As of 15 March 2020, the World Health Organization reports a total of 167,757 cases of 2019-nCoV infection, and 6456 deaths. A rapid response to this pandemic would be greatly helped by the possibility to repurpose old drugs as novel antiviral medications.
The 2019-nCoV is a new human betacoronavirus. Coronaviruses are enveloped, non-segmented positive-sense RNA viruses. Their surface displays club-shaped protrusions made by trimers of the spike (S) protein. The initial attachment of the virion to the host cell is initiated by interactions between the S-protein and its receptor, which varies according to the specific virus.1 The S-protein/receptor interaction is the primary determinant for a coronavirus to infect a host species and also governs the tissue tropism of the virus. Infectivity assays have demonstrated that 2019-nCoV uses the angiotensin-converting enzyme 2 (ACE2) for entry into human cells, as in the case of SARS-CoV (whose genome is >99.9% similar).2 Other mechanisms of attachment to the cell of the 2019-nCoV have not been described so far.
Following receptor binding, the virus is taken up by receptor-mediated endocytosis, ending in an acidic endosomal compartment where the S-protein undergoes an acid-dependent proteolytic cleavage by cathepsin L. The S-protein then triggers the mixing of viral and endosomal membranes, causing the release of the viral genome into the cytoplasm. A mildly acidic pH environment in late endosomes/lysosomes (LE/Lys) seems to be important, since infection can be blocked by lysomotropic agents such as NH4Cl or chloroquine.1
Cationic amphiphilic drugs (CADs) such as amiodarone are characterized by a hydrophobic aromatic ring or ring system and a hydrophilic side chain containing an ionizable amine functional group.3 Because of their structure, CADs accumulate into acidic compartments such as LE/Lys, reducing their luminal acidity, altering the trafficking of membrane components and inducing in several cell types, for example, alveolar macrophages, a Niemann–Pick C-like phenotype.3 This may affect cell activities important for an efficient viral internalization, such as partial hydrolysis of viral surface proteins, macro- and/or micro-pinocytosis, the organization of the membrane invagination systems, and the vesicular transport of material to the Lys.3
Amiodarone and its main metabolite (mono-n-desethyl amiodarone) inhibited the entry of filoviruses (a family of single-stranded negative-sense RNA virus that includes Ebola virus (EBOV)) at concentrations close to those found in the sera of patients treated for arrhythmias.4 Amiodarone also proved able to block the spreading of SARS CoV infection in cell cultures without modifying the density of ACE2 receptors on the cell surface or interfering with the attachment of SARS-CoV to the cells.5 Interestingly, amiodarone displayed antiviral activity even when SARS-CoV could deliver its genome into the cytoplasm through the plasma membrane, thus bypassing the endocytic compartment.5 Therefore, although the antiviral activity of amiodarone is most likely due to interference with the endocytic pathway (Figure 1), further mechanisms cannot be excluded. Indeed, amiodarone inhibits hepatitis C virus infection by downregulating the CD81 receptor, but inhibition of virus assembly and release has also been proposed.6
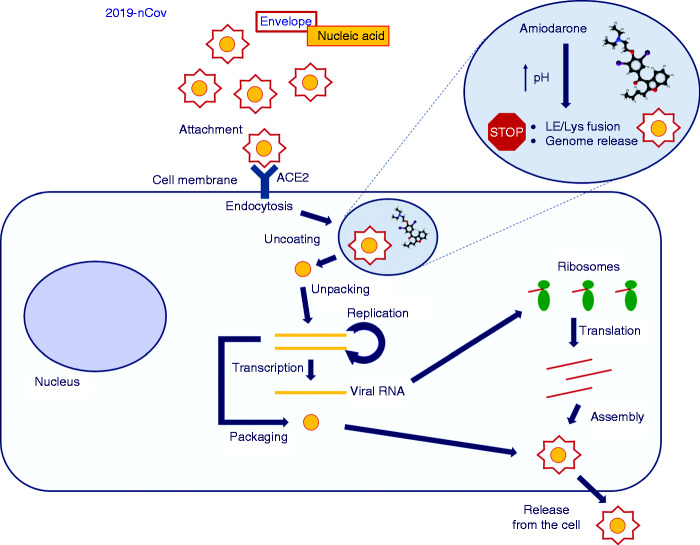
Figure 1.
Proposed mechanism of action of amiodarone on coronavirus replication.
Viruses bind to receptors on the plasma membrane and are internalized by endocytosis. Amiodarone accumulates into late endosomes/lysosomes (LE/Lys) and increases the pH of these organelles. Through this mechanism, amiodarone blocks the mixing of viral and endosomal membranes, and then the release of the viral genome into the cytoplasm, and ultimately virus replication. Further inhibitory effects of amiodarone on virus replication cannot be excluded.
ACE2: angiotensin-converting enzyme 2.
During the EBOV epidemic in West Africa, a systematic screen of Food and Drug Administration-approved drugs was performed to identify compounds with in vitro antiviral activities against EBOV. Amiodarone increased survival of mice infected with EBOV, although this effect was not very reproducible. Amiodarone was self-administered by a 38-year-old doctor with EBOV infection four days after symptom onset at a 400 mg oral dose, followed by 1200 mg intravenous infusion around 6 and 24 h later, but was discontinued due to concerns of cardiac, hepatic and pulmonary adverse effects.7,8 A phase III trial of amiodarone (NCT02307591) was stopped shortly after initiation, despite an apparent reduction in case–fatality rates.3
Other CADs with antiarrhythmic properties have proven effective against RNA viruses in vitro; most notably, dronedarone, verapamil and the calcium channel blocker bepridil inhibited filovirus infection in cell cultures and mouse models.3,9,10 It is then reasonable to postulate a class effect of CADs, a category of drugs including also antidepressants, antibiotics, antipsychotics, cholesterol-lowering and fertility-regulator drugs, and antimalarial medications3 (including chloroquine, which has been very recently reported to inhibit 2019-nCoV infection in vitro).11 We may envisage the assessment of the therapeutic potential of amiodarone and other CADs in cell cultures and animal models, then the evaluation of the most promising drugs in human patients. Since drugs such as amiodarone have been used for many decades on millions of patients, their safety profile is well known, and they appear to be a good candidate for phase III trials, as previously done for amiodarone during the EBOV epidemic. Notably, CADs appear to be not strong antivirals, and it may be speculated that they are more effective with low viral loads and at the start of infection, when virus entry into target cells is the dominant step. Amiodarone could then be usefully evaluated in adequately powered trials on people exposed to the virus but currently asymptomatic. Alternatively, amiodarone or other CADs might be evaluated as part of a combination regimen including, for example, protease inhibitors.12
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Fehr AR, Perlman S.. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol Biol 2015; 1282: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 2020; 9: 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salata C, Calistri A, Parolin C, et al. Antiviral activity of cationic amphiphilic drugs. Expert Rev Anti Infect Ther 2017; 15: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salata C, Baritussio A, Munegato D, et al. Amiodarone and metabolite MDEA inhibit Ebola virus infection by interfering with the viral entry process. Pathog Dis 2015; 73: ftv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stadler K, Ha HR, Ciminale V, et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol 2008; 39: 142–149. [DOI] [PubMed] [Google Scholar]
- 6. Cheng YL, Lan KH, Lee WP, et al. Amiodarone inhibits the entry and assembly steps of hepatitis C virus life cycle. Clin Sci 2013; 125: 439–448. [DOI] [PubMed] [Google Scholar]
- 7. Wolf T, Kann G, Becker S, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: Treatment of a patient in intensive care. Lancet 2015; 385: 1428–1435. [DOI] [PubMed] [Google Scholar]
- 8. Mendoza EJ, Qiu X, Kobinger GP.. Progression of Ebola therapeutics during the 2014–2015 outbreak. Trends Mol Med 2016; 22: 164–173. [DOI] [PubMed] [Google Scholar]
- 9. Gehring G, Rohrmann K, Atenchong N, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother 2014; 69: 2123–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. Epub ahead of print 4 February 2020. DOI: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed]
- 11. DeWald LE, Dyall J, Sword JM, et al. The calcium channel blocker bepridil demonstrates efficacy in the murine model of Marburg virus disease. J Infect Dis 2018; 218: S588–S591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004; 59: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]