L., D.A.S., N.M., G.N., L.D., D.N.-D., A.Z., and C.S. could benefit financially if any of the bridged homopiperazine inhibitors are licensed as part of a reward scheme for employees at ICR and the University of Manchester rewards to inventors scheme.
Supplementary Material
References
- Bremner J. B. Seven-Membered Rings. Prog. Heterocycl. Chem. 2005, 16, 431–450. 10.1016/S0959-6380(05)80058-3. [DOI] [Google Scholar]
- Marais R.; Springer C.; Niculescu-Duvaz D.; Miller N.; Aljarah M.; Zambon A.; Leung L.; Smithen D.; Brown M.; Tang H.. Lysyl Oxidase Inhibitors. WO 2019/073251 A1, 2019.
- Taylor R. D.; MacCoss M.; Lawson A. D. G. Rings in Drugs. J. Med. Chem. 2014, 57 (14), 5845–5859. 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- Kantorowski E. J.; Kurth M. J. Expansion to Seven-Membered Rings. Tetrahedron 2000, 56 (26), 4317–4353. 10.1016/S0040-4020(00)00218-0. [DOI] [Google Scholar]
- Dickerman S. C.; Lindwall H. G. Studies In Piperidone Chemistry. I. A Synthesis Of 5-Homopiperazinones. J. Org. Chem. 1949, 14 (4), 530–536. 10.1021/jo01156a005. [DOI] [Google Scholar]
- Carraher C. E. Synthesis of Caprolactam and Nylon 6. J. Chem. Educ. 1978, 55 (1), 51. 10.1021/ed055p51. [DOI] [Google Scholar]
- Schmidt K. F. Aus Den Sitzungen Der Abteilungen. Angew. Chem. 1923, 36 (67), 506–523. 10.1002/ange.19230366703. [DOI] [Google Scholar]
- Schmidt K. F. Über Den Imin-Rest. Ber. Dtsch. Chem. Ges. B 1924, 57 (4), 704–706. 10.1002/cber.19240570423. [DOI] [Google Scholar]
- Smith P. A. S. The Schmidt Reaction: Experimental Conditions and Mechanism. J. Am. Chem. Soc. 1948, 70 (1), 320–323. 10.1021/ja01181a098. [DOI] [Google Scholar]
- Rahman M. T.; Wirth T. Safe Use of Hazardous Chemicals in Flow. Top. Heterocycl. Chem. 2018, 56, 343–373. 10.1007/7081_2018_17. [DOI] [Google Scholar]
- Gutmann B.; Obermayer D.; Roduit J.-P.; Roberge D. M.; Kappe C. O. Safe Generation and Synthetic Utilization of Hydrazoic Acid in a Continuous Flow Reactor. J. Flow Chem. 2012, 2 (1), 8–19. 10.1556/jfchem.2012.00021. [DOI] [Google Scholar]
- Movsisyan M.; Delbeke E. I. P.; Berton J. K. E. T.; Battilocchio C.; Ley S. V.; Stevens C. V. Taming Hazardous Chemistry by Continuous Flow Technology. Chem. Soc. Rev. 2016, 45 (18), 4892–4928. 10.1039/C5CS00902B. [DOI] [PubMed] [Google Scholar]
- Gálvez N.; Moreno-Mañas M.; Sebastián R. M.; Vallribera A. Dimethoxyethane as an Alternative Solvent for Schmidt Reactions. Preparation of Homochiral N-(5-Oxazolyl)Oxazolidinones from N-Acetoacetyl Derivatives of Oxazolidinones. Tetrahedron 1996, 52 (5), 1609–1616. 10.1016/0040-4020(95)00989-2. [DOI] [Google Scholar]
- Chen Y.; Liu B.; Liu X.; Yang Y.; Ling Y.; Jia Y. Schmidt Reaction of Ketones in DME Solution in a Continuous-Flow Microreactor. Org. Process Res. Dev. 2014, 18 (11), 1589–1592. 10.1021/op500269j. [DOI] [Google Scholar]
- Chen Y.; Gutmann B.; Kappe C. O. Continuous-Flow Electrophilic Amination of Arenes and Schmidt Reaction of Carboxylic Acids Utilizing the Superacidic Trimethylsilyl Azide/Triflic Acid Reagent System. J. Org. Chem. 2016, 81 (19), 9372–9380. 10.1021/acs.joc.6b02085. [DOI] [PubMed] [Google Scholar]
- Painter T. O.; Thornton P. D.; Orestano M.; Santini C.; Organ M. G.; Aubé J. In Situ Generation and Intramolecular Schmidt Reaction of Keto Azides in a Microwave-Assisted Flow Format. Chem. - Eur. J. 2011, 17 (35), 9595–9598. 10.1002/chem.201100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Dong C.; Du C.; Luo G. Organocatalyzed Beckmann Rearrangement of Cyclohexanone Oxime in a Microchemical System. Org. Process Res. Dev. 2015, 19 (2), 352–356. 10.1021/op500370u. [DOI] [Google Scholar]
- Chen Y.; Cantillo D.; Kappe C. O. Visible Light-Promoted Beckmann Rearrangements: Separating Sequential Photochemical and Thermal Phenomena in a Continuous Flow Reactor. Eur. J. Org. Chem. 2019, 2019 (11), 2163–2171. 10.1002/ejoc.201900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a review that summarizes studies up to 1980, see:; Krow G. R. Nitrogen Insertion Reactions of Bridged Bicyclic Ketones. Regioselective Lactam Formation. Tetrahedron 1981, 37 (7), 1283–1307. 10.1016/S0040-4020(01)92445-7. [DOI] [Google Scholar]
- Hall H. K. Synthesis and Polymerization of Atom-Bridged Bicyclic Lactams. J. Am. Chem. Soc. 1960, 82 (5), 1209–1215. 10.1021/ja01490a044. [DOI] [Google Scholar]
- Morita K.; Suzuki Z. The Beckmann Rearrangement and Fragmentation of Substituted Bicyclo[2.2.2]Octan-2-one Oximes. J. Org. Chem. 1966, 31 (1), 233–237. 10.1021/jo01339a052. [DOI] [Google Scholar]
- Reinisch G.; Bara H.; Klare H. Synthesen Und Konstitutionsaufklärung von Äthano-Caprolactamen. Chem. Ber. 1966, 99 (3), 856–861. 10.1002/cber.19660990318. [DOI] [Google Scholar]
- Krow G. R.; Szczepanski S. W.; Kim J. Y.; Liu N.; Sheikh A.; Xiao Y.; Yuan J. Regioselective Functionalization. 7. Unexpected Preferences for Bridgehead Migration in Schmidt Rearrangement Syntheses of Novel 2,6- Diazabicyclo[3.2.x]Alkan-3-ones (x = 1–3). J. Org. Chem. 1999, 64 (4), 1254–1258. 10.1021/jo981977d. [DOI] [Google Scholar]
- Coleman P. J.; Schreier J. D.; Roecker A. J.; Mercer S. P.; McGaughey G. B.; Cox C. D.; Hartman G. D.; Harrell C. M.; Reiss D. R.; Doran S. M.; Garson S. L.; Anderson W. B.; Tang C.; Prueksaritanont T.; Winrow C. J.; Renger J. J. Discovery of 3,9-Diazabicyclo[4.2.1]Nonanes as Potent Dual Orexin Receptor Antagonists with Sleep-Promoting Activity in the Rat. Bioorg. Med. Chem. Lett. 2010, 20 (14), 4201–4205. 10.1016/j.bmcl.2010.05.047. [DOI] [PubMed] [Google Scholar]
- Krow G. R.; Cheung O. H.; Hu Z.; Lee Y. B. Regioselective Functionalization. 6. Migratory Preferences in Hydroxylamine- O -Sulfonic Acid and Schmidt Rearrangements of 7-Substituted Norcamphors. J. Org. Chem. 1996, 61 (16), 5574–5580. 10.1021/jo960604e. [DOI] [Google Scholar]
- Fikes L. E.; Shechter H. Effects of Acid Strength on Schmidt Reactions of Alkyl Cyclopropyl Ketones and Alkyl Phenyl Ketones. J. Org. Chem. 1979, 44 (5), 741–744. 10.1021/jo01319a017. [DOI] [Google Scholar]
- Oliveros-Desherces E.; Riviere M. M.; Parello J.; Lattes A. Photolyse d’oxazirannes. IV Regioselectivite et Effets de Solvant Lors de l’ouverture d’oxazirannes a Jonction Spirannique. Tetrahedron Lett. 1975, 16 (11), 851–854. 10.1016/S0040-4039(00)72001-6. [DOI] [Google Scholar]
- Lattes A.; Oliveros E.; Riviere M.; Belzeck C.; Mostowicz D.; Abramskj W.; Piccinni-Leopardi C.; Germain G.; Van Meerssche M. Photochemical and Thermal Rearrangement of Oxaziridines. Experimental Evidence in Support of the Stereoelectronic Control Theory. J. Am. Chem. Soc. 1982, 104 (14), 3929–3934. 10.1021/ja00378a024. [DOI] [Google Scholar]
- Aubé J. Oxiziridine Rearrangements in Asymmetric Synthesis. Chem. Soc. Rev. 1997, 26 (4), 269–277. 10.1039/CS9972600269. [DOI] [Google Scholar]
- Wolfe M. S.; Dutta D.; Aubé J. Stereoselective Synthesis of Freidinger Lactams Using Oxaziridines Derived from Amino Acids. J. Org. Chem. 1997, 62 (3), 654–663. 10.1021/jo961670j. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Chackalamannil S.; Aubé J. Stereochemistry of the Oxidation of Imines Derived from Substituted Cyclohexanones: Axial vs Equatorial Attack and Evidence for Delivery by an Adjacent Hydroxyl Group. J. Org. Chem. 2000, 65 (17), 5120–5126. 10.1021/jo991928g. [DOI] [PubMed] [Google Scholar]
- Aube J.; Hammond M.; Gherardini E.; Takusagawa F. Syntheses and Rearrangements of Spirocyclic Oxaziridines Derived from Unsymmetrical Ketones. J. Org. Chem. 1991, 56 (2), 499–508. 10.1021/jo00002a006. [DOI] [Google Scholar]
- Oliveros E.; Riviere M.; Malrieu J. P.; Teichteil C. Theoretical Exploration of the Photochemical Rearrangement of Oxaziridines. J. Am. Chem. Soc. 1979, 101 (2), 318–322. 10.1021/ja00496a007. [DOI] [Google Scholar]
- Loubière K.; Oelgemöller M.; Aillet T.; Dechy-Cabaret O.; Prat L. Continuous-Flow Photochemistry: A Need for Chemical Engineering. Chem. Eng. Process. 2016, 104, 120–132. 10.1016/j.cep.2016.02.008. [DOI] [Google Scholar]
- Politano F.; Oksdath-Mansilla G. Light on the Horizon: Current Research and Future Perspectives in Flow Photochemistry. Org. Process Res. Dev. 2018, 22 (9), 1045–1062. 10.1021/acs.oprd.8b00213. [DOI] [Google Scholar]
- Hook B. D. A.; Dohle W.; Hirst P. R.; Pickworth M.; Berry M. B.; Booker-Milburn K. I. A Practical Flow Reactor for Continuous Organic Photochemistry. J. Org. Chem. 2005, 70 (19), 7558–7564. 10.1021/jo050705p. [DOI] [PubMed] [Google Scholar]
- Cochran J. E.; Waal N. Photochemical Rearrangement of Chiral Oxaziridines in Continuous Flow: Application Toward the Scale-Up of a Chiral Bicyclic Lactam. Org. Process Res. Dev. 2016, 20 (8), 1533–1539. 10.1021/acs.oprd.6b00213. [DOI] [Google Scholar]
- Elliott L. D.; Berry M.; Harji B.; Klauber D.; Leonard J.; Booker-Milburn K. I. A Small-Footprint, High-Capacity Flow Reactor for UV Photochemical Synthesis on the Kilogram Scale. Org. Process Res. Dev. 2016, 20 (10), 1806–1811. 10.1021/acs.oprd.6b00277. [DOI] [Google Scholar]
- Lee D. S.; Sharabi M.; Jefferson-Loveday R.; Pickering S. J.; Poliakoff M.; George M. W. Scalable Continuous Vortex Reactor for Gram to Kilo Scale for UV and Visible Photochemistry. Org. Process Res. Dev. 2020, 24 (2), 201–206. 10.1021/acs.oprd.9b00475. [DOI] [Google Scholar]
- Cambié D.; Bottecchia C.; Straathof N. J. W.; Hessel V.; Noël T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116 (17), 10276–10341. 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]
- Cambié D.; Zhao F.; Hessel V.; Debije M. G.; Noël T. A Leaf-Inspired Luminescent Solar Concentrator for Energy-Efficient Continuous-Flow Photochemistry. Angew. Chem., Int. Ed. 2017, 56 (4), 1050–1054. 10.1002/anie.201611101. [DOI] [PubMed] [Google Scholar]
- Mizuno K.; Nishiyama Y.; Ogaki T.; Terao K.; Ikeda H.; Kakiuchi K. Utilization of Microflow Reactors to Carry out Synthetically Useful Organic Photochemical Reactions. J. Photochem. Photobiol., C 2016, 29, 107–147. 10.1016/j.jphotochemrev.2016.10.002. [DOI] [Google Scholar]
- Sundén H.; Ibrahem I.; Eriksson L.; Córdova A. Direct Catalytic Enantioselective Aza-Diels-Alder Reactions. Angew. Chem., Int. Ed. 2005, 44 (31), 4877–4880. 10.1002/anie.200500811. [DOI] [PubMed] [Google Scholar]
- Beckmann E. Zur Kenntniss Der Isonitrosoverbindungen. Ber. Dtsch. Chem. Ges. 1886, 19 (1), 988–993. 10.1002/cber.188601901222. [DOI] [Google Scholar]
- Gawley R. E.The Beckmann Reactions: Rearrangements, Elimination–Additions, Fragmentations, and Rearrangement–Cyclizations. In Organic Reactions; Wiley: Hoboken, NJ, 1988; pp 1–420. [Google Scholar]
- For practicality, the reported average residence times (tR) were calculated by dividing the reaction volume by the total flow rate. As reported by Jia and co-workers,14 the evolution of gas during the reaction led to a segmented-flow regime, and thus, the actual residence time was slightly shorter.
- Highest yielding example, Table 1, entry 1: 0.1 M, 5 mL min–1, 83%. Reactor volume 130 mL.Cochran J. E.; Waal N. Photochemical Rearrangement of Chiral Oxaziridines in Continuous Flow: Application Toward the Scale-Up of a Chiral Bicyclic Lactam. Org. Process Res. Dev. 2016, 20 (8), 1533–1539. 10.1021/acs.oprd.6b00213. [DOI] [Google Scholar]
- Bailey P. D.; Beard M. A.; Dang H. P. T.; Phillips T. R.; Price R. A.; Whittaker J. H. Debenzylation Using Catalytic Hydrogenolysis in Trifluoroethanol, and the Total Synthesis of (−)-Raumacline. Tetrahedron Lett. 2008, 49 (13), 2150–2153. 10.1016/j.tetlet.2008.01.104. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.