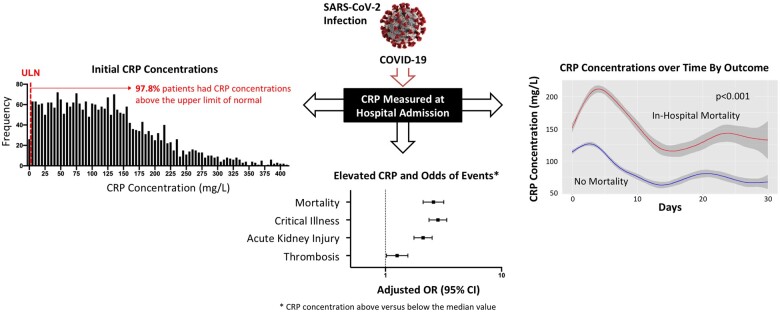
Abstract
Background
A systemic inflammatory response is observed in coronavirus disease 2019 (COVID-19). Elevated serum levels of C-reactive protein (CRP), a marker of systemic inflammation, are associated with severe disease in bacterial or viral infections. We aimed to explore associations between CRP concentration at initial hospital presentation and clinical outcomes in patients with COVID-19.
Methods and results
Consecutive adults aged ≥18 years with COVID-19 admitted to a large New York healthcare system between 1 March and 8 April 2020 were identified. Patients with measurement of CRP were included. Venous thrombo-embolism (VTE), acute kidney injury (AKI), critical illness, and in-hospital mortality were determined for all patients. Among 2782 patients hospitalized with COVID-19, 2601 (93.5%) had a CRP measurement [median 108 mg/L, interquartile range (IQR) 53–169]. CRP concentrations above the median value were associated with VTE [8.3% vs. 3.4%; adjusted odds ratio (aOR) 2.33, 95% confidence interval (CI) 1.61–3.36], AKI (43.0% vs. 28.4%; aOR 2.11, 95% CI 1.76–2.52), critical illness (47.6% vs. 25.9%; aOR 2.83, 95% CI 2.37–3.37), and mortality (32.2% vs. 17.8%; aOR 2.59, 95% CI 2.11–3.18), compared with CRP below the median. A dose response was observed between CRP concentration and adverse outcomes. While the associations between CRP and adverse outcomes were consistent among patients with low and high D-dimer levels, patients with high D-dimer and high CRP have the greatest risk of adverse outcomes.
Conclusions
Systemic inflammation, as measured by CRP, is strongly associated with VTE, AKI, critical illness, and mortality in COVID-19. CRP-based approaches to risk stratification and treatment should be tested.
Keywords: C-reactive protein, Coronavirus, COVID-19, Critical illness, Inflammation, Mortality
Graphical Abstract
See page 2280 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab169)
Introduction
The systemic inflammatory response to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is a hallmark of the 2019 coronavirus disease (COVID-19), and most hospitalized patients with COVID-19 have abnormal inflammatory biomarkers.1 C-reactive protein (CRP), an acute-phase protein first described by Tillet and Francis,2 is synthesized by the liver in response to interleukin-6 (IL-6) and is a widely available biomarker of inflammation.3 Elevated CRP concentrations are associated with cardiovascular disease and acute kidney injury (AKI) in surgical patients,4 with inflammatory rheumatic diseases such as rheumatoid arthritis and gout, and with incident venous thrombo-embolism (VTE) in community cohorts.5 C-reactive protein has also been associated with severe disease in patients with H1N1 influenza pneumonia,6 and a number of recent series have reported an association between higher CRP concentrations and greater disease severity in COVID-19.1 , 7–12 However, most studies were small, and evaluated neither a dose response nor heterogeneity across demographics. Moreover, the association between initial CRP concentration and VTE and AKI in COVID-19 is uncertain. The relationship between CRP concentrations and D-dimer, a fibrin degradation product that is associated with thrombosis in COVID-19, has not been explored.13 The aim of this study is to explore the associations between CRP concentrations at initial hospital presentation and clinical outcomes, including VTE and AKI, in patients with COVID-19 who were hospitalized at a large healthcare system in New York.
Methods
Study participants and data collection
The study was approved by the New York University (NYU) Grossman School of Medicine Institutional Review Board and performed with a waiver of informed consent. We identified consecutive adults age ≥18 years with a nucleic acid amplification test positive for SARS-CoV-2 between 1 March 2020 and 8 April 2020 who were admitted to NYU Langone Health (NYULH), a multi-hospital health system in New York. At all inpatient facilities, CRP surveillance was standard of care for individuals with suspected or confirmed COVID-19 diagnoses because it was included in the electronic admission order sets during the pandemic. Due to the high prevalence of inflammation in COVID-19, elevated CRP concentration (Siemens Dimension C-Reactive Protein, Siemens, Washington DC; Abbot Architect C-Reactive Protein, Chicago, IL) was defined as a measurement above the median value among patients hospitalized with COVID-19 at NYU. Patients were also divided into subgroups by quartile of initial CRP concentration. Demographics, comorbidities, outpatient medications, clinical presentations, and other laboratory data recorded for clinical purposes were abstracted from the electronic health record. Only the initial in-hospital measurements for each laboratory test were recorded. Relevant comorbidities, including hypertension, hyperlipidaemia, diabetes mellitus, heart failure, coronary artery disease, atrial fibrillation, cancer, and chronic kidney disease, were defined by International Classificatiion of Diseases 10th Revision (ICD-10) codes.
Outcomes
Venous thrombo-embolism was defined by deep vein thrombosis or pulmonary embolism. Thrombotic events were identified from radiology reports and clinical documentation using a natural-language processing tool (simpleNLP) with sensitivity and specificity >95%, ICD-10 diagnosis codes assigned during hospitalization, and chart review of echocardiogram reports, as described previously.13 All thrombotic events were confirmed by manual medical record review. Acute kidney injury was defined as an increase in serum creatinine by ≥0.3 mg/dL within a 48 h period or a 50% increase in serum creatinine compared with the baseline value. Critical illness was defined by treatment in an intensive care unit, need for mechanical ventilation, transfer to a hospice, or in-hospital death. All-cause in-hospital mortality was determined for all patients. Follow-up was complete through 13 May 2020.
Statistical analysis
Categorical variables are reported as frequencies and percentages, and were compared by χ2 tests. Continuous variables are presented as mean (SD) and median [interquartile range (IQR)] and compared using t-tests or non-parametric Mann–Whitney test for all non-normally distributed data. Patients were categorized by the median CRP concentration, or the quartile of CRP concentration. Logistic regression models were generated to estimate the odds of the clinical endpoints, adjusted for demographics and clinical comorbidities. Covariates included in the multivariable models included age, sex, race/ethnicity, body mass index, tobacco use, hypertension, hyperlipidaemia, chronic kidney disease, coronary artery disease, heart failure, malignancy, and baseline laboratory values. Subgroup analyses were performed to evaluate the consistency of the study findings by age, sex, race, and the presence or absence of obesity [body mass index (BMI) ≥30 kg/m2]. To evaluate trends in CRP over time, CRP trajectory plots were generated and stratified by clinical outcomes. Differences in CRP values by group were compared by repeated measures analysis of variance (ANOVA). Since D-dimer is associated with thrombosis in patients with COVID-19, subgroup analyses were also performed to evaluate relationships between CRP and outcomes by D-dimer level (above vs. below the median value of 384 ng/mL).13 Finally, we investigated the relationship between IL-6 and outcomes in patients with both CRP and IL-6 measured at hospital presentation. Statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests are two-sided, and P-values <0.05 were considered to be statistically significant.
Results
Patient characteristics
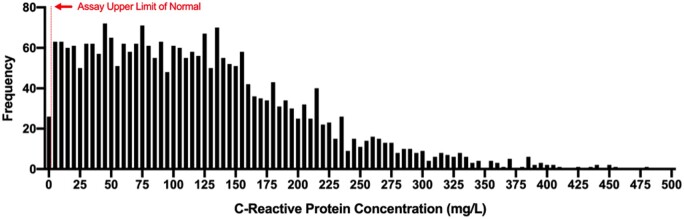
A total of 2782 consecutive adults with COVID-19 were admitted to NYULH between 1 March and 8 April 2020, and 2601 (93.5%) had a measurement of CRP. The median initial CRP concentration was 108 mg/L (IQR 53–169) (Figure 1). Only 58 patients (2.2%) had an initial normal CRP <5 mg/L (the upper reference limit for the assay). Clinical characteristics of patients stratified by quartile of initial CRP concentration are shown in Table 1. Patients with CRP concentrations in the highest quartiles were more likely to be men (71.3% in the highest CRP quartile vs. 52.6% in the lowest CRP quartile, P < 0.001) and of Hispanic ethnicity (30.4% in the highest quartile vs. 20.5% in the lowest quartile, P < 0.001) than patients with lower CRP concentrations. Fewer patients with the highest CRP values had a history of heart failure (9.6% in the highest quartile vs. 16.4% in the lowest quartile, P = 0.0004), coronary artery disease (18.5% vs. 27.5%, P = 0.0002), or chronic kidney disease (16.8% vs. 23.9%, P = 0.0019). Patients with the highest CRP concentrations were also less likely to have been prescribed statin therapy (11.6% in the highest quartile vs. 17.4% in the lowest quartile, P = 0.0035) or a beta-blocker (10% vs. 15.3%, P = 0.005) prior to hospital admission. Elevated CRP concentrations at presentation were associated with higher temperatures and lower oxygen saturation at presentation, as well as higher initial white blood cell and platelet counts, higher initial D-dimer levels, and higher initial ferritin concentrations (Figure 1). Clinical characteristics of patients stratified by median initial CRP concentration are shown in Supplementary material online Table S1. In a sensitivity analysis of patients who were admitted and discharged alive with a hospital length of stay ≤1 day, the median initial CRP was 45.9 mg/L (IQR 21.8–107.4).
Figure 1.
Frequency distribution of initial CRP concentrations in patients hospitalized with COVID-19.
Table 1.
Characteristics of patients with COVID-19 based on the quartile of initial CRP
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value | |
|---|---|---|---|---|---|
| (≤53 mg/L) | (>53 to ≤108 mg/L) | (>108 to ≤169 mg/L) | (>169 mg/L) | ||
| (n = 648) | (n = 655) | (n = 650) | (n = 648) | ||
| Age, years; median (IQR) | 64 (51–75) | 64 (51–75) | 64 (53–74) | 62 (52–72) | 0.451 |
| Male sex | 341 (52.62%) | 389 (59.39%) | 432 (66.46%) | 462 (71.30%) | <0.001 |
| Race/ethnicity | 0.036 | ||||
| Non-Hispanic white | 278 (42.90%) | 247 (37.71%) | 260 (40.00%) | 252 (38.89%) | |
| African American | 107 (16.51%) | 94 (14.35%) | 87 (13.38%) | 85 (13.12%) | |
| Hispanic | 133 (20.52%) | 181 (27.63%) | 179 (27.54%) | 197 (30.40%) | |
| Asian | 50 (7.72%) | 50 (7.63%) | 48 (7.38%) | 31 (4.78%) | |
| Other/multiracial | 58 (8.95%) | 59 (9.01%) | 49 (7.54%) | 55 (8.49%) | |
| Unknown | 22 (3.40%) | 24 (3.66%) | 27 (4.15%) | 28 (4.32%) | |
| Tobacco use | <0.001 | ||||
| Current | 56 (8.64%) | 32 (4.89%) | 23 (3.54%) | 30 (4.63%) | |
| Former | 140 (21.60%) | 128 (19.54%) | 133 (20.46%) | 131 (20.22%) | |
| Never | 452 (69.75%) | 495 (75.57%) | 494 (76.00%) | 487 (75.15%) | |
| BMI, kg/m2, mean (SD) | 28.5 (25.1–32.9) | 29.0 (25.5–34.0) | 29.3 (25.8–34.5) | 28.98 (25.5–33.0) | 0.011 |
| Clinical comorbidities | |||||
| Hypertension | 412 (63.58%) | 413 (63.05%) | 425 (65.38%) | 374 (57.72%) | 0.029 |
| Hyperlipidaemia | 284 (43.83%) | 286 (43.66%) | 264 (40.62%) | 284 (43.83%) | 0.575 |
| Diabetes mellitus | 245 (37.81%) | 254 (38.78%) | 272 (41.85%) | 229 (35.34%) | 0.114 |
| Heart failure | 106 (16.36%) | 85 (12.98%) | 85 (13.08%) | 62 (9.57%) | 0.004 |
| Coronary artery disease | 178 (27.47%) | 146 (22.29%) | 133 (20.46%) | 120 (18.52%) | 0.001 |
| Atrial fibrillation | 57 (8.91%) | 70 (10.75%) | 48 (7.41%) | 50 (7.78%) | 0.137 |
| Cancer | 70 (10.80%) | 80 (12.21%) | 72 (11.08%) | 61 (9.41%) | 0.446 |
| Chronic kidney disease | 155 (23.92%) | 143 (21.83%) | 145 (22.31%) | 109 (16.82%) | 0.012 |
| Medications at baseline | |||||
| Statin | 113 (17.44%) | 85 (12.98%) | 90 (13.85%) | 75 (11.57%) | 0.017 |
| Beta-blocker | 98 (15.31%) | 90 (13.82%) | 71 (10.96%) | 64 (9.95%) | 0.012 |
| ACE-I or ARB | 107 (16.72%) | 114 (17.51%) | 115 (17.75%) | 88 (13.69%) | 0.176 |
| Anticoagulation | 52 (8.12%) | 61 (9.37%) | 54 (8.33%) | 53 (8.24%) | 0.844 |
| Clinical characteristics at presentation | |||||
| Temperature at presentation (°C) | 37.3 (36.8–38.1) | 37.4 (36.9–38.2) | 37.5 (37–38.2) | 37.5 (37–38.3) | 0.001 |
| Oxygen saturation (%) | 96 (94–98) | 94 (91–96) | 93 (89–95) | 91 (86–95) | <0.001 |
| Initial laboratory markers [median (IQR)] | |||||
| First creatinine, mg/dL | 1.0 (0.8–1.4) | 1.0 (0.8–1.3) | 1.0 (0.8–1.4) | 1.0 (0.8–1.4) | 0.694 |
| First WBC | 2.0 (1.0–7.0) | 3.0 (1.0–8.5) | 4 (2.0–12.3) | 5.0 (2.0–12.5) | <0.001 |
| First CRP | 27.5 (13.4–40.9) | 80.6 (67.5–94.0) | 136.0 (123.0–151.0) | 218.0 (191.1–267.9) | <0.001 |
| First haemoglobin, g/dL | 13.2 (11.8–14.4) | 13.2 (11.9–14.4) | 13.3 (12–14.4) | 13.3 (12.1–14.3) | 0.94 |
| First platelet | 180 (142–230) | 189 (152–244.5) | 200 (160–255) | 229 (175–285) | <0.001 |
| First D-dimer | 301 (197.96–563) | 335 (213–630) | 404 (261–740) | 484 (296–963) | <0.001 |
| First ferritin | 379 (167–730.6) | 658 (355.4–1310.2) | 811 (433.5–1646) | 1173.6 (637.35–1903.5) | <0.001 |
| First lymphocyte | 1 (0.7–1.4) | 0.8 (0.6–1.2) | 0.8 (0.6–1.1) | 0.8 (0.5–1.1) | <0.001 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; WBC, white blood cell count.
Clinical outcomes
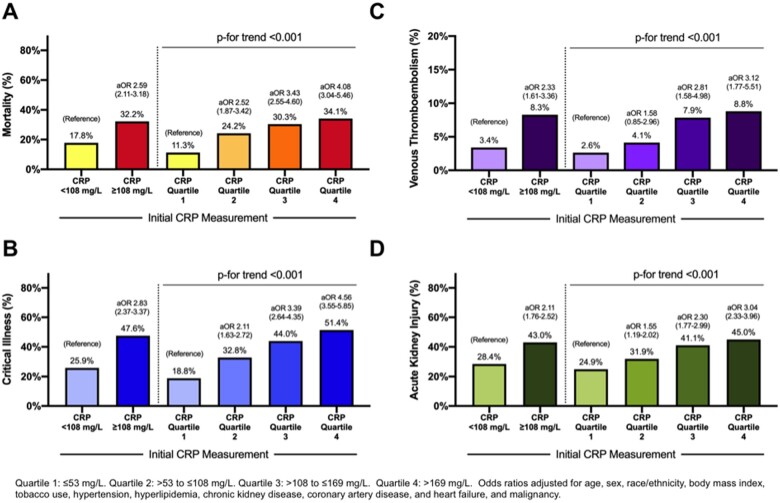
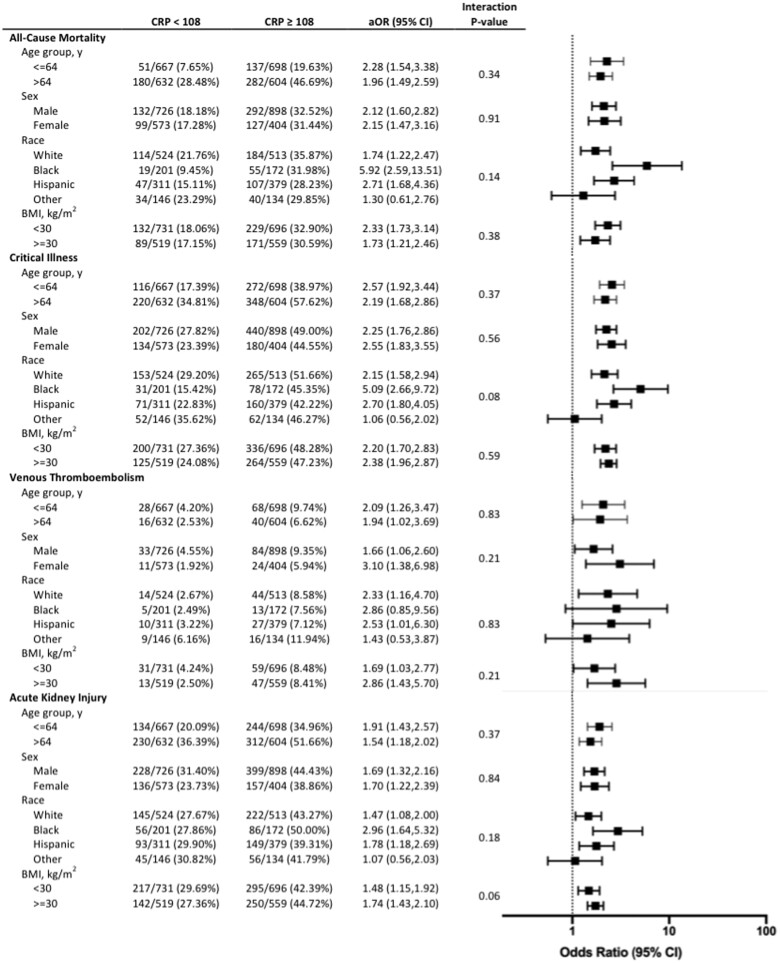
Initial CRP concentrations were associated with clinical outcomes in patients with COVID-19. An initial CRP value above the median measurement was associated with VTE [8.3% vs. 3.4%; adjusted odds ratio (aOR) 2.33, 95% confidence interval (CI) 1.61–3.36], AKI (43.0% vs. 28.4%; aOR 2.11, 95% CI 1.76–2.52), critical illness (47.6% vs. 25.9%; aOR 2.83, 95% CI 2.37–3.37), and in-hospital mortality (32.2% vs. 17.8%; aOR 2.59, 95% CI 2.11–3.18) compared with patients with an initial CRP value below the median (Figure 2). Patients with the highest quartiles of CRP measured had the greatest likelihood of VTE, AKI, critical illness, and mortality (Figure 2). Associations between CRP concentration and adverse outcomes were consistent in subgroups defined by age, sex, race, and obesity (Figure 3).
Figure 2.
Mortality (A), critical illness (B), venous thrombo-embolism (C), and acute kidney injury (D) among patients with COVID-19, stratified by initial CRP measurement. Quartile 1: ≤53 mg/L. Quartile 2: >53 to ≤108 mg/L. Quartile 3: >108 to ≤169 mg/L. Quartile 4: >169 mg/L. Odds ratios adjusted for age, sex, race/ethnicity, body mass index, tobacco use, hypertension, hyperlipidaemia, chronic kidney disease, coronary artery disease, heart failure, and malignancy.
Figure 3.
Associations between CRP and all-cause mortality, critical illness, venous thromb-oembolism, and acute kidney injury in subgroups by age, sex, race, and body mass index. Odds ratios adjusted for age, sex, race/ethnicity, body mass index, tobacco use, hypertension, hyperlipidaemia, chronic kidney disease, atrial fibrillation, coronary artery disease, heart failure, malignancy, initial ferritin, absolute lymphocyte count, D-dimer, and baseline use of statins, beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and anticoagulants.
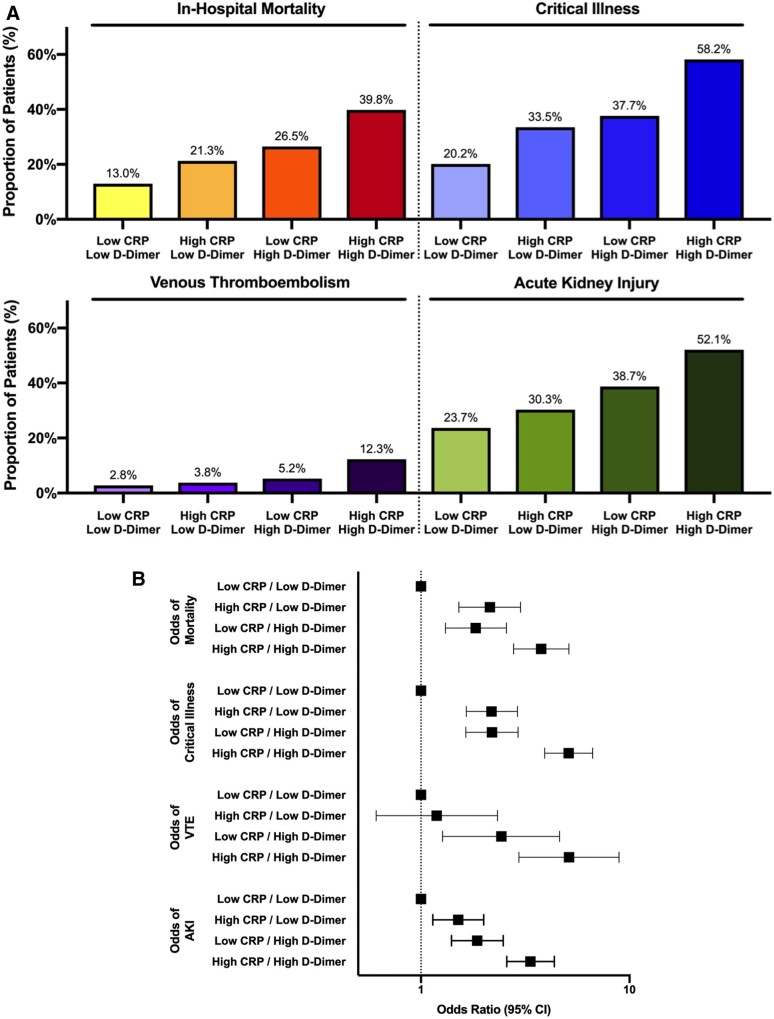
Levels of D-dimer increased concordantly with higher quartiles of initial CRP concentration (Table 1). Associations between CRP concentration and VTE, AKI, critical illness, and mortality were consistent in patients with low and high D-dimer (data not shown). In a subgroup analyses by D-dimer level, patients with low values of both CRP and D-dimer were at low risk for in-hospital adverse events. In contrast, the incidences of VTE (12.3% vs. 2.8%, P < 0.001), AKI (52.1% vs. 23.7%, P < 0.001), critical illness (58.2% vs. 20.2%, P < 0.001), and mortality (39.8% vs. 13.0%, P < 0.001) were substantially higher in patients with concomitant elevations in CRP and D-dimer compared with those with low CRP and D-dimer values (Figure 4A). While both CRP and D-dimer increased the adjusted odds of an adverse outcome, patients with elevation of both CRP and D-dimer concentrations had the greatest risk of adverse events (Figure 4B).
Figure 4.
Associations between CRP and all-cause mortality, critical illness, venous thrombo-embolism, and acute kidney injury stratified by initial D-dimer measurement. The incidence (A) and adjusted odds (B) of adverse outcomes are shown. (low CRP <108 mg/dL; high CRP ≥108 mg/dL; low D-dimer ≤384 ng/mL; high D-dimer >384 ng/mL). (A) *P for trend <0.001 for all outcomes. (B) Odds ratios adjusted for age, sex, race/ethnicity, body mass index, tobacco use, hypertension, hyperlipidaemia, chronic kidney disease, atrial fibrillation, coronary artery disease, heart failure, malignancy, initial ferritin, absolute lymphocyte count, baseline use of statins, beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers,and anticoagulants.
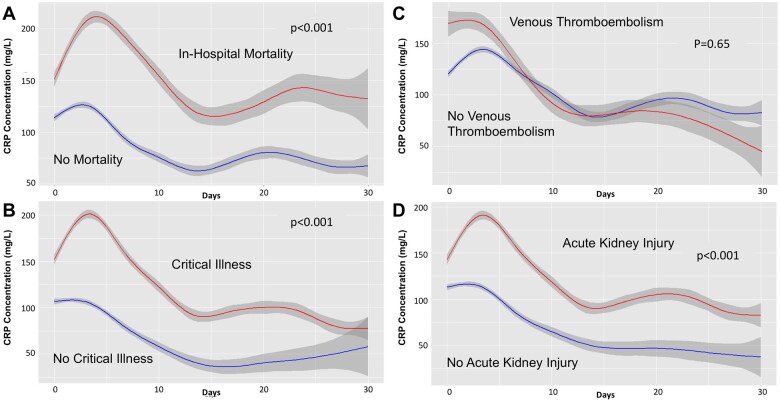
Among 2601 adults with COVID-19 and CRP concentration measured at presentation, 2224 (85.5%) had serial CRP measurements, with a median of 5 (IQR 3–10) CRP measurements reported during hospital admission. The median peak CRP concentration was 164 mg/L (91.09–251.5), measured on median hospital day 2 (IQR 0–5). Higher peak CRP concentrations were reported among patients with fatal COVID-19 (248.1 mg/L, IQR 169.0–395.0 vs. 141.0 mg/L, IQR 74.7–216.8 in patients who survived, P < 0.001). Levels of CRP were significantly higher over time in patients who developed AKI, critical illness, or who died during hospitalization. Levels of CRP over time are shown in patients with and without fatal disease, critical illness, VTE, and AKI (Figure 5A–D).
Figure 5.
Trajectory of CRP levels over time among patients with and without fatal disease (A), critical illness (B), VTE (C), and AKI (D).
Since IL-6 is upstream of CRP in the inflammatory cascade, we investigated the relationship between IL-6 and outcomes in 1693 patients with COVID-19 who had CRP and IL-6 measured at hospital presentation. Elevated initial IL-6 and CRP concentrations in COVID-19 were each associated with increased odds of mortality [IL-6 above the median concentration (>13 pg/mL), aOR 2.29, 95% CI 1.75–2.98; CRP above the median, aOR 1.93, 95% CI 1.50–2.49] and critical illness (IL-6, aOR 3.05, 95% CI 2.43–3.84; CRP, aOR 1.98, 95% CI 1.57–2.49) after adjustment for clinical covariates. When stratified by initial CRP concentration, an initial IL-6 above the median value (>13 pg/mL) was associated with critical illness and mortality among patients with CRP above (aOR for critical illness, 3.04, 95% CI 2.27–4.06; aOR for mortality, 1.91, 95% CI 1.39–2.62) and below (aOR for critical illness, 3.16, 95% CI 2.15–4.64; aOR for mortality, 2.51, 95% CI 1.58–4.00) the median concentration. These data suggest that IL-6 may provide additional prognostic information in patients hospitalized with COVID-19.
Discussion
In an analysis of COVID-19 patients hospitalized in a large New York health system, nearly all patients had evidence of a systemic inflammatory response to SARS-CoV-2 infection with a median CRP concentration of 108 mg/L, a value nearly 40-fold higher than the laboratory upper limit of normal. Patients with elevated CRP concentrations above the median value at the time of initial presentation were more likely to have VTE, AKI, critical illness, and in-hospital mortality during the subsequent hospital stay than those with lower initial measurements, and patients with the highest CRP values had the worst clinical outcomes.
C-reactive protein is well established as a marker of systemic inflammation and severe infection. As an acute-phase reactant, CRP binds to phosphocholine in pathogens and membranes of host cells, and acts as an opsonin to enhance phagocytosis and facilitate clearance. Ligand-bound CRP also efficiently activates the classical pathway of the complement system, an important component of innate host defence.14 Prior to the COVID-19 global pandemic, up to 90% of all marked elevations in CRP concentration were attributed to an infectious aetiology, most often from bacterial pathogens.15 , 16 Elevated CRP concentrations have also been reported in severe viral infections, including H1N1 influenza pneumonia, and now in SARS-CoV-2 infection.6–9 In a prior study of 298 patients with COVID-19, patients who died had an initial CRP that was 10-fold higher than that of survivors (100.0 vs. 9.7 mg/L, P < 0.001), and CRP concentrations were associated with mortality, with an area under the receiver operating characteristic curve (AUC) of 0.896.9 Recent reports also identified associations between CRP concentrations and respiratory failure requiring mechanical ventilation, with a nearly five-fold greater risk of acute respiratory distress syndrome (ARDS) reported in patients with high-sensitivity CRP >5 mg/L compared with those with lower CRP values.12 , 17 CRP is associated with extra-pulmonary disease in COVID-19, and correlations between CRP concentrations and myocardial injury have been reported in multiple series.18–21 In contrast, prior studies have not reported on the relationship between CRP and AKI and VTE in COVID-19, nor on serial measures of CRP over time.
Other inflammatory markers are also associated with adverse outcomes in COVID-19. In our cohort, IL-6 was independently associated with adverse outcomes in patients with high and low initial CRP concentrations. In a separate analysis of 1400 patients hospitalized with COVID-19, IL-6 and tumour necrosis factor (TNF)-α cytokine levels at the time of hospitalization were associated with survival after adjustment for clinical comorbidities and CRP, D-dimer, and ferritin concentrations.22 These data confirm the observed relationships between markers of inflammation and adverse clinical outcomes in COVID-19. Ultimately, CRP may be preferred as a biomarker since it is inexpensive and widely available at most medical centres, facilitating rapid implementation of routine biomarker measurement into clinical care of patients with COVID-19.
In contrast to CRP measurement for cardiovascular risk stratification, in which inflammation may contribute to accelerated atherosclerosis and instability of atherosclerotic plaque, CRP concentrations in COVID-19 infection reflect disease severity and the magnitude of the acute inflammatory response. The role of systemic inflammation in the pathogenesis of COVID-19 remains incompletely understood, and causal relationships between inflammation measured by CRP and adverse clinical outcomes are speculative. However, the detrimental inflammatory response observed in some individuals with COVID-19 parallels secondary haemophagocytic lymphohistiocytosis (also known as macrophage activation syndrome), and may independently contribute to multiorgan damage in COVID-19.23 , 24 Earlier studies of patients with ARDS prior to COVID-19 demonstrated that glucocorticoid administration reduces CRP concentrations.25 In a recent randomized trial of dexamethasone immunosuppression in patients with severe COVID-19, steroid therapy reduced the incidence of death among those who required supplemental oxygen and in critically ill patients requiring invasive mechanical ventilation. The clinical benefit of immunosuppression further supports the hypothesis that inflammation in response to viral infection contributes to poor outcomes in COVID-19.26 Thrombo-inflammation has been proposed as a mediator of adverse events in COVID-19, with dysregulation of the normal antithrombotic function of the endothelium in the response to inflammatory stress, leading to leucocyte recruitment, complement and platelet activation, and enhanced coagulation in the microvasculature.27 , 28 This is supported by autopsy studies of decedents with COVID-19, in which platelets with fibrin microthrombi were identified within the pulmonary, renal, hepatic, and cardiac microcirculation.29 Thrombotic events, particularly pulmonary embolism, are common in COVID-19 and are associated with systemic inflammation.13 , 30 In the present analysis, CRP and D-dimer were each independently associated with adverse events. However, patients were at highest risk when they presented with concomitant elevations in CRP and D-dimer concentrations, providing additional support for the synergistic role of inflammation and thrombosis in the pathogenesis of disease associated with SARS-CoV-2 infection. Given the role of micro- and macrothrombosis in disease pathogenesis, an NIH-funded multicentre, adaptive, randomized clinical trial [Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-4] is ongoing to determine optimal antithrombotic dosing for patients hospitalized with COVID-19 (NCT04505774).
Limitations of this study include its retrospective observational study design. The first measured CRP concentration at hospital admission was used for the primary analyses. Patients were hospitalized at a single healthcare system in New York during a 6-week period of rapid viral spread, although a diverse cohort of COVID-19 patients across four inpatient sites is represented. The time period studied preceded the finding that immunosuppression improves clinical outcomes in patients with severe COVID-19,26 and steroids were administered at the discretion of the treating providers. Knowledge of the initial CRP concentration by the admitting physician may have altered subsequent patient care and could confound outcomes. Although data on steroid use were not available, few patients were likely to receive steroids prior to the initial CRP measurement during hospitalization. In some cases, steroids or tocilizumab, an IL-6 inhibitor, were administered to critically ill patients. However, the timing of drug administration, duration of use, and dosing of these agents were not recorded in this dataset. Venous thrombo-embolism was determined according to treating physicians based on available clinical imaging and laboratory data, and were not independently adjudicated. Finally, only in-hospital outcomes were recorded, and associations between CRP and long-term outcomes following discharge could not be determined.
Conclusions
Systemic inflammation, as measured by CRP, is strongly associated with VTE, AKI, critical illness, and in-hospital mortality in patients with COVID-19. Inflammatory biomarker-based approaches to risk stratification and treatment should be evaluated to improve outcomes of patients with SARS-CoV-2 infection.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Funding for this project was supported in part by New York University (NYU) CTSA grant UL1TR001445 from the National Center for Advancing Translational Sciences.
Conflict of interest: J.S.B. is funded in part by the National Heart, Lung, and Blood Institute (grants R01HL139909 and R35HL144993). N.R.S. is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL150315, and reports consulting for Abbott Vascular. The remaining authors have no disclosures to report.
Supplementary Material
Contributor Information
Nathaniel R Smilowitz, Leon H. Charney Division of Cardiology, Department of Medicine, New York University School of Medicine, New York, NY, USA; Department of Medicine, VA New York Harbor Healthcare System, New York, NY, USA.
Dennis Kunichoff, Department of Population Health, New York University Langone Health, New York, NY, USA.
Michael Garshick, Leon H. Charney Division of Cardiology, Department of Medicine, New York University School of Medicine, New York, NY, USA.
Binita Shah, Leon H. Charney Division of Cardiology, Department of Medicine, New York University School of Medicine, New York, NY, USA; Department of Medicine, VA New York Harbor Healthcare System, New York, NY, USA.
Michael Pillinger, Department of Medicine, VA New York Harbor Healthcare System, New York, NY, USA; Division of Rheumatology, Department of Medicine, New York University School of Medicine, New York, NY, USA.
Judith S Hochman, Leon H. Charney Division of Cardiology, Department of Medicine, New York University School of Medicine, New York, NY, USA.
Jeffrey S Berger, Leon H. Charney Division of Cardiology, Department of Medicine, New York University School of Medicine, New York, NY, USA; Department of Surgery, New York University School of Medicine, New York, NY, USA.
References
- 1. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369 : m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of Pneumococcus. J Exp Med 1930;52:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci 1982;389:406–418. [DOI] [PubMed] [Google Scholar]
- 4. Murashima M, Nishimoto M, Kokubu M, Hamano T, Matsui M, Eriguchi M, Samejima KI, Akai Y, Tsuruya K. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci Rep 2019;9:20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Folsom AR, Lutsey PL, Astor BC, Cushman M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost 2009;102:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasileva D, Badawi A. C-reactive protein as a biomarker of severe H1N1 influenza. Inflamm Res 2019;68:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, Ye L, Xiong J, Jiang Z, Liu Y, Zhang B, Yang W. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis 2020;71:2174–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Zhu Y, Liu Y, Wang X, Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 2020;92:791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang W, Liang H, Ou L, Chen B, Chen A, Li C, Li Y, Guan W, Sang L, Lu J, Xu Y, Chen G, Guo H, Guo J, Chen Z, Zhao Y, Li S, Zhang N, Zhong N, He J; China Medical Treatment Expert Group for COVID-19. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 2020;180:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, Li B, Song X, Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY,, Xiang J,, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J,, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020;324:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol 2001;38:189–197. [DOI] [PubMed] [Google Scholar]
- 15. Vanderschueren S, Deeren D, Knockaert DC, Bobbaers H, Bossuyt X, Peetermans W. Extremely elevated C-reactive protein. Eur J Intern Med 2006;17:430–433. [DOI] [PubMed] [Google Scholar]
- 16. Landry A, Docherty P, Ouellette S, Cartier LJ. Causes and outcomes of markedly elevated C-reactive protein levels. Can Fam Physician 2017;63:e316–e323. [PMC free article] [PubMed] [Google Scholar]
- 17. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT,, Rajan M, Reshetnyak E, Hupert N, Horn EM,, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lala A,, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V; Mount Sinai Covid Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 Infection. J Am Coll Cardiol 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020;41:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020;26:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007;131:954–063. [DOI] [PubMed] [Google Scholar]
- 26.Recovery Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med 2020;doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost 2020;18:1559–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019;133:906–918. [DOI] [PubMed] [Google Scholar]
- 29. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet–fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine 2020;24:100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, Benmansour O, Godeau G, Mecheri Y, Lebourdon R, Yvorel C, Massin M, Leblon T, Chabbi C, Cugney E, Benabou L, Aubry M, Chan C, Boufoula I, Barnaud C, Bothorel L, Duceau B, Sutter W, Waldmann V, Bonnet G, Cohen A, Pezel T; Critical Covid-19 France Investigators. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.