Abstract
Aim
To study the characteristics and outcome among cardiac arrest cases with COVID-19 and differences between the pre-pandemic and the pandemic period in out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA).
Method and results
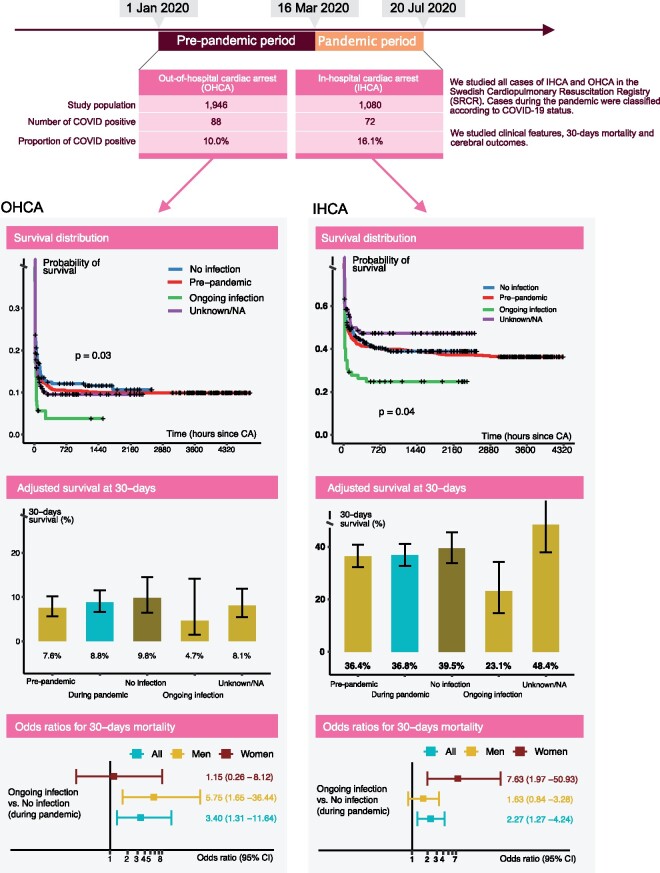
We included all patients reported to the Swedish Registry for Cardiopulmonary Resuscitation from 1 January to 20 July 2020. We defined 16 March 2020 as the start of the pandemic. We assessed overall and 30-day mortality using Cox regression and logistic regression, respectively. We studied 1946 cases of OHCA and 1080 cases of IHCA during the entire period. During the pandemic, 88 (10.0%) of OHCAs and 72 (16.1%) of IHCAs had ongoing COVID-19. With regards to OHCA during the pandemic, the odds ratio for 30-day mortality in COVID-19-positive cases, compared with COVID-19-negative cases, was 3.40 [95% confidence interval (CI) 1.31–11.64]; the corresponding hazard ratio was 1.45 (95% CI 1.13–1.85). Adjusted 30-day survival was 4.7% for patients with COVID-19, 9.8% for patients without COVID-19, and 7.6% in the pre-pandemic period. With regards to IHCA during the pandemic, the odds ratio for COVID-19-positive cases, compared with COVID-19-negative cases, was 2.27 (95% CI 1.27–4.24); the corresponding hazard ratio was 1.48 (95% CI 1.09–2.01). Adjusted 30-day survival was 23.1% in COVID-19-positive cases, 39.5% in patients without COVID-19, and 36.4% in the pre-pandemic period.
Conclusion
During the pandemic phase, COVID-19 was involved in at least 10% of all OHCAs and 16% of IHCAs, and, among COVID-19 cases, 30-day mortality was increased 3.4-fold in OHCA and 2.3-fold in IHCA.
Keywords: Cardiac arrest, COVID-19
Graphical abstract
See page 1107 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab051)
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus causing COVID-19) has infected 27 219 847 and killed 890 687 individuals worldwide as of 9 September 2020, with infection rates on the rise in many regions.1 The full impact of COVID-19 on the healthcare system, health behaviours, disease presentation, and ultimately individual and public health remains to be unravelled.2
Reports have demonstrated increased risk of out-of-hospital cardiac arrest (OHCA) during the pandemic. A study from Paris demonstrated a transient two-fold increase in OHCA incidence, along with a reduction in survival.3 The incidence of resuscitation attempts tripled in New York during the pandemic,4 and a similar increase in OHCA was reported in northern Italy.5 However, these studies were only able to reliably identify patients with COVID-19 in a limited number of patients3 or not at all.4 , 5
Although COVID-19 manifests primarily as a severe respiratory infection, numerous studies demonstrate that cardiovascular complications are common, and pre-existing cardiovascular conditions are predictors of survival in COVID-19.6–8
The first cases of COVID-19 in Sweden were reported in early February 2020. The Swedish Public Health Authority declared community spread in Sweden on 16 March. As a consequence, updated guidelines from the European Resuscitation Council (ERC) and the Swedish Resuscitation Council recommended that bystanders avoid ventilation and focus their resuscitation attempts on chest compressions only in case of suspected COVID-19.9
The Swedish Registry for Cardiopulmonary Resuscitation (SRCR) is a nationwide quality registry. The registry started collecting data on COVID-19 on 1 April 2020. All out-of-hospital and in-hospital cardiac arrests (OHCAs and IHCAs, respectively) are assessed for COVID-19. This report is, to the best of our knowledge, the first detailed report on characteristics and outcomes in COVID-19 patients suffering cardiac arrest. We studied characteristics and outcome in OHCA and IHCA before and during the pandemic.
Methods
This is an observational registry-based study using data from the SRCR. Ethics approval for the study has been obtained by the Swedish ethical review authority (#2020-02017). The study complies with the Declaration of Helsinki.
Study population
We included all cases of OHCA and IHCA registered in the SRCR from 1 January to 20 July 2020. Information on COVID-19 was included in the registry from 1 April 2020, i.e during the initial phase of the pandemic in Sweden. The study population was subdivided into a pre-pandemic group (before 16 March 2020) and a pandemic group (16 March 2020 and later). From 2 April onwards, we stratified the pandemic group into the following three categories: ongoing infection, no infection, or unknown/not assessed. The group defined as having ongoing infection included cases with confirmed infection, suspected infection, or recent infection (this subgroup constituted 5% of cases for OHCA and 11% of cases for IHCA). Thus, during the pandemic period, cases enrolled between 16 March and 1 April were eligible for analyses comparing the pandemic and pre-pandemic period, but not for analyses requiring information on COVID-19 status.
Swedish Registry for Cardiopulmonary Resuscitation (SRCR)
The SRCR is a national quality registry that has monitored the management and outcomes in cardiac arrest in Sweden since 1990. Registration is designed to comply with the Utstein style of reporting for all variables and outcomes.10 This registry has been described in detail elsewhere.11 , 12 Each report includes a detailed description of characteristics, circumstances, time delays, immediate intervention, and subsequent management.
The data are collected from patient medical records and transmitted electronically to the registry. The criteria for inclusion are unresponsive cases in whom cardiopulmonary resuscitation (CPR) and/or defibrillation is performed. IHCA is defined as cases occurring within the hospital perimeter. OHCA is defined as all cases occurring beyond the hospital perimeter. All cases are registered in two steps, with the initial registration taking place immediately after the event and the second registration completed during or after in-hospital care. This allows for information from the first registration to be reassessed before the case is submitted to the registry.
Vital status data were obtained from the Swedish Population Registry.
Statistical analyses
Baseline characteristics are reported using appropriate measures of central tendency and dispersion. Standardized mean differences were used for selected comparisons.
Overall mortality
We calculated overall mortality using the Cox proportional hazards model. In order to avoid excess ties, we used hours since collapse as the time scale. Ties were handled using Efron’s approximation. The assumption of proportional hazards was fulfilled. Adjustment was made for age, sex, and initial rhythm (shockable vs. non-shockable). Age was expanded into a restricted cubic spline with five knots in order to capture non-linear associations in age.
Thirty-day mortality and ROSC
We calculated odds ratios for 30-day mortality despite the fact that not all patients had been observed for 30-days post-cardiac arrest (5.3% of OHCA cases and 1.8% of IHCA cases had not been observed for 30 days at the end of follow-up on 20 July 2020). Odds ratios were derived using logistic regression with the same covariate adjustment as for the Cox model. We also used logistic regression to calculate odds ratios for ROSC (return of spontaneous circulation), adjusting for age, sex, and initial rhythm.
Adjusted survival rates
In order to obtain adjusted absolute risk estimates, we computed the probability of survival using the equation from the logistic regression.
Survival distributions
The Kaplan–Meier estimator was used to delineate survival curves, and the log-rank test was used to test for differences.
Cerebral performance category (CPC) score
The CPC score was assessed at discharge and ranged from 1 to 5 (1 = no sequelae; 2 = mild sequelae; 3 = severe sequelae; 4 = vegetative state; 5 = brain death).
Sensitivity analysis for misclassification of COVID-19 status
We performed a sensitivity analysis in order to address whether misclassification of COVID-19 status may affect our conclusion. We used the method described by Chu et al. 13 , 14 to calculate odds ratios for 30-day survival in COVID-19-positive patients, compared with COVID-19-negative patients. We assume non-differential misclassification of the exposure, COVID-19, with probability density functions for sensitivity and specificity among positive and negative cases equal to uniform distributions with a minimum of 0.7 and a maximum of 0.95 for sensitivity, and a minimum of 0.9 and a maximum of 0.99 for specificity; confidence intervals (CIs) were bootstrapped using 50 000 replicates.
Relative importance and interactions
For patients with IHCA, we had sufficient clinical data to justify calculating the relative importance of various clinical features. Relative importance is defined as each variable’s contribution to the model’s predictive performance and is thus an indirect measure of clinical relevance. Relative importance was computed using random forest with conditional variable importance, as proposed by Strobl et al.15 The predictors included were COVID-19 status, age, sex, location of cardiac arrest, time from arrest to alert, time from arrest to CPR, previous acute myocardial infarction (MI), ongoing MI, previous stroke, ongoing stroke, previous cancer, previous diabetes, ECG monitoring, previous heart failure, witnessed status, initial rhythm, defibrillation, need for adrenaline, intubation, ventilation, antiarrhythmic drugs, mechanical CPR, active temperature control, angiography, county, clock time, and cause of cardiac arrest. Finally, in order to assess whether COVID-19 status interacted with any clinical characteristic, we used gradient boosting to obtain partial dependence plots of such interactions.
Statistical analyses were done in R version 4.0.2 and Python version 3.8.5.
Results
A total of 1946 patients with OHCA and 1080 patients with IHCA were included during the period. Overall mean age for OHCA was 70.2 years and for IHCA 68.9 years. The proportion of women was 33.8% for OHCA and 37.3% for IHCA. A total of 1746 patients (89.7%) died in OHCA and 680 (63.0%) in IHCA. The proportion presenting with shockable rhythm was 20.4% for OHCA and 25.9% for IHCA.
Prevalence of COVID-19 in cardiac arrest
The prevalence of COVID-19 in OHCA during the pandemic was 20.9% (88 out of 422) among cases with available information and 10.0% overall, i.e. including patients with information on COVID-19 unknown or not available (88 out of 877). Corresponding figures for IHCA were 20.2% (72 out of 357) and 16.1% (72 out of 446), respectively.
Characteristics of OHCA cases
With regards to OHCA, 930 cases were reported before the pandemic and 1016 cases during the pandemic, of which 422 had data on COVID-19 status (Table 1). COVID-19 cases were ∼4 years younger than cases without the infection. Overall age during the pandemic was 69.6 years, as compared with 70.8 years before the pandemic. Females represented 33.3% of COVID-19-positive cases, 28.4% of COVID-19-negative cases, and 33.6% of cases with unknown COVID-19 status. COVID-19-positive cases suffered cardiac arrest at home in 87.5% of cases, as compared with 76.9% among COVID-19-negative cases.
Table 1.
Characteristics of OHCA patients in relation to period and COVID-19 status
| Period |
During pandemica
|
||||||
|---|---|---|---|---|---|---|---|
| Pre-pandemic | Pandemic | SMD | No infection | Ongoing infection | Unknown/NA | SMD | |
| n | 930 | 1016 | 334 | 88 | 455 | ||
| Age, mean (SD) | 70.8 (16.6) | 69.6 (17.8) | 0.071 | 70.6 (16.4) | 66.5 (18.4) | 70.0 (17.6) | 0.130 |
| Female sex, n (%) | 326 (35.1) | 319 (32.6) | 0.055 | 93 (28.4) | 29 (33.3) | 146 (33.6) | 0.073 |
| Location of cardiac arrest, n (%) | 0.021 | 0.249 | |||||
| Home | 710 (76.3) | 784 (77.2) | 257 (76.9) | 77 (87.5) | 337 (74.1) | ||
| Public place | 129 (13.9) | 138 (13.6) | 35 (10.5) | 5 (5.7) | 82 (18.0) | ||
| Other place | 91 (9.8) | 94 (9.3) | 42 (12.6) | 6 (6.8) | 36 (7.9) | ||
| Time to EMS arrival, min, median (IQR) | 12.00 (7.00–19.00) | 12.00 (7.00–20.00) | 0.030 | 12.00 (7.00–21.00) | 11.00 (6.00–19.00) | 12.00 (7.00–19.00) | 0.065 |
| Time to CPR, min, median (IQR) | 2.00 (0.00–8.00) | 2.00 (0.00–9.00) | 0.004 | 2.00 (0.00–9.00) | 4.00 (0.00–10.00) | 2.00 (0.00–9.00) | 0.103 |
| Time to defibrillation, min, median (IQR) | 13.00 (8.00–20.00) | 14.00 (9.00–24.00) | 0.087 | 14.00 (8.00–23.00) | 15.00 (10.50–25.00) | 15.00 (10.00–24.00) | 0.105 |
| Aetiology, n (%) | 0.337 | 0.433 | |||||
| Medical condition | 785 (90.8) | 640 (80.2) | 230 (81.3) | 50 (73.5) | 271 (81.1) | ||
| Trauma | 19 (2.2) | 26 (3.3) | 7 (2.5) | 1 (1.5) | 15 (4.5) | ||
| Intoxication | 26 (3.0) | 34 (4.3) | 10 (3.5) | 0 (0.0) | 18 (5.4) | ||
| Drowning | 1 (0.1) | 12 (1.5) | 3 (1.1) | 0 (0.0) | 4 (1.2) | ||
| Electrical accident | 0 (0.0) | 2 (0.3) | 1 (0.4) | 0 (0.0) | 1 (0.3) | ||
| Asphyxia | 34 (3.9) | 84 (10.5) | 32 (11.3) | 17 (25.0) | 25 (7.5) | ||
| Witnessed arrest, n (%) | 534 (57.5) | 571 (57.6) | 0.002 | 203 (61.5) | 53 (63.1) | 243 (55.1) | 0.095 |
| Bystander witnessed, n (%) | 453 (85.8) | 454 (94.0) | 0.275 | 158 (95.8) | 37 (94.9) | 201 (93.1) | 0.189 |
| Bystander defibrillated, n (%) | 13 (22.0) | 12 (32.4) | 0.235 | 4 (28.6) | 0 (0.0) | 8 (42.1) | 0.622 |
| Initial rhythm, n (%) | 0.017 | 0.232 | |||||
| Ventricular fibrillation/pulseless ventricular tachycardia | 168 (20.6) | 170 (20.2) | 63 (22.8) | 6 (7.5) | 80 (21.6) | ||
| Pulseless electrical activity | 139 (17.0) | 148 (17.6) | 49 (17.8) | 19 (23.8) | 62 (16.8) | ||
| Asystole | 510 (62.4) | 522 (62.1) | 164 (59.4) | 55 (68.8) | 228 (61.6) | ||
| Bystander CPR, n (%) | 0.256 | 0.282 | |||||
| No | 2 (0.4) | 10 (1.7) | 3 (1.6) | 3 (5.9) | 3 (1.1) | ||
| Chest compressions | 354 (66.2) | 439 (74.8) | 142 (74.3) | 40 (78.4) | 200 (75.8) | ||
| Ventilation | 1 (0.2) | 2 (0.3) | 1 (0.5) | 0 (0.0) | 1 (0.4) | ||
| Compressions + ventilation | 178 (33.3) | 136 (23.2) | 45 (23.6) | 8 (15.7) | 60 (22.7) | ||
| Defibrillated, n (%) | 260 (28.2) | 275 (30.2) | 0.044 | 102 (33.6) | 16 (21.3) | 124 (30.2) | 0.146 |
| Adrenaline given, n (%) | 683 (73.5) | 770 (80.0) | 0.153 | 257 (80.3) | 71 (85.5) | 344 (80.0) | 0.152 |
| Amiodarone given, n (%) | 94 (10.2) | 110 (13.1) | 0.089 | 42 (15.1) | 3 (4.2) | 53 (13.9) | 0.207 |
| Active temperature control, n (%) | 31 (34.4) | 11 (26.2) | 0.180 | 3 (14.3) | 2 (33.3) | 1 (25.0) | 0.272 |
| Coronary angiography, n (%) | 44 (38.9) | 13 (25.0) | 0.302 | 9 (36.0) | 1 (16.7) | 0 (0.0) | 0.641 |
| Follow-up | |||||||
| ROSC, n (%) | 310 (33.4) | 297 (30.3) | 0.067 | 106 (32.3) | 22 (25.9) | 126 (29.1) | 0.094 |
| Treatment completed before hospital arrival, n (%) | 1.097 | 0.612 | |||||
| No | 469 (50.4) | 508 (50.2) | 180 (54.1) | 42 (48.3) | 217 (47.8) | ||
| Yes | 51 (5.5) | 398 (39.3) | 120 (36.0) | 37 (42.5) | 186 (41.0) | ||
| Unknown | 410 (44.1) | 106 (10.5) | 33 (9.9) | 8 (9.2) | 51 (11.2) | ||
| Discharged alive, n (%) | 65 (38.9) | 17 (33.3) | 0.117 | 9 (36.0) | 0 (0.0) | 1 (16.7) | 0.641 |
| Death within 30 days, n (%) | 833 (89.6) | 905 (89.1) | 0.016 | 291 (87.1) | 84 (95.5) | 408 (89.7) | 0.151 |
| Death, overall, n (%) | 838 (90.1) | 908 (89.4) | 0.024 | 293 (87.7) | 84 (95.5) | 408 (89.7) | 0.144 |
All 139 cases who were enrolled during the pandemic before data on COVID-19 were recorded have been excluded.
Abbreviations: SMD = standardized mean difference; CPR = cardiopulmonary resuscitation; EMS = emergency medical services (ambulance and first responders); ROSC = return of spontaneous circulation.
The variable discharge alive only includes cases who were hospitalized
Shockable rhythms (ventricular fibrillation, pulseless ventricular tachycardia) were equally common during the two periods. However, COVID-19-positive cases displayed shockable rhythm in 7.5% of cases, as compared with 22.8% among cases without COVID-19.
Bystander-witnessed arrests were more common during the pandemic (94.0% vs. 85.8%), but there was no association with COVID-19 status. Bystander defibrillation increased from 22.0% (before the pandemic) to 32.4% during the pandemic. No patient with COVID-19 received bystander defibrillation. Bystanders provided compression-only CPR more frequently during the pandemic period (74.8% vs. 66.2%), leading to a reduction in cases receiving both compressions and ventilation (23.2% vs. 33.3%). Defibrillation at any time was less common in COVID-19 cases (21.3% vs. 33.6%), whereas adrenaline was more commonly used (85.5% vs. 80.3%).
ROSC (return of spontaneous circulation) was slightly less common during the pandemic (30.3% vs. 33.4%). COVID-19-positive cases exhibited ROSC in 25.9% of cases, as compared with 32.3% among cases without COVID-19. The emergency medical services (EMS) terminated treatment before hospital arrival in 42.5% of COVID-19-positive cases, as compared with 36.0% in cases without COVID-19.
The rate of cases discharged alive decreased from 38.9% to 33.0% from the pre-pandemic to pandemic period. As of 20 July 2020, no patient with COVID-19 has been discharged alive, with only four patients (4.5%) surviving 30 days.
Characteristics of IHCA cases
A total of 532 IHCA patients were registered in the pre-pandemic period and 548 patients during the pandemic, of which 357 had data on COVID-19 status (Table 2). Mean age in the pre-pandemic period was 70.1 years, as compared with 67.8 years during the pandemic. Female sex was less common during the pandemic (36.1% vs. 38.6%). Cardiac arrest in the emergency room (ER) was more common during the pandemic (16.2% vs. 10.2%); COVID-19-positive cases experienced cardiac arrest in the ER in 20.8% of cases.
Table 2.
Characteristics of IHCA patients in relation to COVID-19 status and period
| Period |
During pandemica
|
||||||
|---|---|---|---|---|---|---|---|
| Variable | Pre-pandemic | Pandemic | SMD | No infection | Ongoing infection | Unknown/NA | SMD |
| n | 532 | 548 | 285 | 72 | 89 | ||
| Age, mean (SD) | 70.1 (18.2) | 67.8 (18.9) | 0.121 | 67.0 (20.8) | 67.8 (13.0) | 69.4 (15.6) | 0.106 |
| Female sex, n (%) | 205 (38.6) | 197 (36.1) | 0.052 | 93 (32.6) | 23 (32.4) | 37 (42.0) | 0.121 |
| Location of cardiac arrest, n (%) | 0.209 | 0.560 | |||||
| Coronary care unit | 75 (14.1) | 76 (13.9) | 50 (17.5) | 5 (6.9) | 12 (13.5) | ||
| Intensive care unit | 58 (10.9) | 52 (9.5) | 29 (10.2) | 10 (13.9) | 5 (5.6) | ||
| Operating room | 13 (2.4) | 11 (2.0) | 7 (2.5) | 0 (0.0) | 2 ( 2.2) | ||
| Emergency room | 54 (10.2) | 89 (16.2) | 36 (12.6) | 15 (20.8) | 20 (22.5) | ||
| Outpatient, lab, radiology ward | 16 (3.0) | 25 (4.6) | 8 (2.8) | 4 (5.6) | 8 (9.0) | ||
| Catheterization laboratory | 54 (10.2) | 49 ( 8.9) | 24 (8.4) | 3 (4.2) | 15 (16.9) | ||
| Intermediary ward | 16 ( 3.0) | 17 (3.1) | 6 (2.1) | 6 (8.3) | 2 (2.2) | ||
| Regular hospital ward | 231 (43.4) | 215 (39.2) | 116 (40.7) | 29 (40.3) | 22 (24.7) | ||
| Other | 15 (2.8) | 14 (2.6) | 9 (3.2) | 0 (0.0) | 3 (3.4) | ||
| Time to alert, median (IQR) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 0.022 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 0.115 |
| Time to CPR, median (IQR) | 0.00 (0.00–1.00) | 0.00 (0.00–1.00) | 0.110 | 0.00 (0.00–1.00) | 0.00 (0.00–0.00) | 0.00 (0.00–1.00) | 0.150 |
| Time to defibrillation, median (IQR) | 2.00 (1.00–6.00) | 2.00 (1.00–5.00) | 0.070 | 2.00 (1.00–6.50) | 2.00 (1.00–4.00) | 1.00 (0.25–2.75) | 0.144 |
| Status at arrival of alarm group | |||||||
| Consciousness, n (%) | 78 (17.0) | 102 (22.7) | 0.142 | 57 (23.7) | 9 (15.5) | 17 (25.0) | 0.146 |
| Breathing, n (%) | 142 (31.2) | 133 (29.6) | 0.035 | 74 (30.7) | 10 (17.2) | 25 (37.3) | 0.232 |
| Palpable pulse, n (%) | 151 (33.4) | 143 (31.9) | 0.032 | 80 (33.3) | 14 (24.1) | 27 (39.7) | 0.169 |
| Comorbidities | |||||||
| Ongoing MI, n (%) | 103 (28.9) | 39 (20.0) | 0.207 | 23 (22.5) | 1 (3.7) | 4 (30.8) | 0.408 |
| Ongoing stroke, n (%) | 10 (2.7) | 5 (2.5) | 0.013 | 2 (1.9) | 1 (3.7) | 0 (0.0) | 0.155 |
| Previous cancer, n (%) | 70 (18.7) | 36 (18.2) | 0.013 | 16 (15.5) | 5 (20.0) | 5 (38.5) | 0.272 |
| Previous diabetes, n (%) | 124 (32.4) | 60 (28.8) | 0.077 | 28 (26.9) | 11 (40.7) | 4 (23.5) | 0.207 |
| Previous heart failure, n (%) | 137 (37.1) | 59 (29.9) | 0.152 | 38 (37.6) | 6 (22.2) | 3 (23.1) | 0.222 |
| Previous MI, n (%) | 81 (21.7) | 37 (18.2) | 0.087 | 20 (19.2) | 3 (11.5) | 2 (13.3) | 0.165 |
| Previous stroke, n (%) | 39 (10.3) | 16 (7.8) | 0.087 | 5 (4.8) | 1 (3.8) | 3 (18.8) | 0.280 |
| Aetiology, n (%) | 0.602 | 1.364 | |||||
| Anaphylactic shock | 1 (0.3) | 1 (0.6) | 1 (1.1) | 0 (0.0) | 0 (0.0) | ||
| Haemorrhage, any | 7 (2.1) | 7 (3.9) | 4 (4.3) | 0 (0.0) | 0 (0.0) | ||
| Acute abdomen | 6 (1.8) | 3 (1.7) | 2 (2.1) | 0 (0.0) | 0 (0.0) | ||
| Sepsis | 18 (5.5) | 5 (2.8) | 0 (0.0) | 4 (16.0) | 0 (0.0) | ||
| Infection, other | 3 (0.9) | 7 (3.9) | 3 (3.2) | 3 (12.0) | 0 (0.0) | ||
| Intoxication | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Suicide attempt | 1 (0.3) | 1 (0.6) | 1 (1.1) | 0 (0.0) | 0 (0.0) | ||
| Cancer | 5 (1.5) | 3 (1.7) | 1 (1.1) | 0 (0.0) | 1 (10.0) | ||
| Technical system failure | 0 (0.0) | 1 (0.6) | 1 (1.1) | 0 (0.0) | 0 (0.0) | ||
| Dehydration | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| COPD, asthma | 5 (1.5) | 2 (1.1) | 1 (1.1) | 0 (0.0) | 0 (0.0) | ||
| Primary arrhythmia | 59 (18.0) | 16 (8.9) | 13 (13.8) | 1 (4.0) | 0 (0.0) | ||
| Cardiomyopathy | 3 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Arrhythmia post-cardiac surgery | 1 (0.3) | 2 (1.1) | 1 (1.1) | 0 (0.0) | 0 (0.0) | ||
| Aspiration | 3 (0.9) | 4 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Respiratory insufficiency | 38 (11.6) | 37 (20.6) | 15 (16.0) | 9 (36.0) | 3 (30.0) | ||
| MI, ischaemia | 111 (33.9) | 43 (23.9) | 24 (25.5) | 1 (4.0) | 5 (50.0) | ||
| Other thrombo-embolism | 10 (3.1) | 7 (3.9) | 3 (3.2) | 1 (4.0) | 0 (0.0) | ||
| Aortic dissection/rupture | 6 (1.8) | 11 (6.1) | 6 (6.4) | 2 (8.0) | 0 (0.0) | ||
| Cardiac tamponade | 4 (1.2) | 2 (1.1) | 2 (2.1) | 0 (0.0) | 0 (0.0) | ||
| Other | 45 (13.8) | 25 (13.9) | 13 (13.8) | 4 (16.0) | 1 (10.0) | ||
| Stroke | 1 (0.3) | 3 (1.7) | 3 (3.2) | 0 (0.0) | 0 (0.0) | ||
| Witnessed arrest, n (%) | 440 (82.9) | 445 (82.6) | 0.008 | 236 (83.7) | 55 (76.4) | 76 (89.4) | 0.179 |
| ECG monitored, n (%) | 310 (58.9) | 307 (57.4) | 0.031 | 177 (62.8) | 36 (50.7) | 44 (53.0) | 0.142 |
| CPR before arrival of alarm group, n (%) | 415 (90.2) | 416 (91.8) | 0.057 | 218 (91.6) | 55 (93.2) | 64 (90.1) | 0.064 |
| Defibrillated before arrival of alarm group, n (%) | 90 (20.6) | 72 (16.4) | 0.109 | 43 (18.6) | 5 (8.5) | 16 (23.5) | 0.218 |
| Shockable rhythm, n (%) | 138 (28.0) | 116 (23.8) | 0.096 | 68 (26.4) | 12 (18.5) | 20 (25.3) | 0.117 |
| Defibrillated, n (%) | 181 (35.1) | 152 (29.7) | 0.115 | 86 (31.5) | 15 (22.4) | 26 (33.3) | 0.148 |
| Intubated, n (%) | 271 (52.8) | 239 (47.6) | 0.104 | 113 (42.0) | 35 (52.2) | 42 (55.3) | 0.135 |
| Ventilated, n (%) | 318 (81.7) | 250 (62.3) | 0.443 | 140 (66.7) | 20 (37.0) | 35 (56.5) | 0.528 |
| Adrenaline given, n (%) | 327 (63.2) | 336 (65.6) | 0.050 | 176 (64.2) | 49 (73.1) | 49 (63.6) | 0.109 |
| Antiarrhythmics given, n (%) | 74 (15.0) | 59 (12.1) | 0.085 | 34 (12.9) | 6 (9.2) | 10 (14.1) | 0.094 |
| Mechanical compressions, n (%) | 67 (13.1) | 62 (12.4) | 0.022 | 33 (12.2) | 6 (8.8) | 10 (13.7) | 0.082 |
| Temperature control, n (%) | 17 (7.2) | 5 (5.0) | 0.093 | 3 (5.6) | 0 (0.0) | 0 (0.0) | 0.257 |
| Coronary angiography, n (%) | 62 (26.1) | 27 (27.3) | 0.028 | 16 (30.2) | 1 (11.1) | 2 (22.2) | 0.257 |
| Follow-up | |||||||
| ROSC, n (%) | 251 (47.2) | 257 (46.9) | 0.006 | 150 (52.6) | 22 (30.6) | 40 (44.9) | 0.236 |
| 30-day mortality, n (%) | 318 (59.8) | 334 (60.9) | 0.024 | 166 (58.2) | 54 (75.0) | 46 (51.7) | 0.253 |
| Overall mortality, n (%) | 340 (63.9) | 340 (62.0) | 0.039 | 169 (59.3) | 54 (75.0) | 46 (51.7) | 0.263 |
All 102 cases who were enrolled during the pandemic before data on COVID-19 were recorded have been excluded.
Abbreviations: SMD = standardized mean difference; MI = myocardial infarction; CPR = cardiopulmonary resuscitation; EMS = emergency medical service (ambulance); ROSC = return of spontaneous circulation; COPD = chronic obstructive pulmonary disease.
IHCA due to MI decreased from 33.9% in the pre-pandemic period to 23.9% during the pandemic. Previous heart failure, MI, and stroke were less common in COVID-19 cases, whereas diabetes was more common in COVID-19 cases (40.7% vs. 26.9%). Witnessed arrests were less common in COVID-19 cases (76.4% vs. 83.7%), as was in-hospital ECG monitoring (50.7% vs. 62.8%), shockable rhythm (18.5% vs. 26.4%), and defibrillations (22.4% vs. 31.5%).
ROSC emerged in 30.6% of COVID-19 cases, as compared with 52.6% in patient without COVID-19, and 44.9% among cases without data on COVID-19. A total of 54 (75.0%) COVID-19 cases died during follow-up, as compared with 169 (59.3%) of remaining patients.
Survival in OHCA
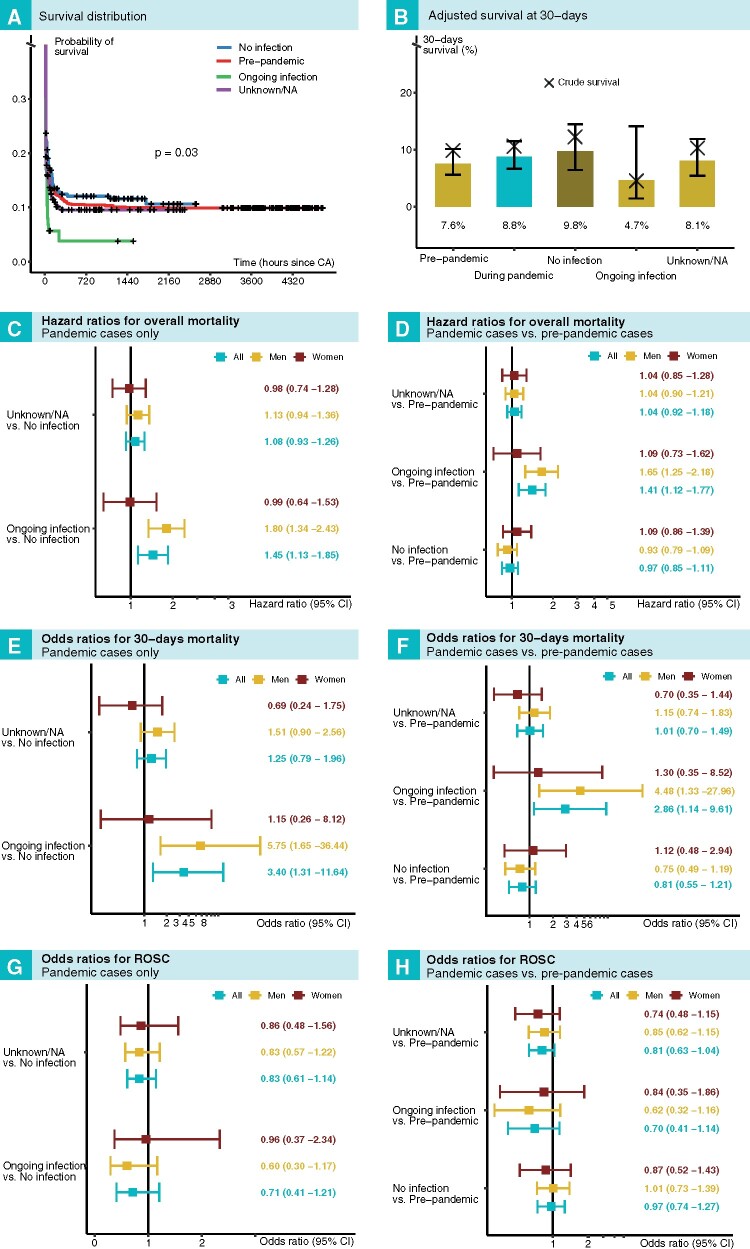
Figure 1A shows survival curves for OHCA. COVID-19-positive patients displayed the most pronounced drop in survival (long rank P-value = 0.03), such that 83.4% had died within 24 h. The predicted 30-day survival probability (Figure 1B) was 4.7% for patients with COVID-19, as compared with 9.8% for patients without COVID-19. Overall survival during the pandemic was 8.8%, as compared with 7.6% during the pre-pandemic period.
Figure 1.
Outcomes after out-of-hospital cardiac arrest in relation to COVID-19 status and period. (A) Kaplan–Meier plot for all groups. (B) Adjusted (predicted) 30-day survival for all groups. Survival in relation to COVID-19 status during the pandemic is shown in (C), (E), and (G). Survival in relation to COVID-19 status during the pandemic, as compared with the pre-pandemic period, is shown in (D), (F), and (H).
During the pandemic, the hazard ratio for death in COVID-19-positive patients, compared with COVID-19-negative patients, was 1.45 (95% CI 1.13–1.85) overall, 1.80 (95% CI 1.34–2.43) for men, and 0.99 (95% CI 0.64–1.53) for women (Figure 1C). As compared with the pre-pandemic cases, the hazard ratio for death for patients with COVID-19 was 1.41 (95% CI 1.12–1.77) overall, 1.65 (95% CI 1.25–2.18) for men, and 1.09 (95% CI 0.73–1.62) for women, as presented in Figure 1D.
Odds ratios for 30-day mortality among COVID-19-positive patients, as compared with COVID-19-negative patients during the pandemic, were 3.40 (95% CI 1.31–11.64) overall, 5.75 (95% CI 1.65–36.44) for men, and 1.15 (95% CI 0.26–8.12) for women (Figure 1E). When comparing COVID-19-positive cases with pre-pandemic cases, odds ratios for 30-day survival were 2.86 (95% CI 1.14–9.61) overall, 4.48 (95% CI 1.33–27.96) for men, and 1.30 (95% CI 0.35–8.52) for women (Figure 1F).
Odds ratios for ROSC did not differ for any comparison (Figure 1G and H); refer to Figure 1 for additional details.
Survival in IHCA
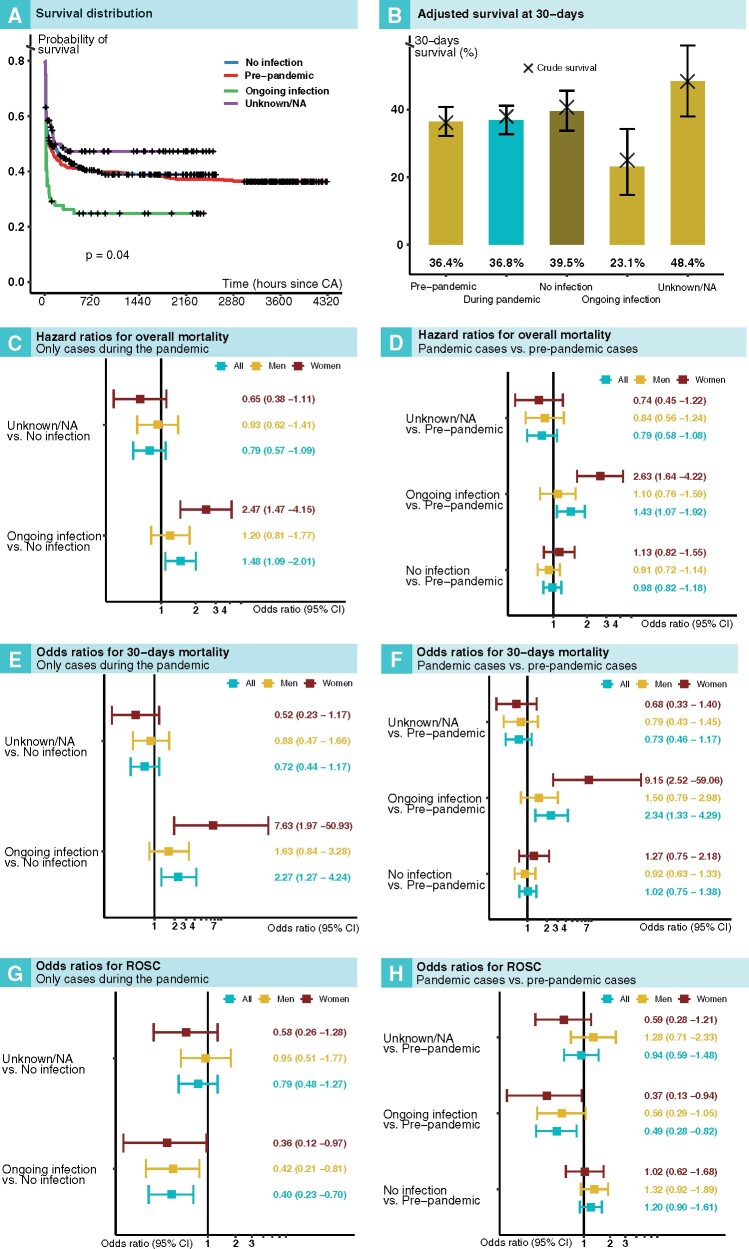
COVID-19-positive patients had the most unfavourable survival curve (Figure 2A), such that 60.5% had died within 24 h (long rank P-value = 0.04). In terms of adjusted probability of 30-day survival (Figure 2B), the lowest probability was noted for COVID-19 patients (23.1%), whereas patients without COVID-19 during the pandemic had a slightly greater survival rate (39.5%) than cases during the pre-pandemic period (36.4%).
Figure 2.
Outcomes after in-hospital cardiac arrest in relation to COVID-19 status and period. (A) Kaplan–Meier plot for all groups. (B) Adjusted (predicted) 30-day survival for all groups. Survival in relation to COVID-19 status during the pandemic is shown in (C), (E), and (G). Survival in relation to COVID-19 status during the pandemic, as compared with the pre-pandemic period, is shown in (D), (F), and (H).
The hazard ratio for death in COVID-19-positive patients, compared with patients without COVID-19 during the pandemic, was 1.48 (95% CI 1.09–2.01) overall, 1.20 (95% CI 0.81–1.77) in men, and 2.47 (95% CI 1.47–4.15) in women (Figure 2C). Corresponding hazard ratios for COVID-19-positive patients, as compared with pre-pandemic patients, were 1.43 (95% CI 1.07–1.92), 1.10 (95% CI 0.76–1.59), and 2.63 (95% CI 1.64–4.22), respectively (Figure 2D).
Odds ratios for 30-day mortality in COVID-19-positive patients, compared with COVID-19-negative patients, during the pandemic, were 2.27 (95% CI 1.27–4.24) overall, 1.63 (95% CI 0.84 –3.28) for men, and 7.63 (1.97–50.93) for women (Figure 2E). Corresponding odds ratios for 30-day mortality in COVID-19-positive patients, as compared with pre-pandemic cases, were 2.34 (95% CI 1.33–4.29) overall, 1.50 (95% CI 0.79–2.98) for men, and 9.15 (95% CI 2.52–59.06) for women (Figure 2F).
The odds ratio for ROSC was 0.40 (95% CI 0.23–0.70) in patients with COVID-19, as compared with patients without COVID-19 during the pandemic (Figure 2G). As compared with pre-pandemic cases, the odds ratio for ROSC in COVID-19-positive cases was 0.49 (95% CI 0.28–0.82); refer to Figure 2 for additional details.
Time delays in OHCA and IHCA
With regards to OHCA, there were no significant difference in time to EMS arrival, time to CPR, or time to defibrillation (Supplementary material online, Figure S1A–C). Similarly, there were no differences in time to alert, time to CPR, or time to defibrillation in IHCA (Supplementary material online, Figure S1D–F).
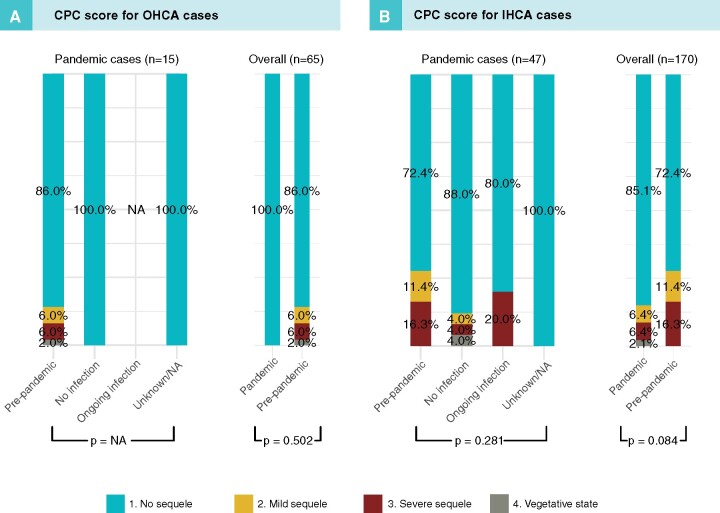
CPC score at discharge
All OHCA patients with CPC data at discharge during the pandemic had CPC category 1 (no sequelae), as compared with 86% during the pre-pandemic period (P = 0.502). CPC category 1 was also more common in IHCA (P = 0.084); refer to Figure 3 for details.
Figure 3.
Cerebral performance category (CPC) in patients with OHCA (A) and IHCA (B) in relation to period and COVID-19 status.
Sensitivity analyses
With regards to misclassification of IHCA cases, the crude odds ratio for 30-day mortality for COVID-19-positive vs. COVID-19-negative cases was 2.15 (95% CI 1.20–3.85). Accounting for misclassification bias yielded an odds ratio of 3.13 (95% CI 1.56–6.91). Corresponding figures for OHCA were 3.10 (95% CI 1.08–8.89) and 5.35 (95% CI 1.62–19.32).
Relative importance and interactions
Out of the 27 predictors included to study relative importance, COVID-19 status was the sixth most important predictor of survival. Need for adrenaline, intubation, location of cardiac arrest, age, and initial rhythm had greater importance than COVID-19 status. We found no interactions between COVID-19 status and other clinical characteristics.
As an ancillary analysis, we assessed the same calendar period (January–July) for each year during 2010–2020 in order to assess whether there are periodic variations in survival. Such periodicity may suggest that there are always monthly differences in survival that may bias our comparison of the pre-pandemic and pandemic period. We noted monthly variations in absolute numbers, but these were not statistically significant (Supplementary material online, Figure S2).
Discussion
This is, to the best of our knowledge, the most detailed report on characteristics and outcome in COVID-19 patients suffering cardiac arrest within and beyond the hospital perimeter. We report a number of unexpected findings, many of which highlight the severity of COVID-19 and the potential shift in the epidemiology of cardiac arrest brought by this pandemic. As of writing this report, no patient with COVID-19 has been discharged alive after suffering an OHCA; as a comparator, 36% of cases without COVID-19 who were hospitalized have been discharged alive.
Our results are of relevance to all healthcare systems handling patients with COVID-19. Cardiac arrest is the ultimate complication of COVID-19, with thousands of cases occurring every day globally. The pandemic is still raging, although with large regional variations. Several countries are now (October) reporting that the number of new cases is rising again, most notably in Europe. Furthermore, the CDC (US Center for Disease Control) predicts that the number of deaths in the USA will double during the winter. It is imperative that policy makers, healthcare professionals, and citizens plan and prepare for the challenges ahead.
With regards to OHCA, there are several noteworthy findings, such as a 2.7-fold increase in the proportion of arrests due to respiratory insufficiency, and a 8.6% increase in compression-only CPR. We also report that only 7.5% of patients with COVID-19 presented with shockable rhythm, as compared with 22.8% among patients without COVID-19. COVID-19 was associated with a 3.4-fold increased risk of 30-day mortality in OHCA and a 2.3-fold increased risk of 30-day mortality in IHCA. Our study clearly shows that cardiac arrest and COVID-19 is a very lethal combination, thus warranting intensive monitoring and measures to prevent the development of cardiac arrest.
With regards to OHCA, we observe some indications of increased survival among COVID-19-negative patients, as compared with pre-pandemic cases (Figure 1B), although corresponding odds ratios and hazard ratios were not statistically significant. If there is an actual increase in survival during the pandemic, it may be explained, at least partly, by the 8.2% (overall) increase in bystander-witnessed arrests and the 47% increase in bystander defibrillations noted here.
It is difficult to disentangle how various societal effects of the pandemic has influenced survival in OHCA. Swedish authorities did not implement a lockdown to mitigate the pandemic. However, as in other countries struck by the pandemic, a substantial proportion of the Swedish population have increased the time spent at home working, studying, vacationing, etc. This could explain the increase in the proportion of cardiac arrests occurring at home, as well as the increase in the rate of witnessed arrests (in households with more than one member) and, subsequently, the increase in bystander defibrillations.
The fact that compression-only CPR has increased may be disadvantageous for patients with COVID-19, given that they frequently suffer respiratory insufficiency, and may therefore benefit from early ventilation. Although previous studies have indicated that compression-only CPR delivered by bystanders may be as effective as compressions and ventilation combined, this may not apply to cases with COVID-19 since they primarily suffer from respiratory failure.16
With regards to IHCA, mean age at cardiac arrest declined by 4 years and ongoing acute MI dropped by 29% during the pandemic. Similar findings were recently reported by the Swedish Coronary Angiography and Angioplasty Registry, which reported a 20% decline in angiography for acute MI during the pandemic.17 We observe a 2.3-fold increased risk of 30-day mortality overall, compared with the pre-pandemic period, which was driven by a 9-fold increased risk among women. Interestingly, COVID-19-positive patients were less frequently ECG monitored and they had fewer witnessed arrest. Given the poor outcomes and high risk of cardiac arrest, we believe that patients with COVID-19 should be provided with monitoring of ECG and oxygen saturation, which would allow for prompt recognition of ventricular arrhythmias and oxygen desaturation.18
Because reporting to the registry lags behind (due to administrative reasons), we are not yet able to perform incidence analyses. However, we report that COVID-19 cases represented 10.0% of all OHCAs and 16.1% of all IHCAs during the studied period. Provided that other causes of cardiac arrest have remained stable during the pandemic, it is likely that the overall incidence has increased in Sweden. Such an increase was reported from New York,4 Paris,3 and northern Italy5. However, we find it unlikely that we will observe a three-fold increase, or even a two-fold increase. The difference may be due to different policies and timing for lockdown, changes in behaviour, health system-related differences, and differences in the population burden of comorbidities and other risk factors for cardiac arrest.
Limitations
Stockholm county has not yet reported data regarding OHCA due to ongoing implementation of new IT systems. This has reduced the number of COVID-19 patients in the current study. Stockholm county has been hit hard by the pandemic, presumably because it is the largest and most densely populated county in Sweden. We do not, however, find any reason to believe this has biased our results. Moreover, we did not have data regarding basis for the diagnosis of COVID-19, which may result in some misclassification of cases. This is of particular concern for OHCA cases. However, we did perform sensitivity analyses to check the reliability of the classification and confirmed that the results were robust. With regards to the aetiology of OHCA, which was introduced in the SRCR in December 2019, we believe that the increase in the prevalence of asphyxia during the pandemic (3.9% before the pandemic vs. 10.5% during the pandemic) is likely to reflect misclassifications. We have not yet provided clinicians who report to the SRCR with a definition of recent COVID-19 infection and included these patients in the COVID-19-positive group in this study. This should not, however, have had any material effect on our results since this group represented only 5% of cases for OHCA and 11% of cases for IHCA.
To conclude, during the pandemic period, COVID-19 has caused a shift in the epidemiology of cardiac arrest in Sweden. The vast majority of parameters studied here—from key patient characteristics, to multiple outcome measures—display a shift during the pandemic, and extremely poor outcomes among patients with COVID-19. This is highlighted by the fact that as of 20 July no patient with COVID-19 has been discharged alive after OHCA.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the Swedish Research Council [2019-02019] and the Swedish Heart and Lung Foundation [20200261].
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Pedram Sultanian, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Peter Lundgren, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Anneli Strömsöe, Centre for Clinical Research Dalarna, Uppsala University, S-79182 Falun, Sweden.
Solveig Aune, Unit for Health Care Coordination, Head Office, Region Västra Götaland, Sweden.
Göran Bergström, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Eva Hagberg, Region Västra Götaland, Sahlgrenska University Hospital, Department of Clinical Physiology, Gothenburg, Sweden.
Jacob Hollenberg, Department of Medicine, Centre for Resuscitation Science, Karolinska Institutet, Solna, Sweden.
Jonny Lindqvist, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Therese Djärv, Department of Medicine, Centre for Resuscitation Science, Karolinska Institutet, Solna, Sweden.
Albert Castelheim, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Anna Thorén, Department of Medicine, Centre for Resuscitation Science, Karolinska Institutet, Solna, Sweden.
Fredrik Hessulf, Department of Anesthesiology and Intensive Care Medicine, Halland Hospital, Halmstad, Sweden.
Leif Svensson, Department of Medicine, Centre for Resuscitation Science, Karolinska Institutet, Solna, Sweden.
Andreas Claesson, Department of Medicine, Centre for Resuscitation Science, Karolinska Institutet, Solna, Sweden.
Hans Friberg, Lund University, Skane University Hospital, Department of Clinical Sciences, Anesthesia & Intensive Care, Malmö, Sweden.
Per Nordberg, Department of Medicine, Centre for Resuscitation Science, Karolinska Institutet, Solna, Sweden.
Elmir Omerovic, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Annika Rosengren, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Johan Herlitz, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
Araz Rawshani, University of Gothenburg, Institute of Medicine, Department of Molecular and Clinical Medicine, Gothenburg, Sweden.
References
- 1.Johns Hopkins University and Medicine COVID-19 map. Johns Hopkins Coronavirus Resource Centre; 2020.
- 2. Rosenbaum L. The untold toll – the pandemic’s effects on patients without Covid-19. N Engl J Med 2020;382:2368–2371. [DOI] [PubMed] [Google Scholar]
- 3. Marijon E, Karam N, Jost D, Perrot D, Frattini B, Derkenne C, Sharifzadehgan A, Waldmann V, Beganton F, Narayanan K, Lafont A, Bougouin W, Jouven X. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health 2020;5:e437–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai PH, Lancet EA, Weiden MD, Weiden MD, Webber MP, Zeig-Owens R, Hall CB, Prezant DJ. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol 2020;5:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, Beretta G, Reali F, Parogni P, Facchin F, Bua D, Rizzi U, Bussi D, Ruggeri S, Visconti LO, Savastano S. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med 2020;383:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma L, Song K, Huang Y. Coronavirus disease-2019 (COVID-19) and cardiovascular complications. J Cardiothorac Vasc Anesth 2020;doi: org/10.1053/j.jvca.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 9. Nolan JP, Monsieurs KG, Bossaert L, Böttiger BW, Greif R, Lott C, Madar J, Olasveengen TM, Roehr CC, Semeraro F, Soar J, Van de Voorde P, Zideman DA, Perkins GD. European Resuscitation Council COVID-19 guidelines executive summary. Resuscitation 2020;153:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langhelle A, Nolan J, Herlitz J, Castren Maaret, Wenzel V, Soreide E, Engdahl J, Steen PA. Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: the Utstein style. Resuscitation 2005;66:271–283. [DOI] [PubMed] [Google Scholar]
- 11. Strömsöe A, Svensson L, Axelsson ÅB, Göransson K, Todorova L, Herlitz J. Validity of reported data in the Swedish Cardiac Arrest Register in selected parts in Sweden. Resuscitation 2013;84:952–956. [DOI] [PubMed] [Google Scholar]
- 12. Hasselqvist-Ax I, Riva G, Herlitz J, Rosenqvist M, Hollenberg J, Nordberg P, Ringh M, Jonsson M, Christer A, Lindqvist J, Karlsson T, Svensson L. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. N Engl J Med 2015;372:2307–2315. [DOI] [PubMed] [Google Scholar]
- 13. Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. Dordrecht: Springer; 2009. [Google Scholar]
- 14. Chu H, Wang Z, Cole SR, Greenland S. Sensitivity analysis of misclassification: a graphical and a Bayesian approach. Ann Epidemiol 2006;16:834–841. [DOI] [PubMed] [Google Scholar]
- 15. Strobl C, Boulesteix AL, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics 2008;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Svensson L, Bohm K, Castrèn M, Pettersson H, Engerström L, Herlitz J, Rosenqvist M. Compression-only CPR or standard CPR in out-of-hospital cardiac arrest. N Engl J Med 2010;363:434–442. [DOI] [PubMed] [Google Scholar]
- 17. Mohammad MA, Koul S, Olivecrona GK, Götberg M, Tydén P, Rydberg E, Scherstén F, Alfredsson J, Vasko P, Omerovic E, Angerås O, Fröbert O, Calais F, Völz S, Ulvenstam A, Venetsanos D, Yndigegn T, Oldegren J, Sarno G, Grimfjärd P, Persson J, Witt N, Ostenfeld E, Lindahl B, James SK, Erlinge D. Incidence and outcome of myocardial infarction treated with percutaneous coronary intervention during COVID-19 pandemic. Heart 2020;106:1812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thorén A, Rawshani A, Herlitz J, Engdahl J, Kahan T, Gustafsson L, Djärv T. ECG-monitoring of in-hospital cardiac arrest and factors associated with survival. Resuscitation 2020;150:130–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.