Abstract
The role of comprehensive cardiac rehabilitation is well established in the secondary prevention of cardiovascular diseases such as coronary artery disease and heart failure. Numerous trials have demonstrated both the effectiveness as well as the cost-effectiveness of comprehensive cardiac rehabilitation in improving exercise capacity and quality of life, and in reducing cardiovascular mortality and morbidity. However, the current COVID-19 pandemic has led to closure of many cardiac rehabilitation centres in Europe resulting in many eligible patients unable to participate in the optimisation of secondary prevention and physical performance. This elicits an even louder call for alternatives such as cardiac telerehabilitation to maintain the delivery of the core components of cardiac rehabilitation to cardiovascular disease patients. The present call for action paper gives an update of recent cardiac telerehabilitation studies and provides a practical guide for the setup of a comprehensive cardiac telerehabilitation intervention during the COVID-19 pandemic. This set up could also be relevant to any cardiovascular disease patient not able to visit cardiac rehabilitation centres regularly after the COVID-19 pandemic ceases.
Keywords: COVID-19, cardiovascular disease, telerehabilitation, comprehensive cardiac rehabilitation
Cardiac rehabilitation
Cardiovascular disease (CVD) remains a leading cause of death worldwide.1 Fortunately, premature CVD mortality has declined in most European countries due to better medical care and prevention.2 Forty per cent of major coronary events occur in patients with known coronary artery disease (CAD).3 Furthermore, one-fifth of patients admitted for heart failure (HF) are rehospitalised within one year due to a HF exacerbation.4 The improved survival following a cardiac event results in a growing number of patients living with a heart disease.5 Therefore, there is need for optimal lifelong secondary prevention.6 Secondary prevention for CVD consists of three pillars: guideline-directed medical therapy (GDMT), adopting a healthy lifestyle, and patient education to increase health literacy.5–9 The goal of GDMT is prescribed, either to obtain clinically stabilisation of patients or to add preventive medications to secondary prevention strategies.8 It is usually carried out in phase I; however, it can be adapted during phase II.
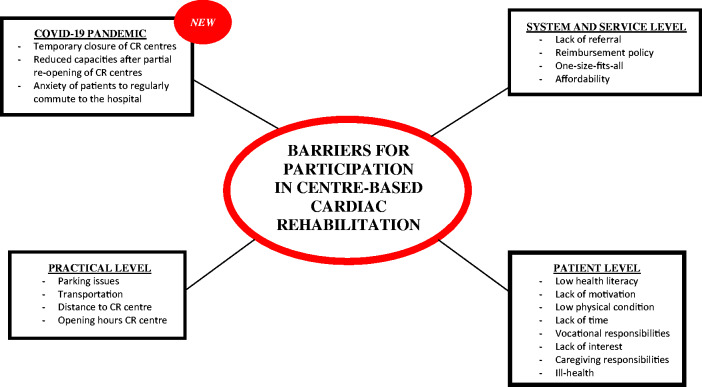
The first step of secondary prevention is often multidisciplinary cardiac rehabilitation (CR). The goals of CR programmes are to reverse the physiological and psychological effects of CVD, to obtain clinical stabilisation (leading to significant reductions in hospitalisations, adverse cardiovascular events and premature death), to optimise cardiovascular risk management, and to enhance the psychosocial and vocational status of participating patients.5,8 Although exercise training is the cornerstone of CR programmes, optimally, CR programmes are comprehensive and comprise all core components such as patient assessment, management and control of cardiovascular risk factors, physical activity counselling and exercise training, patient education, prescription of GDMT, dietary advice, psychosocial management, occupational therapy and vocational support.8 Indeed, multimodal interventions have been shown to elicit greater survival benefits when compared with exercise-only interventions.9,10 CR typically consist of three phases. Phase 1 is the inhospital phase in the first days of hospitalisation after a cardiac event. This phase is important for patient assessment, education and support and to motivate patients to participate in the centre-based CR programme. The core components are routinely delivered during the phase II of CR. This phase II can be provided as an inpatient, outpatient or even home-based service.8 Phase III is the maintenance phase, with the aim of sustaining lifestyle changes. Although the core components and the effectiveness of secondary prevention and CR are well established, rehospitalisation rates for HF and recurrent events for CAD patients remain disappointingly high.3,4 Important causes are the low uptake of CR and poorer long-term adoption of secondary prevention measures, as shown recently in the EuroAspire audits.1,11 An array of factors contributes to these low participation rates. Neubeck et al.12 divided the barriers into three levels. The first level barriers are related to the service and healthcare system such as lack of referral by health professionals, the costs of CR and the local reimbursement policies. Furthermore, most CR programmes are not personalised to the preferences of individual patients or subpopulations. This can lead, for example, to lower participation of women in centre-based CR programmes because women often prefer a more personal and social approach.13 The second level barriers consist of practical issues. Patients choose not to attend CR due to lack of access to transport, time and scheduling constraints often associated with returning to work.12 Thirdly, there are patient barriers such as low health literacy, low motivation, lack of belief that they have the ability to control their CVD, lack of time, ill-health and vocational or caregiving responsibilities.12 What is new, however, and of major impact is the COVID-19 pandemic. Figure 1 gives an overview of common barriers for CR.
Figure 1.
Overview of barriers for participation in centre-based cardiac rehabilitation.
CR in times of the COVID-19 pandemic
The COVID-19 pandemic started in December 2019 in Wuhan and quickly spread throughout the world. COVID-19 infection is a mild viral illness in the vast majority of patients (80%), but may cause severe pneumonia (with subsequent complications) and cardiac complications such as myocarditis with substantial mortality rates in the elderly and individuals with underlying diseases.14
The cause is a novel coronavirus, designated severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which is transmitted mainly by respiratory droplets expelled from the nose or mouth.14 In particular the elderly and patients with comorbidities are at risk of serious complications.15,16 The high infectivity of COVID-19 infections has led in many countries to self-quarantine, the closure of educational settings, non-essential stores and companies. In hospitals all non-urgent outpatient appointments, day cases, inpatient and diagnostic work have been put on hold. This also includes centre-based CR programmes in the majority of European countries. As a result, traditional centre-based CR is impossible in countries with strict lockdown policies. One could argue that patients can enrol in CR after the peak of the pandemic has been achieved, yet available capacity then becomes a major issue. Moreover, there is compelling evidence that delaying the start of CR after a cardiac event is associated with less improvement in cardiopulmonary fitness, and poorer uptake, attendance and completion rates of CR programmes.17,18 Hence, many centres are now exploring feasible options for the remote delivery of personalised CR services to a large and heterogeneous group of patients. The aims of this paper are to: (a) to call for action to start with the remote delivery of CR services in times of the COVID-19 pandemic; (b) to summarise recent cardiac telerehabilitation trials; and (c) to provide a practical guide on how to set up a remote CR programme. This information will also be of benefit to any CVD patient not able to visit outpatient rehabilitation clinics regularly after the COVID-19 pandemic ceases.
Telerehabilitation: a definition
Remote delivery of CR has already been studied for several decades as an alternative intervention or adjunct intervention to overcome common barriers to CR participation such as distance, transport or scheduling constraints.19,20 A meta-analysis by Anderson et al.,21 which included 23 studies with 2890 patients, investigated the effectiveness of home-based CR in comparison with centre-based CR. The authors concluded that home-based CR was equally effective in improving clinical and health-related QoL outcomes.22
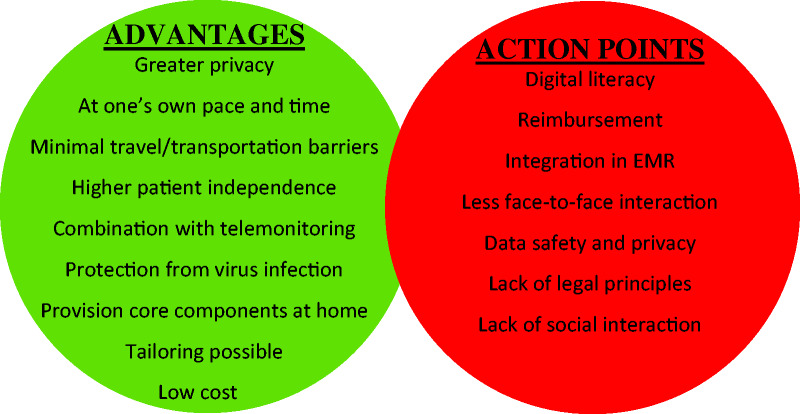
Recent advances in information and communication technologies have facilitated the provision, monitoring and guidance of home-based CR. The use of these technological innovations to deliver CR from a distance is called telerehabilitation or home-based CR.19 Home-based CR and telerehabilitation programmes can overcome many of the practical barriers to CR participation and support long-term adherence to a healthy lifestyle. However, there is up to now no evidence that telerehabilitation can improve participation rates. Telerehabilitation enables the provision of objective feedback and allows patients to track their own progress. This could enhance patients’ self-management skills and, subsequently support more sustainable behavioural change. Figure 2 gives an overview of the advantages and limitations of current telerehabilitation interventions. During the past decade, different formats of telerehabilitation have been tested, yet implementation in clinical practice and in our healthcare systems has remained disappointingly low.22 Furthermore, most telerehabilitation trials have only focussed on the exercise training and physical activity component, whereas ideally telerehabilitation should address all core components of CR tailored to the risk profile of the individual patient.23 At present, a comprehensive telerehabilitation intervention could be used as an alternative mode of CR now the centre-based CR programmes are closed due to the COVID-19 pandemic.
Figure 2.
Overview of advantages and possible concerns of cardiac telerehabilitation. EMR: electronic medical records.
Remotely guided exercise training
Telerehabilitation interventions could be used both in phase II CR as well as in supporting long-term adherence to the physical activity recommendations in phase III CR. The TELEREHAB III trial by Frederix et al.,24 published in 2015, applied accelerometer monitoring in combination with text messaging to deliver telerehabilitation as an add-on to standard CR. The authors found that a 6-month telerehabilitation programme led to a greater improvement in physical fitness and QoL. Moreover, Frederix et al.25 demonstrated that this intervention (CR plus telerehabilitation) induced persistent health benefits and remained cost-effective up to 2 years after the intervention. The TeleRehabilitation in Coronary Heart disease (TRiCH) study compared the effectiveness of a short home-based phase III exercise programme with telemonitoring guidance to a prolonged centre-based phase III programme in CAD patients and to a control group.26 Patients were asked to use a smart watch to record their physical activities and to upload these data to an online web application. Based on the data in the web application, therapists provided feedback by telephone or email.26 Avila et al.26 concluded that the home-based exercise intervention was as effective as prolonged centre-based training and resulted in equal levels of exercise capacity and physical activity. Furthermore, Piotrowicz et al.27 carried out the TELEREH-HF trial, which has been the largest telerehabilitation study for HF patients yet. The authors concluded that the telerehabilitation intervention was effective at 9 weeks, with significantly improving peak oxygen consumption and QoL and it was well tolerated, with no serious adverse events during exercise.27 However, the telerehabilitation intervention was not associated with an increase in the percentage of days alive and out of the hospital and did not reduce mortality and hospitalisation rate over a follow-up period of 14–26 months in comparison with usual care.27 Other studies directly compared centre-based exercise training with home-based exercise training in phase II CR. The randomised controlled Fit@Home trial was conducted in 2014 with 90 CAD patients.28 The trial investigated the effects of a 12-week home-based exercise programme with telemonitoring guidance on exercise capacity and health-related QoL in comparison with a traditional centre-based training programme. Patients in the home-based exercise programme were asked to upload their physical activity data recorded with a wearable heart rate monitor to the internet. The patient’s progress was discussed with their therapist during weekly telephone calls.28 Kraal et al.28 found equal efficacy of the home-based intervention with telemonitoring and the centre-based training on physical fitness, physical activity level or health-related QoL. However, the home-based exercise training resulted in greater patient satisfaction and appeared to be more cost-effective than centre-based training.29
An innovative trial assessing the effectiveness of a smartphone-based exercise programme for CAD patients was conducted in 2019. Maddison et al.30 performed a randomised controlled telerehabilitation trial in 2019 which comprised a smartphone and chest-worn sensor to monitor and educate patients. The authors demonstrated that the intervention was an effective and cost-effective delivery model that could potentially improve overall CR utilisation rates by increasing reach and satisfying unique participant preferences.30 Current evidence suggests that home-based exercise training interventions are effective alternatives or additions for CAD and HF patients that cannot participate in centre-based CR programmes.31 It is important to look critically at these trials. Most trials reveal comparable results in exercise capacity and QoL between centre-based CR and telerehabilitation. These endpoints are prone to spontaneous recovery and do not reflect the three pillars of secondary prevention (e.g. healthy nutrition, adherence to medication, smoking cessation, body composition).26 Only a few trials have investigated the effect of telerehabilitation on recurrent events, rehospitalisations and mortality in HF and CAD patients.25,27 Furthermore, no studies have evaluated the effects of telerehabilitation implementation on participation rates of CR. Table 1 gives an overview of the most recent cardiac telerehabilitation studies for CAD.25,26,28,30,31 Important to mention is the fact that more telerehabilitation research is still needed because studies up to now are limited and recent, conducted on selected and stabilised cardiac patients and all trials have been performed in northern Europe. Research in other parts of Europe should be encouraged to make sure that all findings can be generalised. Furthermore, all trials included in Table 1 focus on phase II or phase III CR. To our knowledge telerehabilitation studies are rarely focused on phase I.
Table 1.
Overview of recent important cardiac telerehabilitation studies for CAD.
| Author | Year | Study design | Participants | Intervention | Physical activity monitoring | Delivery feedback | Conclusion |
|---|---|---|---|---|---|---|---|
| Telerehabilation as add-on to centre-based CR | |||||||
| Frederix et al.25 | 2017 | RCT (1:1) | 139 Mean age: 61 years |
6-Month remote exercise training as add-on to centre based CR for CAD (phase II +III) | Hip-worn pedometer | Text messaging or email | Greater improvements in EC and HRQoL in comparison with centre-based CR |
| Avila et al.26 | 2019 | RCT (1:1:1) | 90 Mean age: 62 years |
12-Week home exercise training for CAD after centre-based CR (phase III) | Smartwatch | Telephone or email | As effective as prolonged centre-based CR with similar levels of EC, PA and HRQoL |
| Claes et al.31 | 2020 | RCT (1:1) | 120 Mean age: 61 years |
6-Month home exercise training for CAD after centre-based CR (phase III) | Microsoft Kinect camera, Microsoft Band 2 heart rate monitor, Zensor 3-lead ECG device | Portal PC + e-learning platform + virtual avatar | Showed positive results on PA time, DBP and CV risk score |
| Telerehabilation as alternative for centre-based CR | |||||||
| Kraal et al.28 | 2017 | RCT (1:1) | 90 Mean age: 59 years |
12-Week home-exercise training for CAD (phase II) | Smartwatch | Telephone | Similar short-term effects on EC capacity and QoL as centre-based patients |
| Maddison et al.30 | 2019 | RCT (1:1) | 162 Mean age: 61 years |
12-Week home-exercise training for CAD (phase II) | Chest sensor + smartphone | Mobile application | Non-inferior and cost-efficient to centre-based CR in improving EC |
CR: cardiac rehabilitation; CAD: coronary artery disease; EC: exercise capacity; HRQoL: health-related quality of life; PA: physical activity; QoL: quality of life; DBP: diastolic blood pressure; CV: cardiovascular; PC: personal computer.
Remote smoking cessation
Smoking is a major modifiable risk factor that should be targeted as part of every primary and secondary CVD programme.32 All European guidelines recommend that smokers should be professionally motivated to stop smoking permanently.33–37 A stepwise strategy is recommended: follow-up, referral to special multidisciplinary programmes and pharmacotherapy.6 Numerous technologies are available to provide remote smoking cessation guidance such as telephone counselling, video consultation, internet-based interventions, text messaging or mobile applications. An extensive meta-analysis researching the effect of telephone counselling for smoking cessation was conducted in 2019.38 The meta-analysis, which included 104 trials totalling 111,653 patients, found moderate certainty evidence that proactive telephone counselling can increase quit rates in smokers.38 The effects of telephone counselling appear more evident when it is provided in combination with print‐based self‐help materials, or brief face‐to‐face advice, and is less pronounced when provided as an adjunct to pharmacotherapy.38 Another medium to provide smoking cessation is video consultation. A meta-analysis conducted in 2019 by Tzelepis et al.39 included two randomised controlled trials (RCTs) with in total 615 patients. The results of the two RCTs do not suggest a significant difference between video counselling and telephone counselling for assisting people to quit smoking.40,41 Both included studies had several methodological limitations so cautious interpretation of the results is needed.39 Internet and email-based interventions are another approach to provide remote smoking cessation guidance. A recent meta-analysis report by McCrabb et al.42 in 2019 on the effectiveness of an internet-based smoking cessation intervention stated that these interventions improved the probability of cessation by 29% in the short term and by 19% in the long term when compared with comparison groups. The Cochrane Review of Taylor et al.43 in 2017 concluded that interactive and tailored internet‐based interventions with or without additional behavioural support are moderately more effective than non‐active controls at 6 months or longer; however there was no evidence that these interventions were better than other active smoking treatments. The use of text messages is an alternative novel way to increase abstinence rates. Numerous studies have showed a beneficial effect of text messaging in adolescents, pregnant women and veterans as a smoking cessation intervention.44–46 This conclusion was confirmed by a RCT with 8000 Chinese patients47 and in two meta-analyses.48,49 A meta-analysis conducted in 2019 by Whittaker et al.50 concluded that there is moderate certainty evidence that automated text message‐based smoking cessation interventions result in greater quit rates than minimal smoking cessation support. There is also moderate certainty evidence for the benefit of text messaging interventions in addition to other smoking cessation support in comparison with smoking cessation support alone. Finally, smartphone applications can also be used for remote smoking cessation guidance.
In 2019, Masaki et al.51 demonstrated that a smartphone application (CureApp smoking cessation) intervention resulted in high long-term continuous abstinence rates, high patient retention rates and improvements in cessation-related symptoms. This suggests that smoking cessation applications have a high potential for delivering effective remote smoking cessation, although a recent meta-analysis of Whittaker et al.50 demonstrated that more RCTs are needed to confirm the effectiveness of these interventions.
In conclusion, effective methods for remote smoking cessation guidance include internet-based, text messaging-based, video consultation and mobile application-based interventions. In particular, text messaging shows great potential. Furthermore, it is a feasible approach for rapid set-up during the COVID-19 pandemic.
Remote hypertension management
Arterial hypertension is also one of the main modifiable risk factors for a wide spectrum of CVDs.32 Blood pressure (BP) is characterised by dynamic fluctuations as a result of the interplay between emotional, physical and environmental factors.52 Therefore, multiple readings are needed to obtain an idea of the average BP. BP telemonitoring is the most popular way to collect various BP readings to diagnose arterial hypertension or to follow-up recent treatment changes.53 These data can be either sent automatically to the health professionals or can be transmitted by the patient via telephone, email, text messaging or via a smartphone application.53 Health professionals can use these BP data to monitor the evolution remotely, to give advice or to suggest treatment changes.53 Two meta-analyses of RCTs revealed that home BP telemonitoring was associated with greater reductions in both office as well as 24-hour ambulatory BP.54,55 One explanation could be that medication adherence is higher in BP telemonitoring. A meta-analysis by Omboni et al.54 demonstrated that the improved BP control obtained with BP telemonitoring was associated with an increased use of antihypertensive medications. Furthermore, BP telemonitoring possibly accelerates the delivery of guideline-directed care because it allows the assessment of real-time data.53 Text messaging and mobile applications are alternatives for the remote management or self-management of arterial hypertension. Text messaging could be used for reminders, education, positive reinforcements and feedback.56 A RCT by Bobrow et al.56 included 1372 patients and demonstrated that a text messaging intervention could elicit a small reduction in systolic BP control compared with usual care at 12 months. Mobile applications can be used for BP monitoring, reminders, lifestyle coaching and education, and patient-directed feedback.53 A recent systematic review in 2018 assessed the effectiveness of mobile applications for hypertension management.57 Ten of the 14 studies reporting the effectiveness of mobile applications in lowering BP demonstrated that the usage of mobile applications could result in a significant decrease in BP. The same systematic review also revealed that mobile applications for BP management were generally very well accepted by participants.57 The same conclusions were drawn in a systematic review by Mohammadi et al.58 Better medication adherence following a mobile application intervention seemed to be the most important effect to lower BP.59 In conclusion, current evidence demonstrates that BP telemonitoring is an effective intervention. There are numerous ways to organise BP telemonitoring such as telephone consultation, text messaging or via a smartphone application. Current evidence suggests that mobile applications are effective in improving BP and medication compliance; however, these studies had only small sample sizes and short durations.53
Remote diabetes management
Type 2 diabetes mellitus (T2DM) is associated with an increased risk of CAD and HF.60,61 Therefore, intensive management of T2DM is required to prevent future or recurrent cardiac events.9,62 Remote technology-based T2DM management may allow real-time monitoring of blood glucose and help to improve medication adherence in comparison with traditional centre-based T2DM management.63 Remote T2DM management could also be provided with various technologies such as telephone follow-up, video consultation, text-messaging or mobile applications. A meta-analysis by Wu et al.64 in 2010 revealed that simple telephone follow‐up interventions delivered only a limited benefit in terms of glycaemic control. However, more interactive approaches including case management, motivational interviewing and more focus on a psychological approach seemed more effective.64 Another meta-analysis conducted in 2014 even concluded that a telephone follow-up intervention was no more effective than standard clinical care.65 Video consultation counselling is a more interactive approach than telephone follow-up. Numerous trials have suggested that interactive video consultations could improve glycaemic control.66–68 Video consultations can be used to monitor glycaemic levels, medication adjustments and potentially even for real-time teleophthalmology.69,70 Text messaging interventions could also have a place in the remote management of T2DM. Text messaging could potentially help to improve therapy adherence with the use of reminders and could contain motivational and educational messages to improve healthy lifestyle choices. Two meta-analyses published in 2019 revealed that messaging results in decreased haemoglobin A1c (HbA1c) and improved blood glucose control. Moreover, it is considered as a low-cost initiative to motivate T2DM patients to adhere to a healthier lifestyle.71,72 Finally, smartphone applications can facilitate remote management of T2DM and promote self-management by providing patients with educational content, self-monitoring and direct communication with health professionals.73 Two recent trials suggest the great potential of smartphone application in T2DM care; however, it seems that a remote management application can support a more sustained glycaemic control than solely using a self-management application.74,75 Unfortunately, firm conclusions cannot be drawn in view of the relatively small trial sample size. A meta-analysis conducted in 2018 reported a reduction in the HbA1c level of 0.57% for T2DM patients using smartphone applications.76 In conclusion, different technology-based interventions seem effective for the remote management of T2DM. Moreover, remote interactive management with a focus on case-management and motivational interviewing seems to be more effective than solely self-management.
Remote dietary counselling and weight management
Both overweight and obesity are modifiable risk factors for most CVDs.32 The European guidelines recommend that weight loss programmes consist of physical activity and exercise training, dietary advice and behaviour modification.6 Dietary advice is often incorporated in weight loss interventions but healthy diet choices also contribute to lower cholesterol, BP and glucose levels.77 Internet-based weight loss and diet interventions have been evaluated in multiple studies. Harden et al.78 revealed in 2015 that an internet-based worksite weight loss programme resulted in clinically significant weight loss (≥5% weight loss) in 22% of the patients. Other trials confirmed the positive effects of internet-based weight loss interventions on diet choices, physical activity or weight.79,80 A meta-analysis by Beleigoli et al.81 demonstrated that internet-based digital interventions led to greater short-term (mean short-term weight loss was 2.13 kg) but not long-term weight loss (mean long-term weight loss was 0.17 kg) than offline interventions in overweight and obese adults. The value of text messaging interventions for weight loss remains controversial. Two meta-analyses showed a small effect of text messaging interventions in short-term weight loss.82,83 However, a lack of long-term results indicates that further long-term studies are necessary. Goldstein et al.84 and Muralidharan et al.85 demonstrated in 2019 that mobile applications can help to accomplish a moderate short-term weight loss. The short-term efficacy of smartphone-based interventions was confirmed in two recent meta-analyses.86,87 However, all of the included RCTs had relatively small sample sizes (between 100 and 833 patients). Nutritional counselling is also a core component of CR. Two studies demonstrated that telephone-based nutritional counselling was effective in improving at least 50% of dietary outcomes.88,89 Moreover, a meta-analysis demonstrated that dietary mobile applications could be effective self-monitoring tools. The meta-analysis concluded that mobile applications can result in positive effects on measured nutritional outcomes in chronic diseases, especially weight loss.90
In conclusion, numerous studies have demonstrated that remote weight and diet management interventions could be an effective alternative for centre-based weight loss programmes. In particular, internet-based interventions and mobile applications seemed to be effective. This suggests that these interventions could be good alternatives when participation in a centre-based programme is not achievable.
Remote psychosocial counselling and vocational support
Modern CR programmes increasingly focus on psychosocial counselling and vocational support. Multimodal behavioural interventions, integrating health education, exercise training and psychological therapy are recommended by the European guidelines because of their importance in affecting cardiovascular prognosis, treatment adherence and QoL.33–37
At this moment, there is limited evidence of remotely delivered psychosocial or vocational support interventions for patients with CVD. However, there are well established digital treatments for depression and most of the anxiety disorders, and for problems such as insomnia for the general population.91 Most digital delivered psychosocial interventions are forms of cognitive behavioural therapy and are based on existing face-to-face treatments.92 Therefore, telephone and video seem the most suitable approaches to deliver remote face-to-face interventions. Less time-consuming alternatives for health professionals could be learning exercises, self-monitoring tools, progress reports, downloadable documents, audio and video files, audio and video feedback, avatars, quizzes and games that are delivered to the patient by email, text messaging or even social media.93
Remote patient education
The education of patients about their heart disease is an essential part of secondary prevention and CR programmes. A recent scientific statement from the American Heart Association7 highlighted that limited health literacy is strongly associated with patient morbidity, mortality, healthcare use and costs. Numerous studies have demonstrated that low health literacy is associated with poorer overall health status and an increased likelihood of rehospitalisation and mortality.94–96 This underlines the importance of patient education in CVD patients. Educational interventions in comprehensive CR programmes may reduce fatal and non-fatal cardiovascular events and improve health-related QoL.97 In centre-based CR, patient education is often organised in group sessions where different lifestyle and disease-related topics are discussed. One of the disadvantages of this approach is that it is one size fits all. Remotely delivered education could be delivered by telephone, email, text messaging, social media or video consultation. The advantage of this approach is that it is easy and quick so the health professional can regularly share small amounts of tailored information with the patient. A meta-analysis by Kotb et al.98 revealed that telephone support interventions during CR may help reduce feelings of anxiety and depression as well as improve BP and smoking cessation. In 2003, Southard and team99 developed and tested an internet-based programme that allows nurse case managers to deliver risk factor management training and education to patients with CAD. The authors demonstrated that this was a cost-effective approach.99 Desteghe et al.100 tested an online education platform for patients with atrial fibrillation (AF). They concluded that it was an effective strategy to improve AF knowledge. Chow et al.101 demonstrated that a lifestyle-focused text messaging service resulted in improvements in various CVD risk factors in CAD patients. Furthermore, Nundy et al.102 reported that a text-message intervention in HF patients is feasible and could possibly lead to improved HF self-management. Multiple strategies can be used for remote education. In particular, telephone counselling and text messaging seem feasible approaches for a quick set-up.
How to set up cardiac telerehabilitation in times of the COVID-19 pandemic
A comprehensive telerehabilitation service requires multiple steps such as patient assessment and patient selection based on contraindications for remote exercise training, patient follow-up and monitoring, and patient direct feedback. The set-up of all these steps is even more difficult in times of the COVID-19 pandemic. Currently, there are four scenarios in Europe:103 (a) totally operating CR centres; (b) partially operating CR centres (reduction of settings and/or programmes); (c) closed CR centres with staff maintenance; (d) closed CR centres with staff redeployment. In all temporarily closed CR centres, initial face-to-face contact for exercise testing and health and risk factor assessment was not possible. Now, exercise testing is possible if local hygienic rules are followed. Moreover, it is also more difficult to follow-up the progress of patients because exercise testing, laboratory testing and conducting patient consultations and questionnaires became challenging. However, in temporarily closed CR centres with staff maintenance remote patient assessment and delivery of telerehabilitation interventions is still possible. Furthermore, it is important still to organise multidisciplinary team meetings. These meetings can be virtual in times of the COVID-19 pandemic by using, for example, video calling. Important to note, these recommendations do not only apply to the most acute phase of the COVID-19 pandemic but will remain relevant in the chronic phase of this pandemic, which will probably last until a vaccination is available.
Remote patient assessment
Almost all centre-based CR and telerehabilitation interventions start with a face-to-face consultation for patient assessment.8 This is an important moment to determine the risk profile of the patient, to estimate exercise capacity, to evaluate psychosocial factors, to set rehabilitation goals and especially for prescribing safe exercise. Many components of patient assessment can be done remotely by careful assessment of the electronic medical records, self-monitoring and multidisciplinary telephone or video consultations. The lack of exercise testing results makes taking safety decisions and training in heart rate zones difficult. There are some studies reporting remote 6-minute walking test for the assessment of (submaximal) exercise capacity.104–106 However, all these studies used self-developed applications which makes it difficult to implement these quickly in all in European centre-based CR. Exercise capacity could be roughly estimated on the basis of the remote consultation with a physical therapist, physical activity questionnaires or non-exercise prediction models such as an assessment tool derived from the HUNT study.107 The HUNT study tool consists of a model that gives a rough assessment of cardiorespiratory fitness in an outpatient setting.107 However, careful interpretation of these results and starting with low-intensity training and resistance training seems advisable. Moreover, patients with acute COVID-19 infections should not perform exercise testing or training. They may gradually increase physical activity after being at least 2 days afebrile. Quarantine times should be respected prior to centre-based visits for risk assessment. Asymptomatic patients may perform exercise training adapted to their cardiovascular risk assessment. Precautions such as social distancing between patients and with health professionals and the use of protective equipment should be taken during centre-based risk assessment to avoid virus spread from asymptomatic carriers. Table 2 gives an overview of remote alternatives for patient assessment in times of the COVID-19 pandemic.108–121
Table 2.
Overview of the patient assessment in CR and options for remote patient assessment in telerehabilitation, if initial face-to-face visit is not possible.
| Components | Patient assessment | Centre-based CR | Remote patient assessment |
|---|---|---|---|
| Clinical history | Cardiovascular risk factors | EMR + Multidisciplinary face-to-face intake consultation |
EMR + Telephone or video intake consultation with MD or case nurse |
| Comorbidities | |||
| Disabilities | |||
| Symptoms | Cardiovascular disease | Multidisciplinary face-to-face intake consultation | Telephone or video intake consultation with MD or case nurse |
| Adherence | Medication | EMR + Multidisciplinaryface-to-face intake consultation |
EMR + Telephone or video intake consultation with MD or case nurse |
| Self-monitoring | Multidisciplinary face-to-face intake consultation | Telephone or video intake consultation with MD or case nurse | |
| Lifestyle | |||
| Physical examination | Cardiovascular | Multidisciplinary face-to-face intake consultation | Recent consultation (EMR) + Video consultation for neurologic and orthopaedic tests + Self-monitoring weight, heart rate and BP |
| Neurologic | |||
| Orthopaedic | |||
| Electrocardiogram | Heart rate, repolarisation changes, heart rhythm | Resting ECG at start of centre-based CR programme | Recent ECG (EMR) Handheld single-lead ECG Smartwatch measured heart rhythm |
| Cardiac imaging | Echocardiography | Last echocardiography | Last echocardiography |
| Blood testing | Lipid values | Last blood test during hospitalisation or new blood test at start of centre-based CR programme | New blood test at general practitioner or last blood test during hospitalisation |
| Renal function | |||
| Glucose levels | |||
| Routine biochemical assay | |||
| Physical activity level | Barriers and social support | Intake face-to-face consultation with physiotherapist e.g. RAPA questionnaire108 |
Telephone or video intake consultation with physiotherapist e.g. TAPA questionnaire109 IPAQ110 |
| Domestic, occupational and recreational level | |||
| Self-confidence + readiness for behaviour change | |||
| Frailty | Frailty | Multidisciplinary face-to-face intake consultation Frailty questionnaire e.g. fried frailty index111 SPPB112 MNA-SF113 Timed up-and-go test114 FRAIL115 |
Telephone or video intake consultation with case nurse + e.g. MNA-SF113 FRAIL115 |
| Peak exercise testing | Exercise capacity | CPET 6MWT SPPB |
Safety decision by MD + e.g. Remote 6MWT Hunt study tool107 |
| Heart rate zones | |||
| Safety assessment | |||
| Education | Literacy level | Intake face-to-face consultation with psychologist e.g. HLS-EU-Q47116 HLQ117 |
Telephone or video intake consultation with psychologist e.g. HLS-EU-Q47116 HLQ117 |
| Patient rehabilitation goals | |||
| Psychosocial | Depression | Intake face-to-face consultation with psychologist e.g. PHQ-9118 BDI-II119 CAQ120 MSPSS121 |
Telephone or video intake consultation with psychologist e.g. PHQ-9118 BDI-II119 CAQ120 MSPSS121 |
| Anxiety | |||
| Social support | |||
| Vocational support |
EMR: electronic medical record; ECG: electrocardiogram; CPET: cardiopulmonary exercise testing; 6MWT: 6-minute walking test; SPPB: short physical performance battery; BP: blood pressure; MD: medical doctor; CR: cardiac rehabilitation; RAPA: rapid assessment of physical activity; TAPA: telephone assessment of physical activity; IPAQ: international physical activity questionnaire; MNA-SF: mini nutritional assessment; PHQ-9: patient health questionnaire-9; BDI-II: Beck depression inventory; CAQ: cardiac anxiety questionnaire; MSPSS: multidimensional scale of perceived social support.
Patient selection
Safety assessment is important even though the frequency of major cardiovascular complications during supervised exercise training for CVD patients is low: about one per 50,000 patient-hours of exercise training.122,123 For a large CR centre including 500–1000 patients per year, this would translate into approximately one major event per year. For home-based CR and telerehabilitation safety assessment is important because patients will exercise without the supervision of health professionals.
During the COVID-19 pandemic, cardiologists can only make a decision on the safety of exercise based on the medical history of the patient, latest electrocardiogram, results of the revascularisation procedure and the left ventricular function on latest echocardiography, as well as personal contact by phone or video call. Screening tools for exercise training such as physical activity anamnesis could aid cardiologists or physical therapists in the decision.124,125 Without exercise testing, extra caution is mainly needed in high-risk patients. It seems reasonable to start with remote low-intensity exercise training for all cardiovascular training in combination with resistance and flexibility exercises. For very high-risk patients, focus only on other core components of comprehensive CR can be an alternative. In this very high-risk population, the presence of a family member or friend during the training could be a reasonable safety measure, eventually with direct supervision of the training session by video link with the rehabilitation centre, so that close control and rapid intervention is possible in case of problems.
Means of delivery of core CR components
There are many telecommunication media that can be used for comprehensive telerehabilitation interventions such as telephones, video consultation, the internet, smartphone applications, text messaging, email and social media. All can be used for bidirectional communication between health professionals and patients. All of them except for self-developed smartphone applications and internet-based interventions are very feasible and can be quickly adopted in every CR centre. Telephone and video consultations are good ways to have remote one-to-one talks with patients to discuss psychosocial topics, medical problems or any questions that patients have. In particular, video consultations have the advantage that patients and health professionals can see each other, which allows non-verbal communication and can potentially improve the trust bond and reduce anxiety and the feeling of being left alone in difficult times. Furthermore, video consultations can also be used for group sessions. For example, health professionals giving a presentation about an educational topic to several patients or a physiotherapist demonstrating exercises. When organising online group sessions, it is important to ensure the privacy of participating patients towards other patients (or to get prior consent) but also to the companies providing the video calling services if applicable. It is therefore preferred to use a general data protection regulation (GDPR) proof system and/or to provide patients with an anonymous account. On top of direct contact, asynchronous telerehabilitation can also be used, sharing commented presentations, films or written material via social media, the website of the hospital or email. This approach can minimise privacy issues but should be combined with moments of interaction between patients and health professionals. Text messaging and email can be used for short educational and motivational messages or to provide quick feedback. Health professionals can share educational and motivational messages and exercise or educational videos via social media. Trustworthy educational resources from national heart foundations or the European Association of Preventive Cardiology (EAPC) (www.healthy-heart.org) can be shared in addition.
Remote monitoring of health status
Remote monitoring of the clinical status and progress of patients is needed to be able to tailor the rehabilitation content better and to adapt the exercise programme to the progression of the patient and to provide objective feedback to patients to improve their self-management skills. Patients can monitor several important parameters at home, such as BP, weight, heart rate, glycaemia, which can be transferred and discussed with the rehabilitation team members. Using short online quizzes or questionnaires, knowledge of the underlying disease or psychosocial status can be assessed, and health professionals can provide feedback, advice and education based on these results. Pictures of patients’ meals, 24-hour food recall questionnaires or diet scores can be used to follow-up healthy diet choices and to tailor the advice based on these tools. Finally, the monitoring of exercise training can be done in multiple ways: self-reporting, interactive training sessions via video consultation, pre-recorded training sessions using the BORG scale,126 or step count measurements. Steps per day can be used for phenotyping of habitual physical activity and for defining physical activity goals (e.g. 5000 steps per day indicate a sedentary lifestyle, 7000–10,000 steps per day are values for healthy adults aged 20 to 65+ years). There is a strong relationship between cadence and intensity. Despite some interindividual variation, 100 steps per minute represents a reasonable value indicative of moderate intensity physical activity.127,128 Devices that assess both accelerometry and heart rate are more accurate and have the advantage that they take into account other activity modes.129 Up-stair steps are included in the daily step count measurements.
Figure 3 gives an overview of the devices that can be used and the CR parameters that can be remotely monitored. Blood and urinary testing can be performed in collaboration with the general practitioner. The results can be reported to the CR centre by the patient or general practitioner.
Figure 3.
Overview of remote monitoring parameters and devices.
Cardiac telerehabilitation in EAPC accredited centres
Table 3 gives an overview of the currently available telerehabilitation services provided in the EAPC accredited centre-based CR programmes. Due to the closure of CR centres during the COVID-19 pandemic the centre-based patient assessment part of following telerehabilitation services was not possible.
Table 3.
Overview of comprehensive telerehabilitation interventions in EAPC accredited CR centres.
| Hasselt, Belgium | Leuven, Belgium | Eindhoven, The Netherlands | Bern, Switzerland | |
|---|---|---|---|---|
| eHealth and mHealth hardware and software |
During COVID-19 Jitsi for real-time education in group. Content shared via social media and website Coroprevention application Currently building a secondary prevention application together with the University of Hasselt and the University of Oulu during a HORIZON 2020 project Exercise training software Expert tool |
Ehealth platform MyNexUZHealth web platform and application are used for video consultations, health dossier for secured storage of patient data, reports, educational material, provision of questionnaires) (https://www.nexuzhealth.be/nl/nexuzhealth) Monitoring devices Physical activity wearables: smartwatch transmission via app/WLAN into Coachbox available in NeXUZ Health |
eHealth platform MijnFLOWportaal (Mibida, Nl) for secured storage of CR-related patient data and chat and video consultation (both individual and group-based for exercise training and psycho-education) Monitoring devices Blood pressure, activity tracker including heart rate and accelerometry (smartwatch). When indicated: scale, thermometer, oxygen saturation Exercise training software FYSIOCARDSS application |
eHealth platform Free web-based health dossier from a certified national provider (Evita.ch) for secured storage of patient data (reports, educational material, vital parameters from monitoring devices) and video consultations Monitored parameters Weight, blood pressure, heart rate, heart rhythm, blood sugar (devices with transmission via mobile network) Steps per days and steps per minute (commercially available fitness tracker, transmission via App/WLAN, integration in health dossier) Exercise training software INSELhealth cardio-fit app (all smartphones) |
| Data privacy | Commercially available Based on companies’ privacy policy (patients acknowledge this with an informed consent) |
Nexuzhealth Encrypted data storage with data only accessible to nexuzhealth hospitals and the patient |
MijnFLOWportaal Encrypted data storage, patient has the full autonomy on granting access to health data Commercially available fitness trackers Data are stored locally on devices and in the portal only. Transfer of data to remote servers is avoided |
Evita.ch Encrypted data storage in a Swiss data centre, patient has full autonomy on granting access to health data Commercially available fitness trackers Based on companies privacy policy (patients acknowledge this by giving informed consent) |
| Reimbursement | In general no specific reimbursement for telerehabilitation. However, during COVID-19 teleconsultations and some parts of telerehabilitation are reimbursed | In general, no specific reimbursement for telerehabilitation. However, during COVID-19 teleconsultations and some parts of telerehabilitation are reimbursed | Full reimbursement of the remote exercise training and psycho-educative prevention modules | Currently no specific reimbursement for telerehabilitation Certain items of the billing catalogue for outpatient medical services can be used (telephone consultations and ECG transmissions by telephone) |
| Patient assessment | Centre-based initial visit with physical examination, laboratory and exercise testing, risk stratification | Centre-based initial visit with physical examination, laboratory and exercise testing, risk stratification | During COVID-19: telephone or video consultation. Laboratory tests on indication. Exercise testing allowed only when urgently needed for diagnostic purposes | Centre-based initial visit with physical examination, laboratory and exercise testing, risk stratification |
| Physical activity and exercise training |
During COVID-19 The different training programmes stay available on the website. During the weekly phonecalls, the therapist gives the patient the advice how he/she can make it easier or harder Coroprevention application • Self-reporting of physical activity • Remote 6MWT • Step count monitoring • Goal-setting |
During COVID-19 All patients receive written material with instructions for exercises Patients are categorised based on phase of CR (phase I or patient was already participating in a phase II program) and based on initial risk screening by cardiologist. Live video group sessions are performed with small groups of patients who are more or less alike to person-tailor the session maximally Exercise logbooks are provided to the patients for registering heart rates and BORG scores |
All patients Individual consultation (video or telephone) by physical therapist Low to moderate risk patients according to Dutch guidelines No exercise test available (COVID-19 only) Live video group sessions (5 patients, 30 min, 2 sessions per week) in 2 fitness categories Exercise test available exercise recommendations based on FITT (intensity based on heart rate, VT1/2 and Borg scale), monitoring of fitness tracker and vital parameters of non-supervised exercise training High-risk patients Individual advice and educational material (video) |
Sport and Physiotherapists One weekly session @ 30 min telephone/video consultation. Distribution of education material, composition of weekly training schedules using INSELhealth cardio-fit app, exercise recommendations based on FITT (intensity based on heart rate, VT1/2 and Borg scale), monitoring of fitness tracker and vital parameters of non-supervised exercise training Three to five non-supervised sessions per week for 8–12 weeks, 20–30 min (monitoring of vital parameters: 2 lead ECG recordings of 40 s, BP, HR, blood sugar in diabetic patients) |
| Psychosocial counselling |
During COVID-19 Educational content delivered via Facebook, Jitsi, LinkedIn and website. Possibility to ask questions to the psychologist via telephone Coroprevention application Stress management module in the application with relaxation exercise |
Psychologist At initiation, all patients have one telephone consultation with the psychologist. Upon patient request or based on HADS questionnaire filled in through myNexUzHealth telephone/video consultations are performed |
Psychologist Screening based on GAD-7 and PHQ. Individual consultations by telephone, group sessions by live |
Psychologists Upon patient request, or based on scores of GAD-7 and PHQ-9 questionnaires, at least two 30 min sessions by |
| Nutrition counselling, weight management |
During COVID-19 Educational content delivered via Facebook, Jitsi, LinkedIn and website. Possibility to ask questions to the dietician via telephone Coroprevention application Behavioural change module in the application based on the MEDDIET score |
Nutritionist At initiation, all patients have one telephone consultation with the dietician. Then, when further indicated or upon patient request telephone/video consultations are being performed |
Nutrition specialist Videoconsultation (when indicated according to Dutch guidelines) |
Nutrition specialists Upon patient request, or if BMI >28 kg/m2, at least two 30 min sessions telephone/video consultations |
| Smoking cessation | During COVID-19 Not available Coroprevention application Behavioural change module in the application |
Psychologist Upon patient request telephone or video consultations are performed Written educational material is provided via mynexuzhealthapp |
Patients who are motivated are referred to Sinefuma (only telephone during COVID-19) | Physicians and psychologists Use of the national smoke quitting telephone line, or individual telephone/ video consultations |
| Guideline-directed medical therapy |
During COVID-19 • Initiation/up-titration during initial visit • Follow-up via telephone Coroprevention application • Guideline-based decision support system • Follow-up by case nurse |
Cardiologist Initiation/up-titration during initial visit Follow-up via scheduled telephone consultations |
During COVID-19 • Initiation/up-titration during initial visit • Follow-up via telephone |
Physicians • initiation/up-titration during initial face-to-face visit • Further up-titration, modification and monitoring of side-effects during remote educational modules |
| Patient education | During COVID-19 • Remote education sessions via Jitsi • Educational content shared via Facebook, LinkedIn and website • Patient can telephone CR centre and ask questions to multidisciplinary team of CR specialist Coroprevention application • Educational videos and animations • Infographics |
During hospitalisation, more time is being devoted to education and all patients can access the education material through mynexuzhealth application and receive written educational material as well. In addition, the 6 educational classes are now replaced by online live stream video classes on • Knowledge on disease/intervention/ medication/risk factors • CVD and physical activity and exercise • CVD, work resumption • CVD, stress, anxiety, partnership • CVD and nutrition |
• Individual education by nurse specialist only • Smartphone app |
Physicians/advanced nurse practitioners Four to five mandatory educational modules @ 30 min by telephone/video-consultation: • Knowledge on disease/intervention • CV risk factors • Medication • Vocational support, leisure-time, partnership • Heart failure (optional) • Distribution of educational material of the Swiss Heart Foundation, including links to websites (e.g. heart groups for phase III) |
EAPC: European Association of Preventive Cardiology; CR: cardiac rehabilitation; CVD: cardiovascular disease; 6MWT: 6-minute walking test.
Data safety and privacy
It is obvious that digital tools and remote management of patients will play a central role in current and future cardiac care. The use of these tools goes hand in hand with the collection of enormous amounts of data. It is important to be aware that collecting these patient data via telerehabilitation interventions such as text messaging, internet-based interventions or mobile applications also brings a great responsibility to healthcare professionals.22 It is crucial that CR centres are aware of the data management of the self-developed or existing commercial mobile applications they want to use. Furthermore, health professionals must be aware that there are health and safety risks related to commercial mobile applications that need to be handled with regard to their clinical evidence, claims on purpose and functions, testing and validation of their performance.22 The National Institute for Health and Care Excellence (NICE) has therefore developed a framework to stratify mobile applications in evidence tiers based on their potential risk for the user. The evidence level needed for each tier is proportionate to the potential risk to users presented by the mobile applications in that tier.130 When starting a telerehabilitation intervention, it is mandatory to inform the patient about the potential data breach risks of self-developed or commercial applications used in telerehabilitation interventions and to obtain an informed consent of the patient.131
Conclusion
The past decade’s telerehabilitation has been investigated in the search for an alternative for centre-based programmes or to support the effectiveness of CR in the longer term. Yet, the COVID-19 pandemic has created the need for cardiac telerehabilitation, as the only possible intervention due to the temporary closure of centre-based CR programmes, limited centre resources, restricted patient travel and limited infrastructure for safe group interventions. Even after the reopening of the CR centres, it will take time before everything goes back to normal. As many centre-based CR is now exploring options to keep delivering the essential core components of CR to their patients we aimed to provide some tools and guidance on how this can be achieved. Considering the fact that many centres do not have a ready to implement internet-based or mobile-based intervention at their disposal, they have to be creative. Text messaging, telephone and video consultation, and social media are simple and affordable ways to deliver the core components of CR to the patient. This offers the possibilities to get experience with these digital interventions among a large and heterogeneous group of patients across Europe, which could support the future study and implementation of telerehabilitation in standard care.
Author contribution
MS, MW, DH, HV and PD contributed to the conception or design of the work. All authors drafted the manuscript. All authors critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bansilal S, Castellano M, Fuster V.. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol 2015; 201: S1–S7. [DOI] [PubMed] [Google Scholar]
- 2. Savarese G, Lund LH.. Global public health burden of heart failure. Cardiac Fail Rev 2017; 3: 7–11. DOI: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Briffa TG, Hobbs MS, Tonkin A, et al. Population trends of recurrent coronary heart disease event rates remain high. Circ Cardiovasc Qual Outcomes 2011; 4: 107–113. DOI: 10.1161/CIRCOUTCOMES.110.957944 [DOI] [PubMed] [Google Scholar]
- 4. Moita B, Marques AP, Camacho AM, et al. One-year rehospitalisations for congestive heart failure in Portuguese NHS hospitals: a multilevel approach on patterns of use and contributing factors. BMJ Open 2019; 9: e031346. DOI: 10.1136/bmjopen-2019-031346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dalal HM, Doherty P, Taylor RS.. Cardiac rehabilitation. BMJ 2015; 351.8027.H5000. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dendale P, Scherrenberg M, Sivakova O, et al. Prevention: from the cradle to the grave and beyond. Eur J Prev Cardiol 2019; 26: 507–511. 10.1177/2047487318821772 [DOI] [PubMed] [Google Scholar]
- 7. Magnani JW, Mujahid MS, Aronow HD, et al.; American Heart Association Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Peripheral Vascular Disease, Council on Quality of Care and Outcomes Research, Stroke Council. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation 2018; 138: e48–e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ambrosetti M, Abreu A, Corrà U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 Update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020. Epub ahead of print 30 March 2020. DOI: 10.1177/2047487320913379. [DOI] [PubMed] [Google Scholar]
- 9.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Müller-Riemenschneider F, Meinhard C, Damm K, et al. Effectiveness of nonpharmacological secondary prevention of coronary heart disease. Eur J Cardiovasc Prev Rehabil 2010; 17: 688–700. [DOI] [PubMed] [Google Scholar]
- 11.Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol 2019; 26: 824–835. [DOI] [PubMed] [Google Scholar]
- 12. Neubeck L, Freedman BS, Clark AM, et al. Participating in cardiac rehabilitation: a systematic review and meta-synthesis of qualitative data. Eur J Prev Cardiol 2012; 19: 494–503. Web. [DOI] [PubMed] [Google Scholar]
- 13. Moore S, et al. Women's views of cardiac rehabilitation programs. J Cardiopulm Rehabil 1996; 16: 123–129. [DOI] [PubMed] [Google Scholar]
- 14.Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020; 382: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z and McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. Epub ahead of print 24 February 2020. JAMA. 2020. DOI: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 16.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. Epub ahead of print 23 March 2020. JAMA. DOI: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 17. Fell J, Dale V, Doherty P.. Does the timing of cardiac rehabilitation impact fitness outcomes? An observational analysis. Open Heart 2016; 3: e000369. Published 2016 Feb 8. DOI: 10.1136/openhrt-2015-000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marzolini S, Blanchard C, Alter DA, et al. Delays in referral and enrolment are associated with mitigated benefits of cardiac rehabilitation after coronary artery bypass surgery. Circ Cardiovasc Qual Outcomes 2015; 8: 608–620. DOI: 10.1161/CIRCOUTCOMES.115.001751. [DOI] [PubMed] [Google Scholar]
- 19. Frederix I, Vanhees L, Dendale P, et al. A review of telerehabilitation for cardiac patients. J Telemed Telecare 2015; 21: 45–53. [DOI] [PubMed] [Google Scholar]
- 20. Thomas RJ, Beatty AL, Beckie TM, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation 2019; 140: e69–e89. [DOI] [PubMed] [Google Scholar]
- 21. Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6: CD007130. Published 2017 Jun 30. DOI: 10.1002/14651858.CD007130.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frederix I, Caiani EG, Dendale P, et al. ESC e-Cardiology Working Group Position Paper: Overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol 2019; 26: 1166–1177. 10.1177/2047487319832394 [DOI] [PubMed] [Google Scholar]
- 23. Janssen V, De Gucht V, Dusseldorp E, et al. Lifestyle modification programmes for patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2013; 20: 620–640. [DOI] [PubMed] [Google Scholar]
- 24. Frederix I, Hansen D, Coninx K, et al. Medium-term effectiveness of a comprehensive internet-based and patient-specific telerehabilitation program with text messaging support for cardiac patients: randomized controlled trial. J Med Internet Res 2015; 17: e185. Published 2015 Jul 23. DOI: 10.2196/jmir.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frederix I, Solmi F, Piepoli M, et al. Cardiac telerehabilitation: a novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur J Prev Cardiol 2017; 24: 1708–1717. Web. [DOI] [PubMed] [Google Scholar]
- 26. Avila A, Claes J, Buys R, et al. Home-based exercise with telemonitoring guidance in patients with coronary artery disease: does it improve long-term physical fitness? Eur J Prev Cardiol 2020; 27, 367–377. [DOI] [PubMed] [Google Scholar]
- 27.Piotrowicz E, Pencina MJ, Opolski G, et al. Effects of a 9-Week Hybrid Comprehensive Telerehabilitation Program on Long-term Outcomes in Patients With Heart Failure: The Telerehabilitation in Heart Failure Patients (TELEREH-HF) Randomized Clinical Trial. JAMA Cardiol 2019; 5: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kraal J, Peek N, Van Den Akker-Van Marle E, et al. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: short-term results of the FIT@Home Study. Eur J Prev Cardiol 2014; 21 (2 Suppl.): 26–31. Web. [DOI] [PubMed] [Google Scholar]
- 29. Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, et al. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: results of the FIT@Home study. Eur J Prev Cardiol 2017; 24: 1260–1273. DOI: 10.1177/2047487317710803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maddison R, Rawstorn J, Stewart R, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019; 105: 122–129. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Claes J, Cornelissen V, McDermott C, et al. Feasibility, acceptability, and clinical effectiveness of a technology-enabled cardiac rehabilitation platform (Physical Activity Toward Health-I): randomized controlled trial. J Med Internet Res 2020; 22: e14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yusuf S, Hawken S, Ounpuu S.. Erratum: Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART Study): case–control Study. Lancet 2004; 364: 937–952. [DOI] [PubMed] [Google Scholar]
- 33. Knuuti J, Wijns W, Saraste A, et al.; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020; 41: 407–477. 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 34.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2016 Dec 30]. Eur Heart J. 2016; 37(27): 2129–2200. doi:10.1093/eurheartj/ehw128 [DOI] [PubMed]
- 35. Ibanez B, James S, Agewall S, et al.; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 36. Roffi M, Patrono C, Collet J, et al.; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 37. De Vries H,. Kemps HMC, Van Engen-Verheul MM, et al. Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur Heart J 2015; 36: 1519–1528. Web. [DOI] [PubMed] [Google Scholar]
- 38. Matkin W, Ordóñez-Mena JM, Hartmann-Boyce J.. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2019; 5: CD002850. DOI: 10.1002/14651858.CD002850.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tzelepis F, Paul CL, Williams CM, et al. Real‐time video counselling for smoking cessation. Cochrane Database Syst Rev 2019; 10: CD012659. DOI: 10.1002/14651858.CD012659.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim SS, Darwish S, Lee SA, Sprague C, et al. A randomized controlled pilot trial of a smoking cessation intervention for US women living with HIV: telephone‐based video call vs voice call. Int J Women's Health 2018; 10: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res 2015; 17: e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCrabb S, Baker AL, Attia J, et al. Internet-based programs incorporating behavior change techniques are associated with increased smoking cessation in the general population: a systematic review and meta-analysis. Ann Behav Med: a publication of the Society of Behavioral Medicine 2019; 53: 180–195. [DOI] [PubMed] [Google Scholar]
- 43. Taylor GMJ, Dalili MN, Semwal M, et al. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev 2017; 9: CD007078. Published 2017 Sep 4. DOI: 10.1002/14651858.CD007078.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Naughton F, Cooper S, Bowker K, et al. Adaptation and uptake evaluation of an SMS text message smoking cessation programme (MiQuit) for use in antenatal care. BMJ Open 2015; 5: E008871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Müssener U, Bendtsen M, Karlsson N, et al. Effectiveness of short message service text-based smoking cessation intervention among university students: a randomized clinical trial. JAMA Intern Med 2016; 176: 321–328. DOI: 10.1001/jamainternmed.2015.8260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cobos-Campos R, Apiñaniz Fernández De Larrinoa A, Sáez De Lafuente Moriñigo A, et al. Effectiveness of text messaging as an adjuvant to health advice in smoking cessation programs in primary care. A randomized clinical trial. Nicotine Tobacco Res 2017; 19: 901–907. [DOI] [PubMed] [Google Scholar]
- 47. Augustson E, Engelgau MM, Zhang S, et al. Text to quit China: an mHealth smoking cessation trial. Am J Health Promot 2017; 31: 217–225. DOI: 10.4278/ajhp.140812-QUAN-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spohr SA, Nandy R, Gandhiraj D, et al. Efficacy of SMS text message interventions for smoking cessation: a meta-analysis. J Substance Abuse Treatm 2015; 56: 1–10. [DOI] [PubMed] [Google Scholar]
- 49. Ybarra ML, Jiang Y, Free C, et al. Participant-level meta-analysis of mobile phonebased interventions for smoking cessation across different countries. Prev Med 2016; 89: 90–97. DOI: 10.1016/j.ypmed.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Whittaker R, McRobbie H, Bullen C, et al. Mobile phone text messaging and app‐based interventions for smoking cessation. Cochrane Database Syst Rev 2019; 10: CD006611. DOI: 10.1002/14651858.CD006611.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Masaki K, Tateno H, Kameyama N, et al. Impact of a novel smartphone app (CureApp smoking cessation) on nicotine dependence: prospective single-arm interventional pilot study. JMIR Mhealth Uhealth 2019; 7: e12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parati G, Stergiou GS, Dolan E, et al. Blood pressure variability: clinical relevance and application. J Clin Hypertens 2018; 20: 1133–1137. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Omboni S. Connected health in hypertension management. Front Cardiovasc Med 2019; 6: 76. Published 2019 Jun 13. DOI: 10.3389/fcvm.2019.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Omboni S, Gazzola T, Carabelli G, et al. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens 2013; 31: 455–467. 10.1097/HJH.0b013e32835ca8dd [DOI] [PubMed] [Google Scholar]
- 55. Duan Y, Xie Z, Dong F, et al. Effectiveness of home blood pressure telemonitoring: a systematic review and meta-analysis of randomised controlled studies. J Hum Hypertens 2017; 31: 427–437. 10.1038/jhh.2016.99 [DOI] [PubMed] [Google Scholar]
- 56. Bobrow KJ, Farmer A, Springer D, et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-Text Adherence Support [StAR]): a single-blind, randomized trial. Circulation 2016; 133: 592–600. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alessa T, Abdi S, Hawley MS, et al. Mobile apps to support the self-management of hypertension: systematic review of effectiveness, usability, and user satisfaction. JMIR Mhealth Uhealth 2018; 6: e10723. 10.2196/10723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mohammadi R, Ayatolahi Tafti M, Hoveidamanesh S, et al. Reflection on mobile applications for blood pressure management: a systematic review on potential effects and initiatives. Stud Health Technol Inform 2018; 247: 306–310. 10.3233/978-1-61499-852-5-306 [PubMed] [Google Scholar]
- 59. Xiong S, Berkhouse H, Schooler M, et al. Effectiveness of mHealth interventions in improving medication adherence among people with hypertension: a systematic review. Curr Hypertens Rep 2018; 20: 86. 10.1007/s11906-018-0886-7 [DOI] [PubMed] [Google Scholar]
- 60. Torp-Pedersen C, Rask-Madsen C, Gustafsson I, et al. Diabetes mellitus and cardiovascular risk: just another risk factor? Eur Heart J 2003; 5 (Suppl. F): F26–F32. 10.1016/S1520-765X(03)90013-7 [DOI] [Google Scholar]
- 61. Kenny HC, Abel ED.. Heart failure in type 2 diabetes mellitus: impact of glucose-lowering agents, heart failure therapies, and novel therapeutic strategies. Circ Res 2019; 124: 121–141. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2020; 41: 255–323. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 63. Kleinman NJ, Shah A, Shah S, et al. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed J E Health 2017; 23: 733–740. [DOI] [PubMed] [Google Scholar]
- 64. Wu L, Forbes A, Griffiths P, et al. Telephone follow‐up to improve glycaemic control in patients with type 2 diabetes: systematic review and meta‐analysis of controlled trials. Diabet Med 2010; 27: 1217–1225. Web. [DOI] [PubMed] [Google Scholar]
- 65. Suksomboon N, Poolsup N, Nge YL.. Impact of phone call intervention on glycemic control in diabetes patients: a systematic review and meta-analysis of randomized, controlled trials. PLoS One 2014; 9: e89207. Published 2014 Feb 19. DOI: 10.1371/journal.pone.0089207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Levin K, Madsen JR, Petersen I, et al. Telemedicine diabetes consultations are cost-effective, and effects on essential diabetes treatment parameters are similar to conventional treatment: 7-year results from the Svendborg Telemedicine Diabetes project. J Diabetes Sci Technol 2013; 7: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Verbosky N, Beckey C, Lutfi N.. Implementation and evaluation of diabetes management via clinical video telehealth. Diabetes Care 2016; 39: e1–e2. [DOI] [PubMed] [Google Scholar]
- 68. Toledo FGS, Ruppert K, Huber KA, et al. Efficacy of the Telemedicine for Reach, Education, Access, and Treatment (TREAT) model for diabetes care. Diabetes Care 2014; 37: e179–e178. [DOI] [PubMed] [Google Scholar]
- 69. McLendon SF. Interactive video telehealth models to improve access to diabetes specialty care and education in the rural setting: a systematic review. Diabetes Spectr 2017; 30: 124–136. DOI: 10.2337/ds16-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Host BKJ, Turner AW, Muir J. . Real‐time teleophthalmology video consultation: an analysis of patient satisfaction in rural Western Australia. Clin Exp Optometry 2018; 101: 129–134. Web. [DOI] [PubMed] [Google Scholar]
- 71. Huang L, Yan Z, Huang H.. The effect of short message service intervention on glycemic control in diabetes: a systematic review and meta-analysis. Postgrad Med 2019; 131: 566–571. [DOI] [PubMed] [Google Scholar]
- 72. Haider R, Likhitha S, Clara KC, et al. Mobile phone text messaging in improving glycaemic control for patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabet Res Clin Pract 2019; 150: 27–37. [DOI] [PubMed] [Google Scholar]
- 73. Fleming G, Petrie J, Bergenstal R, et al. Diabetes digital app technology: benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) Diabetes Technology Working Group. Diabetes Care 2020; 43: 250–260. Web. [DOI] [PubMed] [Google Scholar]
- 74. Zhang L, He X, Shen Y, et al. Effectiveness of smartphone app-based interactive management on glycemic control in chinese patients with poorly controlled diabetes: randomized controlled trial. J Med Internet Res 2019; 21: e15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu Y, Yan Q, Li H, et al. Effects of mobile phone application combined with or without self-monitoring of blood glucose on glycemic control in patients with diabetes: a randomized controlled trial. J Diabetes Investig 2019; 10: 1365–1371. DOI: 10.1111/jdi.13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hou C, Xu Q, Diao S, et al. Mobile phone applications and self‐management of diabetes: a systematic review with meta‐analysis, meta‐regression of 21 randomized trials and GRADE. Diabetes, Obes Metab 2018; 20: 2009–2013. [DOI] [PubMed] [Google Scholar]
- 77. Anand SS, Hawkes C, de Souza RJ, et al. Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol 2015; 66: 1590–1614. DOI: 10.1016/j.jacc.2015.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Harden SM, You W, Almeida FA, et al. Does successful weight loss in an internet-based worksite weight loss program improve employee presenteeism and absenteeism?. Health Educ Behav 2015; 42: 769–774. DOI: 10.1177/1090198115578751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Plaete J, De Bourdeaudhuij I, Verloigne M, et al. Acceptability, feasibility and effectiveness of an ehealth behaviour intervention using self-regulation: ‘MyPlan’. Patient Educ Counsel 2015; 98: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 80. Luger E, Aspalter R, Luger M, et al. Changes of dietary patterns during participation in a web-based weight-reduction programme. Public Health Nutr 2016; 19: 1211–1221. DOI: 10.1017/S1368980015002852. Epub 2015 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Beleigoli AM, Andrade AQ, Cançado AG, et al. Web-based digital health interventions for weight loss and lifestyle habit changes in overweight and obese adults: systematic review and meta-analysis. J Med Internet Res 2019; 21: e298. Published 2019 Jan 8. DOI: 10.2196/jmir.9609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Siopis G, Chey T, Allman-Farinelli M.. A systematic review and meta‐analysis of interventions for weight management using text messaging. J Human Nutr Dietetics 2015; 28: 1–15. [DOI] [PubMed] [Google Scholar]
- 83. Job JR, Fjeldsoe BS, Eakin EG, et al. Effectiveness of extended contact interventions for weight management delivered via text messaging: a systematic review and meta‐analysis. Obesity Rev 2018; 19: 538–549. [DOI] [PubMed] [Google Scholar]
- 84. Goldstein SP, Goldstein CM, Bond DS, et al. Associations between self-monitoring and weight change in behavioral weight loss interventions. Health Psychol 2019; 38: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Muralidharan S, Ranjani H, Mohan Anjana R, et al. Engagement and weight loss: results from the Mobile Health and Diabetes Trial. Diabetes Technol Therapeut 2019; 21: 57–513. [DOI] [PubMed] [Google Scholar]
- 86. Schippers M, Adam PCG, Smolenski DJ, et al. A meta‐analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obesity Rev 2017; 18: 450–459. [DOI] [PubMed] [Google Scholar]
- 87. Park SH, Hwang J, Choi YK.. Effect of mobile health on obese adults: a systematic review and meta-analysis. Healthcare Inform Res 2019; 25: 12–26. DOI: 10.4258/hir.2019.25.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Djuric Z, Vanloon G, Radakovich K, et al. Design of a Mediterranean exchange list diet implemented by telephone counselling. J Am Diet Assoc 2008; 108: 2059–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Parsons JK, Newman V, Mohler JL, et al. The Men's Eating and Living (MEAL) study: a cancer and leukemia group B pilot trial of dietary intervention for the treatment of prostate cancer. Urology 2008; 72: 633–637. [DOI] [PubMed] [Google Scholar]
- 90. Fakih El Khoury C, Karavetian M, Halfens R, et al. The effects of dietary mobile apps on nutritional outcomes in adults with chronic diseases: a systematic review and meta-analysis. J Acad Nutr Dietetics 2019; 119: 626–651. Web. [DOI] [PubMed] [Google Scholar]
- 91. Andersson G, Titov N.. Advantages and limitations of Internet-based interventions for common mental disorders. World Psychiatry 2014; 13: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Andersson G. The internet and CBT: a clinical guide. Boca Raton: CRC Press, 2014. [Google Scholar]
- 93. Fairburn CG, Patel V.. The impact of digital technology on psychological treatments and their dissemination. Behav Res Ther 2017; 88: 19–25. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sentell T, Zhang W, Davis J, et al. The influence of community and individual health literacy on self-reported health status. J Gen Intern Med 2014; 29: 298–304. DOI: 10.1007/s11606-013-2638-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mitchell SE, Sadikova E, Jack BW, et al. Health literacy and 30-day postdischarge hospital utilization. J Health Commun 2012; 17(Suppl. 3): 325–338. DOI: 10.1080/10810730.2012.715233. [DOI] [PubMed] [Google Scholar]
- 96. Bostock S, Steptoe A.. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ 2012; 344: e1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Anderson L, Brown JP, Clark AM, et al. Patient education in the management of coronary heart disease. Cochrane Database Syst Rev 2017; 6: CD008895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kotb A, Hsieh S, Wells GA.. The effect of telephone support interventions on coronary artery disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One 2014; 9: e96581. 10.1371/journal.pone.0096581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Southard BH, Southard DR, Nuckolls J.. Clinical trial of an internet-based case management system for secondary prevention of heart disease. J Cardiopulm Rehabil 2003; 23: 341–348. Web. [DOI] [PubMed] [Google Scholar]
- 100. Desteghe L, Germeys J, Vijgen J, et al. Effectiveness and usability of an online tailored education platform for atrial fibrillation patients undergoing a direct current cardioversion or pulmonary vein isolation. Int J Cardiol 2018; 272: 123–129. Web. [DOI] [PubMed] [Google Scholar]
- 101. Chow CK, Redfern J, Hillis G, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA, the Journal of the American Medical Association 2015; 314: 1255–1263. Web. [DOI] [PubMed] [Google Scholar]
- 102. Nundy S, Razi RR, Dick JJ, et al. A text messaging intervention to improve heart failure self-management after hospital discharge in a largely African-American population: before-after study. J Med Internet Res 2013; 15: e53. Published 2013 Mar 11. DOI: 10.2196/jmir.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Recommendations on how to provide cardiac rehabilitation activities during the COVID-19 pandemic.https://www.escardio.org/Education/Practice-Tools/CVD-prevention-toolbox/recommendations-on-how-to-provide-cardiac-rehabilitation-activities-during-the-c?utm_medium=Email&utm_source=EAPC&utm_campaign=EAPC+-+Bulletin+-+31+March (2020).
- 104. Salvi D, Poffley E, Orchard E, et al. The mobile-based 6-Minute Walk Test: usability study and algorithm development and validation. JMIR Mhealth Uhealth 2020; 8: e13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schulte J, Duc NA, Hoang DB, et al. A remote sensor-based 6-minute functional walking ability test. In: Proceedings of the SENSORS,2012IEEE Conference. 2012 Presented at: SENSORS'12; 28–31 October 2012; Taipei, Taiwan. URL: https://ieeexplore.ieee.org/abstract/document/6411040
- 106. Capela NA, Lemaire ED, Baddour N.. Novel algorithm for a smartphone-based 6-minute walk test application: algorithm, application development, and evaluation. J Neuroeng Rehabil 2015; 12: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nes BM1, Janszky I, Vatten LJ, et al. Estimating VO2peak from a nonexercise prediction model: the HUNT Study, Norway. Med Sci Sports Exerc 2011; 43: 2024–2030. DOI: 10.1249/MSS.0b013e31821d3f6f [DOI] [PubMed] [Google Scholar]
- 108.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis 2006; 3: A118. [PMC free article] [PubMed] [Google Scholar]
- 109. Mayer CJ, Steinman L, Williams B, et al. Developing a telephone assessment of physical activity (TAPA) questionnaire for older adults. Prev Chronic Dis 2008; 5: . [PMC free article] [PubMed] [Google Scholar]
- 110. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 111. Fried LP, Tangen CM, Walston J, et al.; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci 2001; 56A: M146–M156. [DOI] [PubMed] [Google Scholar]
- 112. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014; 63: 747–762. DOI: 10.1016/j.jacc.2013.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rubenstein LZ, Harker JO, Salva A, et al. Screening for undernutrition in geriatric practice: developing the short-form mini nutritional assessment (MNA-SF). J Gerontol 2001; 56A: M366–M377. [DOI] [PubMed] [Google Scholar]
- 114. Podsiadlo D, Richardson S.. The Timed Up & Go: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 115. Morley JE, Malmstrom TK, Miller DK.. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16: 601–608. DOI: 10.1007/s12603-012-0084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sørensen K, Pelikan JM, Röthlin F, et al. Health literacy in Europe: comparative results of the European health literacy survey (HLS-EU). Eur J Public Health 2015; 25: 1053–1058. DOI: 10.1093/eurpub/ckv043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Osborne RH, Batterham RW, Elsworth GR, et al. The grounded psychometric development and initial validation of the Health Literacy Questionnaire (HLQ). BMC Public Health 2013; 13: 658. DOI: 10.1186/1471-2458-13-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. DOI: 10.1046/j.1525-1497.2001.016009606.x\ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Beck AT, Steer RA, Brown GK.. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 120. Eifert GH, Thompson RN, Zvolensky MJ, et al. The Cardiac Anxiety Questionnaire: development and preliminary validity. Behavr Res Therapy 2000; 38: 1039–1053. Web. [DOI] [PubMed] [Google Scholar]
- 121. Zimet GD, Dahlem NW, Zimet SG, et al. The multidimensional scale of perceived social support. J Personal Assess 1988; 52: 30–41. [Google Scholar]
- 122. Pavy B, Iliou MC, Meurin P, et al.; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166: 2329–2334. DOI: 10.1001/archinte.166.21.2329 [DOI] [PubMed] [Google Scholar]
- 123. Smart N, Marwick TH.. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 2004; 116: 693–706. Web. [DOI] [PubMed] [Google Scholar]
- 124. Warburton DE, Jamnik VK, Bredin SS, et al. Evidence-based risk assessment and recommendations for physical activity clearance: an introduction. Appl Physiol Nutr Metab 2011; 36 (Suppl. 1): S1–S2. DOI: 10.1139/h11-060 [DOI] [PubMed] [Google Scholar]
- 125.Warburton D, Jamnik V, Bredin S, et al. The physical activity readiness questionnaire for everyone (PAR-Qfl) and electronic physical activity readiness medical examination (ePARmed-Xfl). Health Fitness J Canada 2011; 4: 3–17. [Google Scholar]
- 126. Williams N. The Borg Rating of Perceived Exertion (RPE) scale. Occupat Med 2017; 67: 404–405. 10.1093/occmed/kqx063 [DOI] [Google Scholar]
- 127. Tudor-Locke C, Craig CL, Brown WJ, et al. How many steps/day are enough? For adults. Int J Behav Nutr Phys Act 2011; 8: 79.21798015 [Google Scholar]
- 128. Bassett DR Jr, Toth LP, LaMunion SR, et al. Step counting: a review of measurement considerations and health-related applications. Sports Med 2017; 47: 1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kraal JJ, et al. Energy expenditure estimation in beta-blocker-medicated cardiac patients by combining heart rate and body movement data.Eur J Prev Cardiol 2016; 23: 1734–1742. Epub 2016 Sep 13 [DOI] [PubMed] [Google Scholar]
- 130.Official Journal of the European Union. REGULATION (EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL, https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN (2016).
- 131.NICE. EVIDENCE STANDARDS FRAMEWORK FOR DIGITAL HEALTH TECHNOLOGIES. https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/evidence-standards-framework/digital-evidence-standards-framework.pdf (2019).