Abstract
Background
Troponin elevation is common in hospitalized COVID-19 patients, but underlying aetiologies are ill-defined. We used multi-parametric cardiovascular magnetic resonance (CMR) to assess myocardial injury in recovered COVID-19 patients.
Methods and results
One hundred and forty-eight patients (64 ± 12 years, 70% male) with severe COVID-19 infection [all requiring hospital admission, 48 (32%) requiring ventilatory support] and troponin elevation discharged from six hospitals underwent convalescent CMR (including adenosine stress perfusion if indicated) at median 68 days. Left ventricular (LV) function was normal in 89% (ejection fraction 67% ± 11%). Late gadolinium enhancement and/or ischaemia was found in 54% (80/148). This comprised myocarditis-like scar in 26% (39/148), infarction and/or ischaemia in 22% (32/148) and dual pathology in 6% (9/148). Myocarditis-like injury was limited to three or less myocardial segments in 88% (35/40) of cases with no associated LV dysfunction; of these, 30% had active myocarditis. Myocardial infarction was found in 19% (28/148) and inducible ischaemia in 26% (20/76) of those undergoing stress perfusion (including 7 with both infarction and ischaemia). Of patients with ischaemic injury pattern, 66% (27/41) had no past history of coronary disease. There was no evidence of diffuse fibrosis or oedema in the remote myocardium (T1: COVID-19 patients 1033 ± 41 ms vs. matched controls 1028 ± 35 ms; T2: COVID-19 46 ± 3 ms vs. matched controls 47 ± 3 ms).
Conclusions
During convalescence after severe COVID-19 infection with troponin elevation, myocarditis-like injury can be encountered, with limited extent and minimal functional consequence. In a proportion of patients, there is evidence of possible ongoing localized inflammation. A quarter of patients had ischaemic heart disease, of which two-thirds had no previous history. Whether these observed findings represent pre-existing clinically silent disease or de novo COVID-19-related changes remain undetermined. Diffuse oedema or fibrosis was not detected.
Keywords: COVID-19, SARS-CoV-2, Cardiovascular magnetic resonance, Myocarditis, Myocardial infarction, Myocardial oedema
Graphical Abstract
See page 1879 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab145)
Introduction
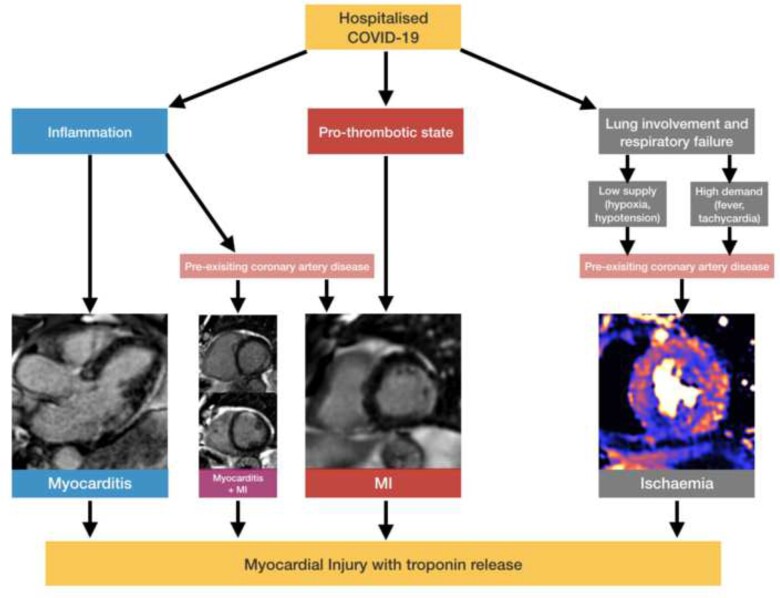
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global pandemic that continues to cause significant mortality and morbidity worldwide.1 Although most cases are mild, a minority of patients sustain severe acute respiratory syndrome, the most frequent cause of death. Involvement of multiple organs including the heart has been reported2 , 3 and concern is growing that survivors may endure long-term sequelae, particularly after intensive care admission.
Acute respiratory infections and sepsis are often associated with elevated serum troponin levels, which are associated with mortality even after recovery.4 , 5 Similarly, elevated troponin is common in hospitalized COVID-19 patients6–11 and is associated with adverse outcomes.8 , 10 , 11 Patients with severe COVID-19 disease frequently have high rates of comorbidity associated with cardiac disease including diabetes, airways disease, and obesity.12 A variety of mechanisms responsible for troponin rise have been proposed including acute coronary syndromes, unmasking occult underlying cardiovascular disease, arrhythmias, myocarditis, or as part of a systemic inflammatory syndrome.13 Furthermore, concern is growing on the long-term sequalae in COVID-19 survivors, which represent an increasing number of patients as the pandemic progresses.14
Cardiovascular magnetic resonance (CMR) is useful to provide a diagnosis in patients with elevated troponin from unclear aetiology14 , 15 and is recommended by position statements.16 Advances in multi-parametric CMR now include quantitative ischaemia assessment, and detailed tissue characterization including scar, diffuse fibrosis, and oedema. Early COVID-19 CMR studies have had necessary limitations given the strain put upon healthcare systems: they have typically considered heterogenous COVID-19 cohorts with a wide range of disease severity and limited troponin data, have been single centre, and have not had a metrology focus (reference ranges, magnetic phantom quality control) for advanced techniques such as T1 and T2 mapping.
The aim of this multicentre study across six acute hospitals scanned at 3 institutions (4 scanners) was to assess the presence, type and extent of myocardial injury using quality-controlled CMR in a well-defined cohort of patients surviving hospital admission with COVID-19 during which they had elevated serum troponin levels.
Methods
Patient population
All patients admitted with a diagnosis of COVID-19 and who were subsequently discharged from hospitals in three NHS trusts [Royal Free London NHS Foundation Trust, Imperial College Healthcare NHS Trust (Hammersmith) and University College London Hospital (UCLH) NHS Foundation Trust] were reviewed. The three NHS trusts comprise six acute hospitals serving a total population of over 3.5 million in North, Central, and West London. Discharges up until 20 June 2020 (first wave) were reviewed (Figure 1). Patients had a diagnosis of COVID-19 made either by (i) a positive combined oro/nasopharyngeal throat swab or tracheal aspirate for SARS-CoV-2 by reverse-transcriptase-polymerase chain reaction or (ii) a negative swab for SARS-CoV-2 but with a triad of symptoms of viral illness (such as one or more of cough, fever, myalgia), typical blood biomarkers (such as one or more of new lymphopenia, high d-dimer, high ferritin, elevated liver transaminases), and reported findings of at least probable likelihood of COVID-19 infection on chest radiograph or computed tomography (CT). A CMR scan appointment was offered to patients discharged following hospital admission with acute COVID-19 symptoms if they had an abnormal high-sensitivity troponin (hsTnT >14 ng/L for Royal Free and UCLH; hsTnI >14 ng/L for females and >34 ng/L for males for Imperial) recorded during admission. Exclusion criteria included patient refusal, severe renal impairment (estimated glomerular filtration rate <30 mL/min/m2, if local hospital policy excluded these patients), pregnancy, medical unsuitability assessed by the referring clinician (including severe comorbid disease and/or frailty in which it was felt that the information acquired would be unlikely to alter clinical management), acute coronary syndrome as the primary reason for hospital admission and standard CMR contraindications. Twenty-nine patients included in the analysis (all from Royal Free) have been reported in a previous study.17 A historical control group (40 patients) was identified from stable outpatients attending for clinical CMR scans at the Royal Free Hospital prior to 1 January 2020. The controls were sequential outpatients matched for age, gender, diabetes, and hypertension but with no clinical suspicion of myocardial injury such as acute myocardial infarction (MI) or myocarditis. The control group was used to assess whether COVID-19 infection is associated with diffuse elevation in native T1 or T2. In addition, 40 healthy volunteer scans performed at the Royal Free Hospital prior to 1 January 2020 were also analysed to derive normal ranges for native T1 and T2. All volunteer participants had no symptoms, no past history of cardiovascular disease, and no history of hypertension. For healthy volunteers and matched controls, only scans performed before January 2020 were considered, to eliminate the risk of previous COVID-19 infection in this group.
Figure 1.
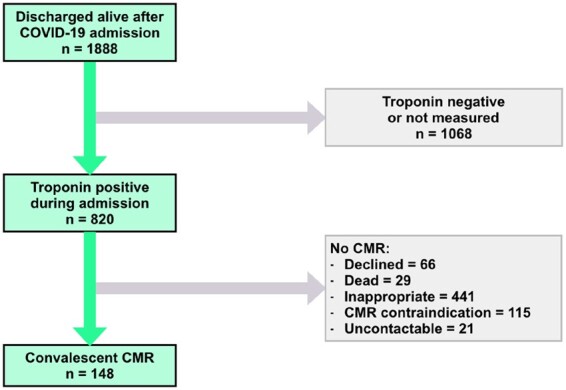
Consort diagram. CMR, cardiovascular magnetic resonance.
All CMR scans on recovered COVID-19 patients were performed for clinical reasons. Ethical approval was obtained from the West Midlands—Edgbaston Research Ethics Committee for the use of patient’s clinical data for research purposes (Royal Free and Imperial sites; REC reference 20/WM/0208) and from the Joint University College London/University College London Hospitals (UCL/UCLH) Research Ethics Committee (UCLH site; REC reference 07/H0715/101). Patients in the control group had provided written informed consent for use of their data for research purposes and ethical approval had previously been obtained from the Joint UCL/UCLH Research Ethics Committee (REC reference 07/H0715/101). All healthy volunteers provided written informed consent and ethical approval was obtained from the South-Central Research Ethics Committee (REC reference 17/SC/0077).
Clinical data
Patient symptoms, medication histories, inpatient blood test results, chest radiographic imaging, electrocardiograms, and (where applicable) coronary angiography data were reviewed.
Cardiovascular magnetic resonance study protocol
Cardiovascular magnetic resonance was performed in accordance with local institutional and international infection control guidelines18 on 1.5T CMR scanners (Magnetom Aera, Siemens Healthcare, Erlangen, Germany). A standard CMR protocol including parametric mapping and post-contrast imaging was used which included:
Standard long- (four-, two-, three-chamber) and short-axis cine images (breath-hold or real-time, as needed).
Native T1 and T2 mapping of three long-axis and at least one mid-ventricular short-axis view. T1 mapping used the modified Look-Locker inversion recovery sequence (MOLLI) after regional shimming with 5s(3s)3s sampling.19 T2 mapping used single-shot T2-prepared images acquired at multiple echo times (TE).20
Following 0.1 mmol/kg gadoterate meglumine (Royal Free and UCLH) or gadobutrol (Imperial), early gadolinium enhancement of a short-axis stack or three long-axis view images was performed to detect intracardiac thrombi. Bright blood and dark blood late gadolinium enhancement (LGE) images were acquired using respiratory motion-corrected sequences with magnitude and phase-sensitive inversion recovery reconstructions.21
Patients with a clinical indication and no contraindications to adenosine also underwent quantitative stress perfusion mapping22 after refraining from caffeine for at least 12 h. Three short-axis views were acquired during vasodilatation (140 µg/kg/min adenosine for 4 min with a further two minutes at 175 µg/kg/min if needed). Acquisition was for 60 heartbeats using 0.05 mmol/kg gadoterate meglumine (Royal Free and UCLH) administered at 4 mL/s followed by a 20-mL 0.9% saline flush.
Cardiovascular magnetic resonance post-processing
Cardiovascular magnetic resonance studies were analysed offline using Osirix MD 9.0.1 (Pixmeo Sarl, Bernex, Switzerland) and CVI42 5.12.1 (Circle Cardiovascular Imaging, Calgary, Canada). All cines, maps, first-pass perfusion images/maps and early gadolinium enhancement/LGE images were analysed by experienced observers (D.S.K., T.K., G.C., T.A.T., M.F. and J.M.). When calculating ventricular volumes and mass, trabeculations and papillary muscles were included in the myocardial mass. Right ventricular insertion point LGE was not included as an abnormal LGE finding. The CMR diagnosis of myocarditis-like injury was made in accordance with published expert recommendations based on the presence of non-ischaemic myocardial injury in a typical distribution (patchy sub-epicardial or mid-wall LGE, which tends to favour the basal to mid inferolateral wall) and myocardial oedema (by T2 mapping).23 Active myocarditis was defined as the presence of non-ischaemic myocarditis-pattern LGE with associated elevation in T1 and T2, or T2 alone, in the same distribution as LGE (i.e. T1 and T2 were measured within areas of LGE). Healed myocarditis was defined as the presence of non-ischaemic myocarditis-pattern LGE with or without elevation in native T1 and with normal T2.24 Native T1 and T2 relaxation times were measured within the myocardial septum in the four-chamber long-axis motion-corrected quantitative maps and away from any areas of LGE (remote myocardium) and also averaged within any area of myocarditis. Controls and healthy volunteers were scanned at the centre contributing the most patients (Royal Free), and a quality assurance standardization process across all centres was used to demonstrate consistency in T1 and T2 mapping values across all participating centres and thus validating the threshold between normal and abnormal.25
Multicentre phantom testing
A quality assurance standardization T1 and T2 phantom assessment was undertaken to detect whether (i) measured T1 and T2 results from the three sites (four magnetic resonance imaging scanners: Royal Free, Hammersmith 1, Hammersmith 2, and UCLH) were comparable (i.e. consistent measurements across sites) and (ii) that pre-pandemic controls could be used to construct a reference range (i.e. no significant magnet system drift or hardware shift events in the time between control and patient scanning). This used the T1 Mapping and ECV Standardization (T1MES) phantom and analysis pipeline.25 , 26 At the time of COVID patient scanning (September to October 2020), the same phantom was transported between the four scanners in this study (all Siemens Aera 1.5T). After a minimum 8 h equilibrium in the magnet room at each site, the phantom was scanned according to the protocol specified in the T1MES user manual.26
Statistical analysis
Analyses were performed using SPSS Statistics, version 26 (IBM, Armonk, NY, USA). Data were examined for normality using the Shapiro–Wilk test. Normally distributed variables were expressed as mean ± standard deviation; non-normal as median (interquartile range). Proportions were expressed as percentages. Student’s t-tests (two-tailed) and the Mann–Whitney U test were used to compare normal and non-normally distributed data between two groups. One-way analysis of variance with Bonferroni correction was used to compare normally distributed data across groups and Kruskal–Wallis test to compare non-normally distributed variables across groups. The χ2 test was used to compare proportions across groups. Binary logistic regression determined relationships between troponin and LGE, a diagnosis of myocarditis or any ischaemic or non-ischaemic diagnosis. Spearman’s correlation was used to compare time to CMR with native T1 and T2. P < 0.05 was considered statistically significant.
Results
Clinical characteristics
In total, 148 recovered COVID-19 patients underwent CMR scans (75 at Royal Free, 40 at Imperial, and 33 at UCLH). One-hundred and thirty-three patients underwent a full tissue characterization protocol (LGE, T1, and T2 mapping) including 76 with additional adenosine stress perfusion. Eleven patients underwent CMR without T1 and T2 mapping and four underwent a non-contrast CMR with T1 and T2 mapping. Patient characteristics are presented in Table 1 and Supplementary material online, Table S1. Mean age was 64 ± 12 years and patients were predominantly male (104/148, 70%). One-hundred and twenty-eight (86%) were polymerase chain reaction positive with the remainder diagnosed clinically based on symptoms, blood biomarkers, and radiological features. Presenting symptoms were typical of a COVID-19 prodrome (one or more of fever, cough, dyspnoea, and myalgia) in 138/148 (93%) patients. Seven patients presented with gastrointestinal symptoms, one with a fall and two were asymptomatic. Median length of inpatient hospital stay was 9 days [interquartile range (IQR) 6–18 days]. Forty-eight patients (32%) required intensive care unit (ICU) for ventilatory support. Sixty-three patients underwent computed tomography pulmonary angiography (CTPA) during admission of which 22/63 (35%) showed pulmonary embolism. The interval between discharge or confirmed diagnosis (defined as a positive COVID-19 swab result or, in the case of swab-negative patients, diagnostic chest radiographic, or CT imaging) and CMR study was 56 days (IQR 30–88 days) and 68 days (IQR 39–103 days), respectively.
Table 1.
Clinical characteristics
| Recovered COVID-19 (n = 148) | Controls (n = 40) | P-value (controls vs. COVID-19) | Healthy Volunteers (n = 40) | P-value (volunteers vs. COVID-19) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 64 ± 12 | 64 ± 9 | 0.86 | 49 ± 6 | <0.001 |
| Female (%) | 44 (30) | 12 (30) | 0.97 | 17 (43) | 0.13 |
| Height (m) | 1.70 ± 0.10 | 1.71 ± 0.10 | 0.84 | 1.73 ± 0.10 | 0.15 |
| Weight (kg) | 82.5 ± 17.9 | 83.3 ± 19.0 | 0.60 | 75.5 ± 14.4 | 0.02 |
| Body mass index (kg/m2) | 28.5 ± 5.6 | 28.8 ± 5.7 | 0.71 | 25.2 ± 3.7 | 0.001 |
| Body surface area (m2) | 1.95 ± 0.24 | 1.99 ± 0.27 | 0.37 | 1.89 ± 0.21 | 0.19 |
| Ethnicity | 0.001 | 0.02 | |||
| Caucasian | 74 (50) | 34 (85) | 25 (63) | ||
| Afro-Caribbean | 26 (18) | 3 (8) | 4 (10) | ||
| Asian | 22 (15) | 2 (5) | 11 (28) | ||
| Other or unspecified | 26 (18) | 1 (3) | 0 (0) | ||
| Past medical history | |||||
| Previous MI (%) | 11 (7) | 7 (18) | 0.08 | 0 | <0.001 |
| Previous PCI or CABG (%) | 17 (12) | 11 (28) | 0.01 | 0 | <0.001 |
| Hypertension (%) | 85 (57) | 25 (63) | 0.56 | 0 | <0.001 |
| Diabetes mellitus (%) | 50 (34) | 11 (28) | 0.45 | 0 | <0.001 |
| Hypercholesterolaemia (%) | 68 (46) | 21 (53) | 0.46 | 0 | <0.001 |
| Smoking history | 35 (24) | 12 (30) | 0.41 | 2 (5) | 0.008 |
BSA, body surface area; CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention. Bold denotes statistically significant values (P < 0.05).
Blood biomarkers
Admission and peak (in cases where more than one measurement was taken) high-sensitivity troponin T levels were 20 ng/L (15–29) and 26 ng/L (19–70) respectively (Royal Free and UCLH), and high-sensitivity troponin I levels were 39 ng/L (21–82) and 43 ng/L (24–125) (Imperial). N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were available in 92 patients and were elevated overall (231 ng/L, 72–878). Median lowest lymphocyte count was 0.70 × 109/L (0.41–0.98). Lymphopenia (<1.0 × 109/L) was present in 115/148 (78%) patients. Median peak C-reactive protein was 186 mg/L (123–309) with C-reactive protein being elevated (>5 mg/L) in all patients on at least one measurement during admission. Blood biomarkers are summarized in Table 2.
Table 2.
Laboratory biomarkers
| Laboratory findings | |
|---|---|
| Peak white-cell count (×109/L) | 10.6 (8.4–15.7) |
| Peak neutrophil count (×109/L) | 8.5 (6.4–13.5) |
| Lowest lymphocyte count (×109/L) | 0.70 (0.41–0.98) |
| Peak lactate dehydrogenase (U/L) | 469 (363–593) |
| Creatinine at discharge (µmol/L) | 77 (66–97) |
| Creatinine at peak troponin (µmol/L) | 92 (72–116) |
| Admission high-sensitivity troponin T (ng/L)a | 20 (15–29) |
| Peak high-sensitivity troponin T (ng/L)a | 26 (19–70) |
| Admission high-sensitivity troponin I (ng/L)b | 39 (21–82) |
| Peak high-sensitivity troponin I (ng/L)b | 43 (24–125) |
| Peak creatine kinase (U/L) | 206 (87–813) |
| Peak fibrinogen (g/L) | 6.7 (6.3–7.8) |
| Peak D-dimer (ng/mL) | 2417 (1172–7548) |
| Peak C-reactive protein (mg/L) | 186 (123–309) |
Royal Free and UCLH patients.
Imperial patients.
Cardiovascular magnetic resonance
Extracardiac findings
Pleural effusions were present in 14/148 (9%) patients and pericardial effusion in 8/148 (5%).
Cardiac function
Cardiac structure, function, and tissue characterization are detailed in Table 3 and Supplementary material online, Table S2. Average left ventricular (LV) systolic function of the COVID-19 recovered group was normal [left ventricular ejection fraction (LVEF) 67% ± 11%] and was no different to matched controls (LVEF 67% ± 9%, P = 0.99) or healthy volunteers (66% ± 5%, P = 0.55). Left ventricular dysfunction was present in 17/148 (11%) patients (13 with LVEF 35–55% and 4 with LVEF <35%), with 9 cases due to myocardial infarction (of which 6 had a known past history of ischaemic heart disease), one case both infarct-pattern and non-ischaemic myocarditis-pattern LGE, one case with non-ischaemic pattern mid-wall LGE and 6 with no LGE. The average right ventricular ejection fraction (RVEF) was 61% ± 9% and was no different to healthy volunteers (RVEF 61% ± 5%, P = 0.85) but was lower than matched controls (RVEF 64% ± 7%, P = 0.032).
Table 3.
Cardiovascular magnetic resonance findings
| Recovered COVID-19 (n = 148) | Controls (n = 40) | P-value (controls vs. COVID-19) | Healthy Volunteers (n = 40) | P-value (volunteers vs. COVID-19) | |
|---|---|---|---|---|---|
| Functional metrics | |||||
| LVEDV indexed (mL/m2) | 67 ± 15 | 60 ± 13 | 0.44 | 78 ± 17 | <0.001 |
| LVESV indexed (mL/m2) | 23 ± 14 | 23 ± 9 | 0.97 | 28 ± 7 | 0.08 |
| LVEF (%) | 67 ± 11 | 67 ± 9 | 0.99 | 66 ± 5 | 0.55 |
| LV mass indexed (g/m2) | 69 ± 18 | 74 ± 27 | 0.19 | 58 ± 11 | <0.001 |
| RVEDV indexed (mL/m2) | 70 ± 12 | 65 ± 13 | 0.025 | 87 ± 21 | <0.001 |
| RVESV indexed (mL/m2) | 28 ± 9 | 23 ± 6 | 0.002 | 37 ± 11 | <0.001 |
| RVEF (%) | 61 ± 9 | 64 ± 7 | 0.032 | 61 ± 5 | 0.85 |
| Multi-parametric myocardial mapping | |||||
| Number of patients with abnormal septal T1 (>1076 ms by MOLLI) | 19 (13%) | 5 (13%) | 0.95 | ||
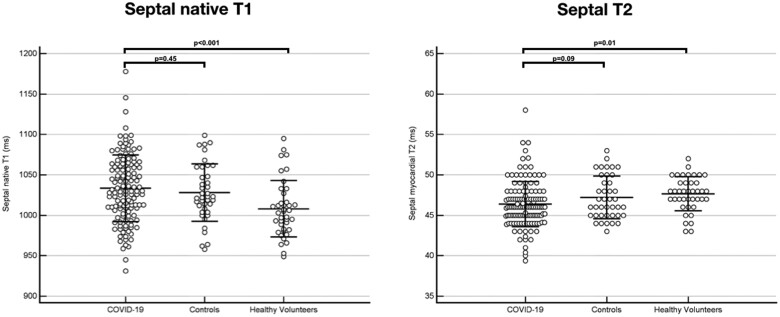
| Remote myocardium native T1 (ms) | 1033 ± 41 | 1028 ± 35 | 0.45 | 1008 ± 35 | <0.001 |
| Number of patients with abnormal septal T2 (>52 ms) | 4 (3%) | 1 (3%) | 0.93 | ||
| Remote myocardium T2 (ms) | 46 ± 3 | 47 ± 3 | 0.09 | 48 ± 2 | 0.01 |
| Late gadolinium enhancement | |||||
| Any LGE | 70 (49%) | 18 (45%) | 0.80 | 0 | |
| Subendocardial or transmural | 28 (16%) | 10 (15%) | 0.40 | 0 | |
| Mid-myocardial | 16 (11%) | 6 (15%) | 0.46 | 0 | |
| Subepicardial | 31 (22%) | 2 (5%) | 0.018 | 0 | |
CMR, cardiovascular magnetic resonance; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic function; LGE, late gadolinium enhancement; LV, left ventricular; MOLLI, modified Look-Locker inversion recovery; RV, right ventricular.
Bold denotes statistically significant values (P < 0.05).
Pattern of myocardial injury
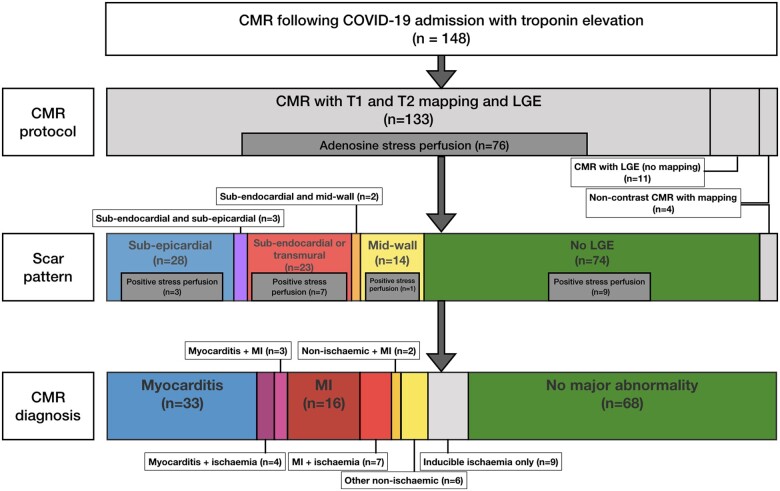
In total, LGE was present in 70/144 patients (49%). There was subendocardial or transmural LGE only in 23/144 (16%), subepicardial LGE only in 28/144 (19%), and mid-wall LGE only in 14/144 (10%). Five of 144 (3%) patients had LGE in more than one distribution (three with sub-endocardial and sub-epicardial; two with sub-endocardial and mid-wall) (Figure 2). Four patients did not receive gadolinium contrast. Nine patients had inducible ischaemia without LGE and one patient undergoing non-contrast CMR had regional elevation in myocardial T2.
Figure 2.
Patterns of myocardial scar and cardiovascular magnetic resonance diagnoses. CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; MI, myocardial infarction.
Cardiovascular magnetic resonance identified a cardiac abnormality in 80/148 (54%) patients, classified as non-ischaemic (including myocarditis-like LGE) in 39/148 (26%), ischaemic heart disease-related (infarction and/or inducible ischaemia) in 32/148 (22%) or dual pathology in 9/148 (6%). Sixty-eight (46%) were classified as normal. Of these normal CMR scans, 35/68 (51%) had a CTPA during their acute admission with 10/35 (29%) demonstrating pulmonary embolism. This compares to 12/28 (43%) with cardiac abnormality on CMR who also had co-existing pulmonary embolism (P = 0.24 for normal CMR vs. abnormal CMR).
Non-ischaemic, myocarditis-like injury pattern
Forty-seven of 148 (32%) patients had a non-ischaemic pattern of myocardial injury (including 9 with dual pathology). Overall, 40/148 (27%) patients had myocarditis-pattern injury including four with co-existing inducible ischaemia on adenosine stress perfusion and three with co-existing myocardial infarction. One patient undergoing non-contrast CMR had regional elevation in native T2 in the absence of regional wall motion abnormality and was classified as myocarditis-pattern injury. Median time from discharge to CMR and COVID-19 diagnosis to CMR in those with myocarditis-pattern injury were 34 days (IQR 19–65) and 45 days (IQR 30–76 days), respectively. Of the patients with myocarditis-pattern LGE, 20 (50%) had involvement of one myocardial segment, 8 (20%) with two myocardial segments, 7 (18%) with three myocardial segments, and 5 (13%) with four or more segments involved. Biventricular function was preserved in patients with myocarditis and no different to those without myocarditis (LVEF 70% ± 6% vs. 66% ± 12%, P = 0.05; RVEF 62% ± 8% vs. 61% ± 9%, P = 0.27). No patients with myocarditis-pattern LGE had regional wall motion abnormalities.
Neither admission nor peak troponin levels were predictive of the diagnosis of myocarditis. Thirteen of the 48 patients (27%) requiring ICU admission had evidence of myocarditis compared with 27 of the 100 patients (28%) not requiring ICU admission (P = 0.99).
Of patients with myocarditis-pattern injury, 12 (30%) had findings consistent with active myocarditis [8 (20%) with regional elevation of both native T1 and T2, 4 (10%) with regional elevation of T2 only, Figure 3]. Twenty-seven patients (68%) had findings consistent with healed myocarditis [11 (28%) with elevated native T1 only and 16 (49%) with normal native T1 and T2]. One patient had myocarditis-pattern LGE but no T1 and T2 mapping was performed.
Figure 3.
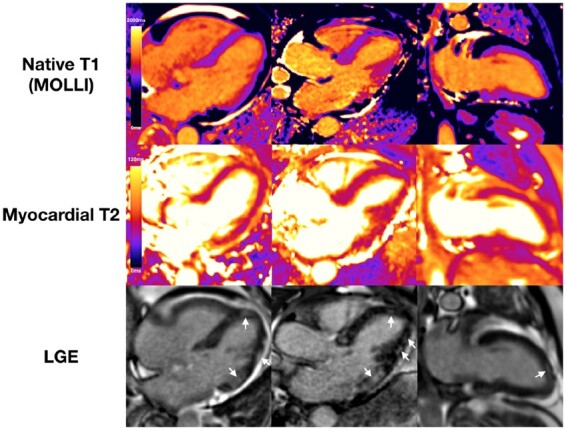
Example of patient with a myocarditis-pattern late gadolinium enhancement and evidence of active inflammation. Native T1 and myocardial T2 were elevated in the inferolateral wall (T1 1261 ms, T2 56 ms) and normal in the basal inferoseptum (T1 983 ms, T2 50 ms). Late gadolinium enhancement imaging shows patchy areas of subepicardial enhancement in the lateral wall and basal inferior wall, and mid-wall enhancement in the distal septum and distal anterior wall (white arrows).
Ischaemic injury pattern
Forty-one of 148 (28%) patients had evidence of ischaemic heart disease-related abnormality on CMR, including: 28/148 (19%) with sub-endocardial and/or transmural LGE consistent with myocardial infarction (Figure 4). Seven patients with evidence of MI also had additional inducible ischaemia on adenosine stress and 13 patients had inducible ischaemia without evidence of MI (Figure 5). Nine patients had a known past history of myocardial infarction, 5 had undergone previous PCI or CABG without previous MI and 27 (66%) had no known history of ischaemic heart disease and were, therefore, first presentations of coronary artery disease diagnosed with CMR. Of patients with ischaemic heart disease-related abnormality, 39/41 (95%) had at least one cardiovascular risk factor.
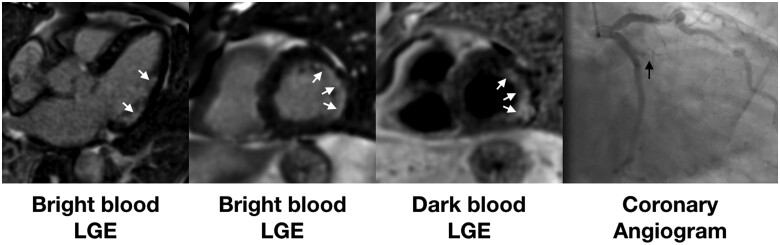
Figure 4.
Example of patient admitted with COVID-19 infection and associated troponin rise. Late gadolinium enhancement (bright blood left two panels and dark blood third panel) shows a lateral infarct (white arrows). Coronary angiography (right panel) showed an occluded obtuse marginal branch (black arrow).
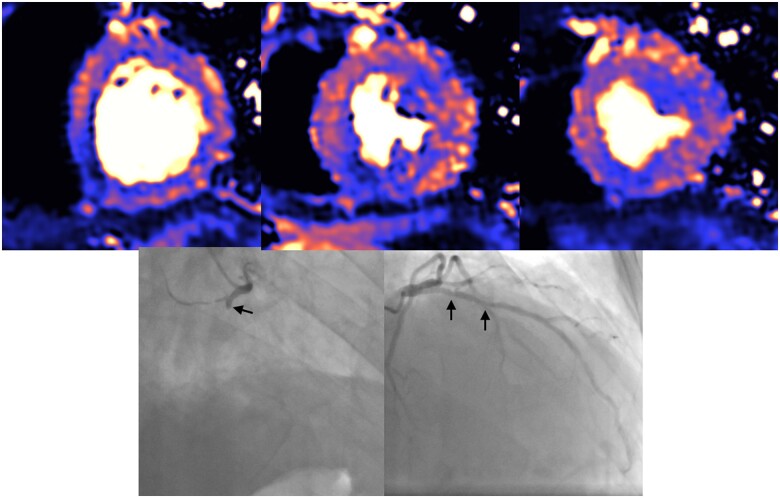
Figure 5.
Example of patient admitted with COVID-19 infection and associated troponin rise. Cardiovascular magnetic resonance with adenosine stress perfusion mapping showed inducible ischaemia in the inferior wall, basal inferoseptum, anterior, and anterolateral walls. Coronary angiography showed occluded right coronary artery and severe disease in proximal-mid LAD (black arrows).
Dual pathology
Dual cardiac pathology was noted in 9/148 (6%) patients, with both ischaemic (5 MI, 4 inducible ischaemia) and non-ischaemic findings (7 myocarditis-pattern LGE and 2 other non-ischaemic LGE) (Figure 6).
Figure 6.
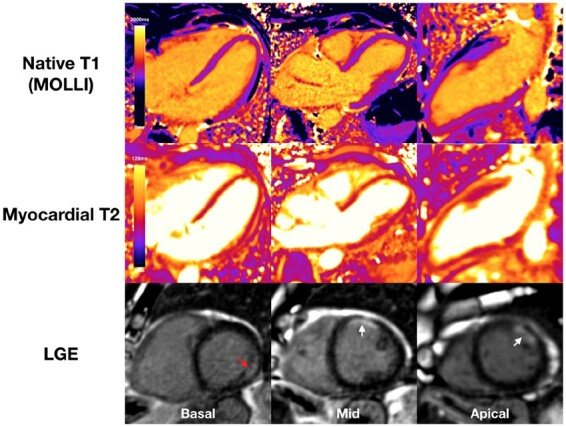
Example of patient with dual pathology. Late gadolinium enhancement showed mid-wall late gadolinium enhancement in the basal inferolateral wall (myocarditis-pattern, red arrow) and sub-endocardial late gadolinium enhancement in the mid-anterior and part of the distal lateral wall (myocardial infarction pattern, white arrows). Native T1 and T2 were normal within the area of myocarditis-pattern late gadolinium enhancement (T1 1061 ms, T2 52 ms).
Tissue characterization of the remote myocardium
Abnormal native T1 and T2 were defined as 1.96 standard deviations above the mean values for the healthy volunteer group (upper limit of native T1 1076 ms, upper limit of myocardial T2 52 ms). Nineteen of 137 (14%) patients had elevated native T1 in the remote myocardium compared with 5/40 (13%) patients in the control group (P = 0.95). Four of 137 (3%) patients had elevated T2 in the remote myocardium compared with 1/40 (3%) in the control group (P = 0.93). There was no difference in mean native T1 in the remote myocardium between controls and COVID-19 patients (COVID-19 patients 1033 ± 41 ms vs. controls 1028 ± 35 ms, P = 0.45) but both groups had higher T1 than healthy volunteers (1008 ± 35 ms, P < 0.001 COVID-19 vs. healthy volunteers and P = 0.013 controls vs. healthy volunteers) (Figure 7). There was no difference in mean myocardial T2 in the remote myocardium between COVID-19 patients and controls although COVID-19 patients had lower T2 compared with healthy volunteers (COVID-19 46 ± 3 ms vs. controls 47 ± 3 ms vs. healthy volunteers 48 ± 3 ms; P = 0.09 for COVID-19 vs. controls and P = 0.01 for COVID-19 vs. healthy volunteers) (Figure 7). There was no difference in native T1 or T2 between patients scanned at each site (T1: Royal Free 1035 ± 37 ms vs. Hammersmith 1030 ± 42 ms vs. UCLH 1032 ± 50 ms, P = 0.80; T2: Royal Free 46 ± 3 ms vs. Hammersmith 47 ± 4 ms vs. UCLH 45 ± 2 ms, P = 0.06). There was no correlation between time from COVID-19 diagnosis to CMR and native T1 (rho −0.135, P = 0.12) or T2 (rho 0.268, P = 0.10).
Figure 7.
Native T1 (left panel) and myocardial T2 (right panel) measured in the remote myocardium. There was no significant difference in native T1 or T2 in the remote myocardium between COVID-19 patients and controls.
Multicentre phantom testing
The phantom temperature, read from the liquid crystal display thermometer fixed to the front of the phantom body, was 21°C at UCLH, 22°C at Royal Free and Hammersmith 1, and 23°C in Hammersmith 2. Phantom T1 and T2 times of tube 4 (representing native myocardium) for UCLH, Hammersmith 1, Hammersmith 2, Royal Free were: T1 = 1052 ms (corrected to 1047 ms), 1047 ms, 1040 ms (corrected to 1045 ms), and 1040 ms; T2 = 48 ms (corrected to 46 ms), 50 ms, 46 ms (corrected to 48 ms), and 49 ms. Therefore, for phantom tube 4 across the four sites, the average (SD, %) of temperature corrected T1 and T2 times (at 22°C), were 1045 ms (SD 3.3 ms, 0.3%) and 48 ms (SD 1.7 ms, 3.6%), respectively. The Royal Free site where healthy volunteers and matched controls were scanned, had phantom T1 and T2 times that deviated from these mean values by 0.5% and 1.5%, respectively. At the Royal Free site where the historical matched control and healthy volunteer cohorts had been scanned, serial data using this same phantom from early 2019 confirmed good longitudinal stability of T1 and T2 reads over 20 months, with mean coefficients of variation of 0.77% and 1.97%, respectively. Phantom results thus supported the cross-site pooling of T1 and T2 patient data, and the use of historical control and healthy volunteer data for reference range construction.
Discussion
Multi-organ involvement in COVID-19 is recognized, with many patients having troponin release indicative of acute myocardial injury.6–11 Here, in a multicentre study across six acute hospitals, we show that myocardial injury during acute COVID-19 infection requiring acute hospital admission is associated with a CMR abnormality in approximately half of patients, with three patterns of injury being observed: non-infarct, myocarditis-pattern injury (27%), ischaemic pathology (22%), and non-ischaemic non-specific scar (5%). Dual pathology with ischaemic and non-ischaemic features was observed in 6%. The different patterns of abnormalities found suggest multiple possible underlying mechanisms including myocarditis (with limited extent and no functional consequence), MI (type 1 or type 2), and inducible myocardial ischaemia.
Cardiovascular magnetic resonance is the most suited non-invasive modality for the assessment of unexplained troponin rise in this context, as it can confirm the presence, type, and extent of myocardial injury, and is able to identify pathology occurring before regional wall motion abnormalities are apparent. Myocarditis has been associated with a range of acute viral illnesses although the true incidence of acute myocarditis in these cohorts is unknown.27 Where viral myocarditis occurs, only a small proportion of patients suffer long-term consequences. Inflammatory myocardial infiltrates have been found at autopsy of patients with COVID-1928 , 29 but the true prevalence of myocarditis in COVID-19 is unknown. A recent preliminary study suggests myocarditis in 15% of competitive athletes with COVID-19, but larger studies are needed to understand the true prevalence.30 Here we show that in patients with COVID-19 hospitalized for severe acute respiratory syndrome and troponin elevation, 27% of patients had myocarditis-pattern LGE and a third of these showed evidence of ongoing active myocardial inflammation at this early stage post-infection. The extent of myocarditis was limited to three or less segments in the majority of cases (88%), there were no associated regional wall motion abnormalities and biventricular function was preserved, an imaging phenotype that would be expected to have a good overall prognosis with a non-COVID myocarditis aetiology. These data suggest that, even in a group who were comparatively ill (all requiring hospital admission, all with positive troponin, one in three intubated and ventilated), inflammation (with changes such as myocardial oedema, inflammatory infiltrates, hyperaemia)31 settle within weeks of acute infection in the majority of cases. Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance. The scale of the COVID-19 pandemic means that following up all patients in the long term with CMR may not be practical. However, those who were very ill (admitted to hospital and having a positive troponin) with evidence of residual inflammation on early convalescent CMR may be an important group to target, especially as it has been shown that inflammation and LGE may play a role in the pathophysiology of dilated cardiomyopathy.32–34 Future follow-up studies would be useful to assess for long-term presence of scar, which has been shown to be associated with adverse cardiac events following myocarditis.31
Of the studied cohort, 26% had CMR findings consistent with ischaemic aetiology either alone or in combination with non-ischaemic pathology. A quarter of patients undergoing adenosine stress perfusion demonstrated inducible ischaemia. Almost all patients with ischaemic-related pathology on CMR (95%) had at least one cardiovascular risk factor. However, 66% of patients with MI or inducible ischaemia had no known past history of ischaemic heart disease. It has previously been shown that patients with COVID-19 requiring hospitalization have high prevalence of comorbidity including hypertension, diabetes, and obesity.35 , 36 It is likely that at least a proportion of the elevated troponins in hospitalized COVID-19 patients could be the result of pre-existing coronary artery disease being unmasked by systemic illness (type 2 MI), where increased myocardial demand due to fever and tachycardia, and reduced supply due to hypoxaemia and hypotension, can result in myocardial ischaemia in vulnerable patients. Even in an asymptomatic population apparently free from cardiovascular disease the prevalence of myocardial scar is 7.9%,37 so it is likely that some of the abnormalities found here represent pre-existing disease. Considering the high proportion of previously undiagnosed MIs, an alternative hypothesis is that a proportion of MIs in these patients could be the result of a pro-thrombotic state in patients with underlying vulnerability due to cardiovascular risk factors resulting in type 1 MI occurring during the acute infection. Regardless of the mechanism underlying ischaemic myocardial injury, these patients are important to identify as they could benefit from prognostic medical therapy and be considered for coronary intervention in the presence of significant obstructive disease.
Pulmonary embolism was detected in 29% of patients with no evidence of myocardial scar or ischaemia who also underwent CTPA scanning, suggesting another possible cause for troponin elevation. However, 43% of patients with cardiac abnormality also had co-existing pulmonary embolism suggesting that the diagnosis of pulmonary embolism during hospital admission should not be assumed to be the only cause for troponin elevation.
It has recently been reported that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in the myocardial septum, in keeping with diffuse fibrosis and oedema.38 These findings were from a cohort with the majority having mild illness (two-thirds recovered at home, <20% requiring invasive or non-invasive ventilation, up to 15% with troponin elevation). Our findings, in a multi-centre cohort of patients requiring hospitalization for respiratory failure and all with troponin elevation, show that both native T1, which may be elevated in diffuse fibrosis or oedema, and T2, which is more specific for myocardial oedema, measured remote to any areas of LGE were within normal limits in the vast majority of patients (87% for T1 and 97% for T2). Importantly, both native T1 and T2 were not elevated when compared with a control group matched for age, gender, and cardiovascular comorbidities either in terms of mean values or proportion of patients with elevated values. Both COVID-19 patients and matched controls had elevated native T1 compared with healthy volunteers suggesting the difference observed in previous literature is likely confounded by chronic disease and comorbidity rather than a direct effect of COVID-19 infection.38 We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis or long-term LV dysfunction is a dominant feature in those surviving COVID-19.
The use of the T1MES phantom across all scanners provided robust quality assurance and confirmed reproducibility on a three-centre scale, fulfilling a key requirement for the use of T1 and T2 mapping in clinical studies involving more than one centre. The results showed that T1 and T2 were remarkably repeatable in the three centres, all of which used the same setup (manufacturer, field strength, magnet, sequence), with very low coefficients of variation.
Limitations
This study reports findings in a group of patients surviving severe COVID-19 infection and with troponin elevation. The study excluded those with contraindications to CMR scanning, those who died in hospital, and those who had no troponin elevation (or where troponin level was not measured). We also excluded those who were assessed by the referring clinician to be sufficiently frail that the findings would be unlikely to inform management. Whilst this introduces some subjectivity, we believe our cohort reflects a group in which clinicians might reasonably request a clinical CMR. The high inpatient mortality of COVID-19 with troponin elevation means conclusions from this study will be prone to survivorship bias; we have no way of knowing, for comparison, what injury pattern occurred in those who died (though recent autopsy data suggest that myocarditis is a relatively uncommon finding at autopsy, being found in <10% of post-mortem hearts29). Ultimately, we cannot definitely establish a link between the abnormalities detected on these CMR scans and the acute COVID-19 infection. However, the high prevalence of myocarditis, high prevalence of ischaemia (likely leading to type 2 MI), and the high proportion of previously unknown MIs (possibly some representing type 1 MIs during acute COVID-19 infection) suggest a likely link with COVID-19 infection. The mechanisms of COVID-19 related injury are still under investigation and the CMR features observed here may reflect injury through pathways other that the classic enteroviral and anti-cardiac myosin autoimmune models.39 Histological confirmation from cardiac biopsies with paired CMR would provide incremental information but was not performed for any of the patients within our cohort and is unlikely to be performed in future studies due to lack of clinical indications.
Troponin-negative patients (and those where no troponin level was measured) with COVID-19 were not included in this study as a comparator group and therefore the incidence of CMR abnormalities in these patients is unknown. However, unless COVID-19 challenges the paradigm that significant acute myocardial injury occurs without troponin release, we believe it is unlikely that more abnormalities would have been seen in a troponin-negative group than we observed in their troponin-positive counterparts in this study.
Our measurement methodology for assessment of diffuse myocardial abnormalities reflected the current guidelines with a septal region of interest measurement of T1 and T2 chosen in order to minimise artefact.40 However, it is possible that we may not have detected subtle abnormalities in non-septal segments. Furthermore, expert criteria for the diagnosis of acute myocarditis have typically focused on short illnesses (<4 weeks),23 whereas our convalescent study group underwent CMR at a later timepoint and this could have led to some degree of underestimation of acute oedema. Additionally, whilst the diagnosis of myocarditis-like injury was made in accordance with published expert recommendations, based on the presence of non-ischaemic myocardial injury in a typical distribution, these criteria are not 100% specific, as these patterns can also be found in patients with other non-ischaemic pathology. However, CMR remains the most sensitive and specific imaging modality to non-invasively confirm a diagnosis of active or healed myocarditis.
High-sensitivity troponin was systematically collected on admission in all patients included in the study, but the quantification of acute myocardial injury using serial troponin measurements was not routinely performed. However, our cohort represents a ‘real-world’ group of patients in whom attending clinicians requested serial troponins if clinically indicated. Cardiovascular magnetic resonance scanning in this cohort was performed relatively early following discharge and further follow-up scanning was not performed within this study to assess the long-term prevalence of scar. This dataset represents the severe end of the spectrum of COVID-19 infection (with one in three patients requiring ventilation) so is likely to be an overestimation of the overall cardiac effects of COVID-19 infection. A further study of asymptomatic and/or mildly symptomatic individuals will be required to understand the possible, if any, cardiac consequences in the wider population. Additionally, the cardiac effects of other severe viral infections are not fully known. It is possible therefore that the effects of COVID-19 infection on the myocardium may not be different from other severe viral illnesses. Finally, the relationship between convalescent CMR phenotype and subsequent arrhythmic and mortality outcomes is not yet known and will require further study.
In summary, myocardial injury during COVID-19 infection severe enough to require acute hospital admission is associated with a CMR abnormality in approximately half of patients. The patterns of abnormalities found suggest multiple underlying mechanisms including myocarditis, MI (type 1 or type 2) and inducible myocardial ischaemia. Whether these represent pre-existing disease or COVID-19-related changes remain undetermined. In those with myocarditis-like scar, the extent is low with no functional consequence. Although 30% of these cases with myocarditis-like scar showed localised oedema, we did not find evidence of diffuse fibrosis. The trajectory and outcome of those with evidence of ongoing localized inflammation remains unknown but would support ongoing research.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The study dataset will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request to the corresponding author, subject to institutional and ethical committee approvals.
Funding
Drs D.S.K. and R.B. are supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre. Prof. J.C.M. is directly supported by the (UCLH) and Barts NIHR Biomedical Research Centres and through a BHF Accelerator Award (Grant number AA/18/6/34223). Drs M.F. and T.A.T. are funded by British Heart Foundation (BHF) Intermediate Fellowships (Grant numbers FS/18/21/33447 and FS/19/35/34374). Dr G.D.C. is supported by the NIHR Imperial Biomedical Research Centre (BRC).
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Tushar Kotecha, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, UK.
Daniel S Knight, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, UK.
Yousuf Razvi, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK.
Kartik Kumar, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK.
Kavitha Vimalesvaran, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK.
George Thornton, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
Rishi Patel, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
Liza Chacko, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
James T Brown, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, UK.
Clare Coyle, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK; National Heart and Lung Institute, Imperial College London, UK.
Donald Leith, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
Abhishek Shetye, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK; University College London Hospitals NHS Trust, London, UK.
Ben Ariff, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK.
Robert Bell, Institute of Cardiovascular Science, University College London, UK; University College London Hospitals NHS Trust, London, UK.
Gabriella Captur, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, UK.
Meg Coleman, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK.
James Goldring, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK.
Deepa Gopalan, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK.
Melissa Heightman, University College London Hospitals NHS Trust, London, UK.
Toby Hillman, University College London Hospitals NHS Trust, London, UK.
Luke Howard, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK; National Heart and Lung Institute, Imperial College London, UK.
Michael Jacobs, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK.
Paramjit S Jeetley, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK.
Prapa Kanagaratnam, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK; National Heart and Lung Institute, Imperial College London, UK.
Onn Min Kon, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK; National Heart and Lung Institute, Imperial College London, UK.
Lucy E Lamb, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Academic Department of Defence Medicine, Royal Centre for Defence Medicine, Edgbaston, Birmingham, UK.
Charlotte H Manisty, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
Palmira Mathurdas, University College London Hospitals NHS Trust, London, UK.
Jamil Mayet, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK; National Heart and Lung Institute, Imperial College London, UK.
Rupert Negus, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK.
Niket Patel, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, UK.
Iain Pierce, Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
Georgina Russell, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK; National Heart and Lung Institute, Imperial College London, UK.
Anthony Wolff, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK.
Hui Xue, National Heart, Lung, and Blood Institute, National Institute of Health, Bethesda, MD, USA.
Peter Kellman, National Heart, Lung, and Blood Institute, National Institute of Health, Bethesda, MD, USA.
James C Moon, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
Thomas A Treibel, Institute of Cardiovascular Science, University College London, UK; Barts Heart Centre, Barts Health NHS Trust, W Smithfield, London EC1A 7BE, UK.
Graham D Cole, Imperial College Healthcare NHS Trust, Du Cane Road, London W12 0HS, UK; National Heart and Lung Institute, Imperial College London, UK.
Marianna Fontana, Royal Free London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; National Amyloidosis Centre, Division of Medicine, University College London, UK.
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation 2020;142:68–78. [DOI] [PubMed] [Google Scholar]
- 3. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368:473–474. [DOI] [PubMed] [Google Scholar]
- 4. Long B, Long DA, Tannenbaum L, Koyfman A. An emergency medicine approach to troponin elevation due to causes other than occlusion myocardial infarction. Am J Emerg Med 2019;38:998–1006. [DOI] [PubMed] [Google Scholar]
- 5. Vestjens SMT, Spoorenberg SMC, Rijkers GT, Grutters JC, Ten Berg JM, Noordzij PG, Van de Garde EMW, Bos WJW, the Ovidius Study Group. High-sensitivity cardiac troponin T predicts mortality after hospitalization for community-acquired pneumonia. Respirology 2017;22:1000–1006. [DOI] [PubMed] [Google Scholar]
- 6. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, Huang H, Luo YC, Zhou X, Liu ZY, Peng Y, Xu YN, Wang B, Yang YY, Liang ZA, Lei XZ, Ge Y, Yang M, Zhang L, Zeng MQ, Yu H, Liu K, Jia YH, Prendergast BD, Li WM, Chen M. Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart 2020;106:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Dunning J, Openshaw PJM, Baillie JK, Semple MG. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. 2020. The European Society for Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC- COVID-19-Guidance (accessed 10 June 2020).
- 13.European Society of Cardiology. ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance. (10 June 2020).
- 14. Bhatia S, Anstine C, Jaffe AS, Gersh BJ, Chandrasekaran K, Foley TA, Hodge D, Anavekar NS. Cardiac magnetic resonance in patients with elevated troponin and normal coronary angiography. Heart 2019;105:1231–1236. [DOI] [PubMed] [Google Scholar]
- 15. Dastidar AG, Baritussio A, De Garate E, Drobni Z, Biglino G, Singhal P, Milano EG, Angelini GD, Dorman S, Strange J, Johnson T, Bucciarelli-Ducci C. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging 2019;12:1973–1982. [DOI] [PubMed] [Google Scholar]
- 16. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De CR, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P, Pharmacotherapy WC. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 17. Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, Wolff A, Moon JC, Xue H, Kellman P, Patel N, Fontana M. COVID-19: myocardial injury in survivors. Circulation 2020;142:1120–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han Y, Chen T, Bryant J, Bucciarelli-Ducci C, Dyke C, Elliott MD, Ferrari VA, Friedrich MG, Lawton C, Manning WJ, Ordovas K, Plein S, Powell AJ, Raman SV, Carr J. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson 2020;22:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kellman P, Arai AE. Cardiac imaging techniques for physicians: late enhancement. J Magn Reson Imaging 2012;36:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kotecha T, Martinez-Naharro A, Boldrini M, Knight D, Hawkins P, Kalra S, Patel D, Coghlan G, Moon J, Plein S, Lockie T, Rakhit R, Patel N, Xue H, Kellman P, Fontana M. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging 2019;12:1958–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 24. von Knobelsdorff-Brenkenhoff F, Schüler J, Dogangüzel S, Dieringer MA, Rudolph A, Greiser A, Kellman P, Schulz-Menger J. Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2017;10:e005242. [DOI] [PubMed] [Google Scholar]
- 25. Captur G, Gatehouse P, Keenan KE, Heslinga FG, Bruehl R, Prothmann M, Graves MJ, Eames RJ, Torlasco C, Benedetti G, Donovan J, Ittermann B, Boubertakh R, Bathgate A, Royet C, Pang W, Nezafat R, Salerno M, Kellman P, Moon JC. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance-the T1 mapping and ECV standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson 2016;18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Captur G, Bhandari A, Brühl R, Ittermann B, Keenan KE, Yang Y, Eames RJ, Benedetti G, Torlasco C, Ricketts L, Boubertakh R, Fatih N, Greenwood JP, Paulis LEM, Lawton CB, Bucciarelli-Ducci C, Lamb HJ, Steeds R, Leung SW, Berry C, Valentin S, Flett A, de Lange C, DeCobelli F, Viallon M, Croisille P, Higgins DM, Greiser A, Pang W, Hamilton-Craig C, Strugnell WE, Dresselaers T, Barison A, Dawson D, Taylor AJ, Mongeon FP, Plein S, Messroghli D, Al-Mallah M, Grieve SM, Lombardi M, Jang J, Salerno M, Chaturvedi N, Kellman P, Bluemke DA, Nezafat R, Gatehouse P, Moon JC, TIMES Consortium. T1 mapping performance and measurement repeatability: results from the multi-national T 1 mapping standardization phantom program (T1MES). J Cardiovasc Magn Reson 2020;22:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol 2016;68:2348–2364. [DOI] [PubMed] [Google Scholar]
- 28. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, Brown TS, Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol 2021;50:107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 2021;6:116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol 2019;74:2439–2448. [DOI] [PubMed] [Google Scholar]
- 32. Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG. Dilated cardiomyopathy. Nat Rev Dis Primers 2019;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verdonschot JAJ, Merlo M, Dominguez F, Wang P, Henkens MTHM, Adriaens ME, Hazebroek MR, Masè M, Escobar LE, Cobas-Paz R, Derks KWJ, van den Wijngaard A, Krapels IPC, Brunner HG, Sinagra G, Garcia-Pavia P, Heymans SRB. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur Heart J 2021;42:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. JACC Cardiovasc Imaging 2018;11:1274–1284. [DOI] [PubMed] [Google Scholar]
- 35. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP, the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, Melzer D. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community cohort. J Gerontol A Biol Sci Med Sci 2020;75:2224–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turkbey EB, Nacif MS, Guo M, McClelland RL, Teixeira PB, Bild DE, Barr RG, Shea S, Post W, Burke G, Budoff MJ, Folsom AR, Liu CY, Lima JA, Bluemke DA. Prevalence and correlates of myocardial scar in a US cohort. JAMA 2015;314:1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2020;https://doi.org/10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB, Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study dataset will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request to the corresponding author, subject to institutional and ethical committee approvals.