Abstract
Acute kidney injury (AKI) is common among hospitalized patients with coronavirus disease 2019 (COVID-19), with the occurrence of AKI ranging from 0.5% to 80%. An improved knowledge of the pathology of AKI in COVID-19 is crucial to mitigate and manage AKI and to improve the survival of patients who develop AKI during COVID-19. In this review, we summarize the published cases and case series of various kidney pathologies seen with COVID-19. Both live kidney biopsies and autopsy series suggest acute tubular injury as the most commonly encountered pathology. Collapsing glomerulopathy and thrombotic microangiopathy are other encountered pathologies noted in both live and autopsy tissues. Other rare findings such as anti-neutrophil cytoplasmic antibody vasculitis, anti-glomerular basement membrane disease and podocytopathies have been reported. Although direct viral infection of the kidney is possible, it is certainly not a common or even widespread finding reported at the time of this writing (November 2020).
Keywords: AKI, ATN, collapsing glomerulopathy, COVAN, COVID-19, pathology, TMA
INTRODUCTION
A novel coronavirus named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in December 2019 in China. This virus causes coronavirus disease 2019 (COVID-19), which is characterized by a diffuse alveolar damage leading to an acute respiratory distress syndrome [1]. Organs other than the lungs may be affected, including the kidneys. The incidence of acute kidney injury (AKI) in hospitalized patients with COVID-19 disease has been reported to range from 0.5% to 80% [2–4]. In the USA, AKI among hospitalized patients with COVID-19 may range between 28% and 46%, with a high risk for in-hospital mortality [5, 6]. Patients who develop AKI have a worse outcome as compared with those with normal kidney function [5].
In this review, we first discuss the controversy behind SARS-CoV-2 and its role in direct kidney infection, then classify AKI in COVID-19 based on the pattern of injury and describe all published histopathological changes in detail. Lastly, we describe biopsy findings in kidney transplant recipients. The pathophysiology of COVID-19-related AKI is discussed in a separate manuscript in this issue of the journal.
DOES SARS-CoV-2 RESULT IN DIRECT KIDNEY INFECTION?
Certain viral infections of the kidney, including BK polyomavirus, adenovirus and cytomegalovirus, are typically associated with viral cytopathic effect, i.e. nuclear or cytoplasmic inclusions and sometimes extensive tissue necrosis and inflammation. None of these changes has been reported in biopsy and autopsy series describing kidney pathology in COVID-19 [7–12]. From the early studies, it has become apparent that the SARS-CoV-2 virus is not associated with such obvious signs of direct viral infection, but its presence in the kidney has become a subject of controversy.
Initial autopsy reports from China included ultrastructural evidence of viral particles and positive immunofluorescence (IF) staining for SARS-CoV-2 nucleoprotein, but these studies were performed in a portion of cases and in some cases with negative results [13]. Several other studies followed, with variable results. Some reported positive identification of viral particles by electron microscopy but with negative results when using more specific tests, like RT-PCR [14, 15]. Others showed a detectable SARS-CoV-2 viral load in all kidney compartments examined by RT-PCR, in situ hybridization (ISH) and indirect IF with confocal microscopy [16–18]. On the other hand, data from large autopsy and biopsy series from Columbia University and Northwell Health in New York, and a multicenter biopsy series report negative results by immunohistochemistry (IHC) or ISH in all tested cases [8–12]. The Columbia group reported a low-intensity dot-like staining of tubule epithelial cells in many cases and this was interpreted as nonspecific or suggestive of low-level viral abundance, particularly when contrasted with the intense staining in lung specimens from autopsies with COVID-19 [9, 11].
Absolute certainty in viral particle identification by electron microscopy is very challenging because it is primarily based on particle morphology and size. Virus-like particles of SARS-CoV-2 were identified on electron microscopy in several studies, but these findings have been strongly challenged by others who proposed that these ultrastructural findings most likely corresponded to intracellular components like clathrin-coated vesicles and multivesicular bodies [17,19–21] (Figure 1). Ultrastructural details in autopsy tissues can also be misinterpreted as coronavirus particles [7]. Most of these images lack sufficient ultrastructure for viral classification. Some could represent endoplasmic reticulum, some multivesicular bodies and some coated vesicles [21]. Although direct viral infection of the kidney is possible, it is certainly not a common or even widespread finding reported thus far. At the time of this writing (November 2020), SARS-CoV-2 is detected uncommonly by IHC or ISH.
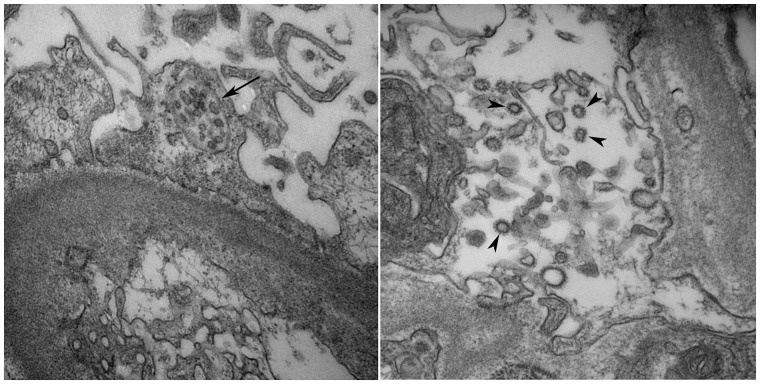
FIGURE 1.
Electron micrograph to the left shows a microvesicular body within the podocyte cytoplasm (black arrow; original magnification ×40 000) and the electron micrograph to the right shows multiple clathrin-coated vesicles in the endothelial cell cytoplasm (black arrowheads; original magnification ×50 000). Both structures have been often confused with viral particles.
HISTOPATHOLOGICAL FINDINGS OF AKI IN COVID-19 BASED ON THE PATTERN OF INJURY
The percentage of patients with COVID-19-associated AKI who undergo a kidney biopsy remains low due to logistical reasons and infection risk. Most of the biopsies done are for indication only so true prevalence of pathological changes may still not be known. Data on the pathology of COVID-19-associated AKI are primarily derived from case reports and case series. While most published case reports discuss glomerular disease findings associated with COVID-19 [collapsing glomerulopathy (CG)], this might be a relative publication bias. Of note, it is common to see more than one compartment of the kidney to be involved. We summarize all the kidney biopsy findings in patients with COVID-19 and AKI that have been published at the time of this writing (November 2020). The three most common kidney biopsy findings to date associated with COVID-19 are acute tubular injury (ATI), CG and thrombotic microangiopathy (TMA). A recent review on this topic by our group summarizes the pathophysiology and the pathology associated with COVID-19-related AKI [22], but the data now have been updated in this review. Table 1 summarizes all commonly reported kidney biopsy pathology seen with COVID-19. Table 2 summarizes the kidney related autopsy data from key manuscripts published in 2020. Supplementary data, Table S1 gives the details of individual cases for all related pathologies.
Table 1.
Summary of commonly noted kidney biopsies findings seen with COVID-19
| Pathology | Median age (IQR), years | Male/ female (n) | Race/ethnicity breakdowna (%) | SCr (IQR), mg/dLb | Initial presentation (%) | KRT (%) | Alive at discharge (%) |
|---|---|---|---|---|---|---|---|
| ATI (n = 9) |
57.0 (55.0–69.0) |
5 M/4 F |
AA—22.2 Black—22.2 Hispanic—33.4 White—22.2 |
4.8 (3.2–6.1) |
AKI—77.8 AKI + proteinuria—22.2 |
Yes—44.4 No—55.5 |
Alive—66.7 Died—22.2 NA—11.1 |
| CG + ATI (n = 28) |
56.0 (46.0–62.7) |
18 M/10 F |
AA—21.4 African—7.1 Asian—3.6 Black—67.9 |
6.6 (4.4–10.6) |
AKI + NS —67.9 AKI + proteinuria—32.1 |
Yes—64.2 No—32.1 N/A—3.6 |
Alive—89.3 Died—7.1 NA—3.6 |
| TMA (n = 3) |
45.0 (34.0–69.0) |
1 M/2 F |
AA—33.3 Hispanic—33.3 White—33.3 |
7.4 (7.8–11.4) |
AKI—33.3 AKI + proteinuria—66.7 |
Yes—100 No—0 |
Alive—33.3 Died—66.7 |
| Vasculitis—ANCA and anti-GBM (n = 9) |
48.0 (39.5–66.5) |
3 M/6 F |
AA—22.2 Asian—22.2 Black—11.1 White—44.4 |
8.2 (4.4–17.8) |
AKI—88.9 AKI + proteinuria—11.1 |
Yes—55.6 No—44.4 |
Alive—100 Died—0 |
SCr, serum creatinine; AA, African American; AKI, acute kidney injury; ANCA, antineutrophilic cytoplasmic autoantibody; ATI, acute tubular injury; CG, collapsing glomerulopathy; crt, creatinine; GBM; glomerular basement membrane; NS, nephrotic syndrome; TMA, thrombotic microangiopathy; KRT: kidney replacement therapy. M, Male; F; Female; F, female.
Race/ethnicity is cited directly from the source article.
SCr values reflect the values of initial presentation.
Table 2.
Summary of autopsy studies associated with COVID-19 and the kidney
| Author (ref #) | Bradley et al. [7] | Golmai et al. [8] | Hanley et al. [23] | Remmelink et al. [18] | Santoriello et al. [11] | Su et al. [13] | Puelles et al. [16] |
|---|---|---|---|---|---|---|---|
| Country | USA | USA | UK | Belgium | USA | China | Germany |
| n | 14 | 12 | 9 | 17 | 42 | 26 | 27 |
| Age, median (IQR), years | 73.5 (67.5–77.2) | 75.0 (57.5–77.2) | 73.0 (52.0–79.0) | 72.0 (62.0–77.0) | 71.5 (38.0–97.0) | 69.0 (39.0–8.07) | NA |
| AKI, % | 42.9 | 100.0 | NA | 82.3 | 94.0 | 34.6 | NA |
| CKD, % | 57.1 | 8.3 | 10.0 | NA | 28.6 | 10.0 | 44.0 |
| DM, % | 35.7 | 33.3 | 20.0 | 52.9 | 42.0 | 15.0 | NA |
| FSGS, % | 7.1 | NA | 0.0 | NA | 3.0 | 7.7 | NA |
| ATI/ATN, % | 78.6 | 100.0 | 100.0 | NA | 62.0 | 100.0 | NA |
| AS, % | 78.6 | 83.3 | 83.3 | NA | 85.0 | 69.2 | NA |
| Endothelial injury, % | NA | 0 | 16.7 | 0 | 14.0 | 11.5 | NA |
| Positive SARS-CoV-2 results/total no. of cases tested | 2/4 IHC |
0/12 IHC 0/4 ISH |
NA | 10/17 virus detected by RT-PCR, IHC not done on kidneys | 0/10 ISH | 3/6 IF | Unclear number tested and number positive by ISH and IF |
| Method of detection | Mouse anti-SARS-CoV-2 nucleocapsid protein (Clone 1C7, Bioss, Woburn, MA, USA) | Mouse anti-SARS-CoV-2 nucleocapsid protein (Clone 1C7, Bioss, Woburn, MA, USA) and The RNAscope® 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, CA, USA) | NA | (Invitrogen, PA1-41098, dilution 1:50) on Dako Omnis (Agilent Technologies, Santa Clara, CA, USA) and Maxwell RSC DNA FFPE Kit (reference: AS1450). Promega Corporation, Madison, WI, USA | The RNAscope® 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, CA, USA) | Anti-SARS-CoV-2 nucleoprotein antibody (40143-T62, Sino Biological, Beijing, China) | The RNAscope® 2.5 HD Duplex Assay (Advanced Cell Diagnostics, Newark, CA) and two commercially available antibodies for SARS-CoV-2 detection: spike glycoprotein antibody [3A2] (Abcam, ab272420) and SARS-CoV-2 SΔ10 within S2 domain protein (Genetex, GTX632604) |
CKD, chronic kidney disease; DM, diabetes mellitus; NA, not available.
The total percentages of biopsy findings may exceed 100% because each kidney biopsy could have multiple findings.
ATI
Evidence to date shows that the vast majority of AKI in patients with COVID-19 is related to ATI. In clinical practice, based on history alone, ATI seems to be the most common finding in patients with COVID-19 and AKI [24]. Several publications examining native kidney biopsy and autopsy tissue have demonstrated ATI as the most common pathologic finding in patients with COVID-19 and concomitant AKI or proteinuria [9, 10]. In a multicenter study of 17 patients, ATI was seen in 14 patients often in association with other glomerular and or endothelial changes [12]. In a case series of 10 patients with COVID-19 and AKI or proteinuria, ATI was demonstrated in all patients [10] (Figure 2). Kudose et al. reported ATI on the kidney biopsy, either as the primary finding or a secondary finding in 12 out of 17 patients with COVID-19 and AKI [9]. In some of these cases, a direct correlation existed between risk factors such as hemodynamic instability and development of AKI. Presence of myoglobin casts and ATI has been reported in two patients who developed rhabdomyolysis in association with COVID-19 [9, 10]. It is important to note that kidney biopsies are typically not done for patients with a clinical diagnosis of ATI. Thus, the kidney biopsy series that have been published reflect ‘for-cause’ biopsies and may not reflect the full extent of ATI in COVID-19 disease.
FIGURE 2.
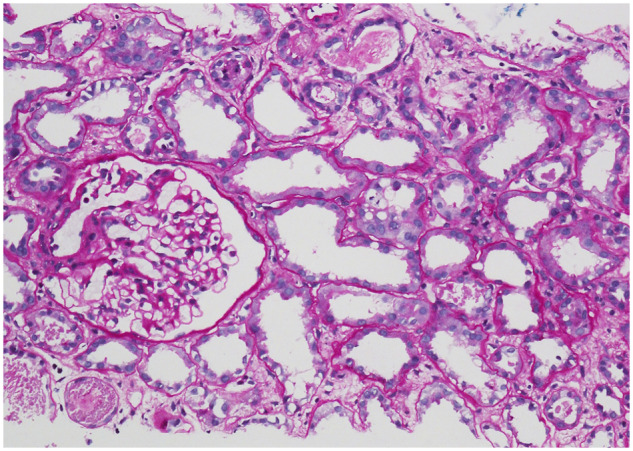
A 56-year-old male with a past medical history of hypertension, coronary artery disease presents with cough and shortness of breath. SARS-CoV-2 infection is confirmed. In 24 h, he gets intubated for worsening pulmonary function. Within 8 h of intubation, AKI ensues. He is on steroids and tocilizumab. Within the next 24 h, he requires kidney replacement therapy. A kidney biopsy is done and confirms ATI. Tubules reveal widespread distension of the lumens and flattening and degenerative changes of the epithelial layer, including vacuolization of the epithelial cell cytoplasm. Some tubules contain cellular debris. The glomerulus does not reveal specific changes (periodic acid–Schiff stain, ×200).
Proximal tubular damage with ATI has been described in patients with COVID-19. Werion et al. reported lab evidence of proximal tubular dysfunction in a subset of patients as suggested by low molecular weight proteinuria (70–80%), neutral aminoaciduria (46%) and defective handling of uric acid (46%) or phosphate (19%). Analysis of six kidney autopsy samples of patients who died of COVID-19 revealed prominent and diffuse proximal tubular damage in all patients with dilation of tubular lumen containing cellular debris, denuded basement membranes and major alterations of brush border [25].
Table 1 and Supplementary data, Table S1 summarize all published cases of ATI from live kidney biopsies. Thus far, there are nine cases with ATI as the primary diagnosis with no other lesions. In those nine patients, 44.4% required renal replacement therapy and 66.7% are alive at the time of this writing (November 2020). In addition, 28 cases of ATI associated with CG, 2 with myoglobin cast nephropathy and 2 with oxalate nephropathy have been reported.
Autopsy data available from histologic evaluation of the kidneys in patients dying from COVID-19 have been notable for the presence of ATI and necrosis, which was the main factor correlating with AKI [8, 11]. In one of the first autopsy series published from China, 26 autopsies were performed for patients with COVID-19 [13]. These cases were all rapid autopsies with a post-mortem interval of ≤6 h, which reduces autolytic artifacts within tissue. All cases in the series showed mild to severe ATI. ATI was characterized by a loss of the proximal tubular brush borders, vacuolar degeneration (non-isometric in most cases), frank epithelial cell necrosis (four cases), pigmented granules within tubular cytoplasm (four cases) and pigmented casts in tubular lumens (three cases). In another retrospective study of 81 patients in a single center in China, a total of 41 (50.6%) patients experienced AKI [26]. In that study, they mentioned a limited autopsy of 10 patients showing pathologic findings consistent with ATI [26]. Combined data from Northwell Health, Columbia University Medical Center and New York University in the USA showed that 76% (38/50) of autopsies had ATI with varying degrees of severity [8]. Another autopsy series from the UK found evidence of ATI in all nine patients examined [23] (Table 2). Artifactual autolysis in autopsy specimens can be difficult to distinguish from ATI, hence more credence should be given to kidney biopsies for this diagnosis.
Glomerular diseases
Several glomerular diseases have been described in association with COVID-19. It is difficult to ascertain if development of the glomerular disease is directly related to the SARS-CoV-2 infection or whether this infection acts as a ‘second hit’ in patients who already have a predilection for glomerular pathology such as those with high-risk Apolipoprotein L 1 (APOL1) genotype. The other possibility is that this represents an incidental finding unrelated to the SARS-CoV-2 infection. Evidence of nephrotic-range proteinuria and CG has been reported in patients with COVID-19 [27, 28]. Tubuloreticular inclusions (TRIs) known as ‘interferon footprints’ have been described in glomerular endothelial cells, which may reflect SARS-CoV-2-induced excessive production of cytokines including interferon. The most common glomerular disease noted with COVID-19 is CG.
Collapsing glomerulopathy (CG)
Collapsing glomerulopathy has emerged as a distinct pathology associated with SARS-CoV-2 infection (Figure 3), which seems to specifically affect individuals of African ancestry who have high-risk APOL1 genotypes (G1/G1, G1/G2 or G2/G2). To date, 32 cases of CG, either in isolation (28 cases) or in combination with other pathology findings (4 cases), have been published in patients with COVID-19 [9, 10, 12, 15, 28–35]. A total of 21 cases mentioned had APOL1 polymorphisms (14 with homozygous G1/G1, 2 with homozygous G2/G2 and 5 with heterozygous G1/G2). All cases presented with AKI or proteinuria; 30/32 (96%) patients were of Black race or of African or African American (AA) heritage, 1 patient was of Asian (Indian) origin and 1 of Hispanic origin. Of the 28 isolated COVID-19-associated CG, 64.2% required dialysis and 89% are alive at the time of this writing. Table 3 describes the four additional cases of CG-associated mixed lesions along with TMA and other pathology findings. Three of those patients are on dialysis and all are alive.
FIGURE 3.
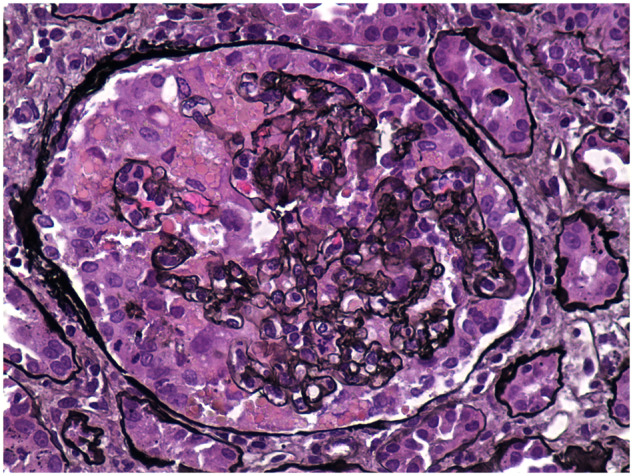
A 45-year-old male presents with cough, shortness of breath and fevers. He also complains of significant new lower extremity swelling and foamy urine. SARS-CoV-2 infection is confirmed and a 24-h urine reveals 15 g of proteinuria. His serum creatinine is 4.2 mg/dL. He is found to be positive for high-risk APOL1 genotype G1/G1. All serological workup is negative. A kidney biopsy reveals CG with ATI. This image reveals proliferation of glomerular epithelial cells over the collapsed segments of the capillary tuft . Some epithelial cells contain prominent intracytoplasmic protein reabsorption droplets (Jones methenamine silver stain, ×400).
Table 3.
Summary of kidney biopsies done in COVID-19 that have combined lesions showing various pathologies (predominant lesion listed first)
| Variable | Pathology | Age, years | Sex | Race/ ethnicitya | Presentation | SCr, mg/dLb | Proteinuria, g/g | KRTc | Kidney outcome | Patient outcome | Other information | Genetics | Country | Reference (#) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CG + TMA + ATI + TIN | 44 | Male | Hispanic | AKI, NS | 12 | 11.4 | Yes | Dialysis- dependent | Alive | APOL1+ | G2/G2 | USA |
Akilesh (AJKD) [12] |
| 2 | CG + TMA + ATI | 58 | Male | Black | AKI, NS | 11.3 | 4 | Yes | 1.5 | Alive | – | – | USA |
Akilesh (AJKD) [12] |
| 3 | TMA + CG + ATI | 47 | Male | Black | AKI, proteinuria | 6.6 | 7.6 | Yes | Dialysis- dependent | Alive | – | – | USA |
Akilesh (AJKD) [12] |
| 4 | TMA + CG + ATI | 63 | Female | Black | AKI, NS | 6 | 7.6 | Yes | Dialysis- dependent | Alive | – | – | USA |
Akilesh (AJKD) [12] |
| 5 | TMA + FSGS + ATI | 77 | Female | Hispanic | AKI, NS | 3.9 | 13.4 | Yes | Dialysis- dependent | Alive | – | – | USA |
Akilesh (AJKD) [12] |
| 6 | PIGN + DN + ATI | 69 | Female | White | AKI | 4 | 5.7 | Yes | Dialysis- dependent | Alive | Urinary tract infection | USA |
Akilesh (AJKD) [12] |
|
| 7 | FSGS + ATN + AIN | 59 | Male | Black | AKI, Proteinuria | 11.9 | 12 | NA | NA | NA | – | – | USA |
Akilesh (AJKD) [12] |
| 8 | DN + FSGS + ATI | 34 | Female | White | AKI, NS | 1.2 | 7 | No | 1.1 | Alive | – | – | USA |
Akilesh (AJKD) [12] |
| 9 | MCD + ATI | 25 | Male | Black | AKI, NS | 2.2 | 21.0 | No | 0.8 | Alive | APOL1+ | G1/G1 | USA |
Kudose (JASN) [9] |
AIN, acute interstitial nephritis; AKI, acute kidney injury; APLO1; apolipoprotein L1; ATI, acute tubular injury; CG, collapsing glomerulopathy; DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; KRT, kidney replacement therapy; MCD, minimal change disease; NA, not available; NS, nephrotic syndrome; PIGN, post-infection glomerulonephritis; TMA, thrombotic microangiopathy; SCr, serum creatinine; AJKD, American Journal of Kidney Diseases; JASN, Journal of American Society of Nephrology.
Race/ethnicity are cited directly from the source article.
Values refer to the peak SCr (mg/dL).
Values refer to SCr (mg/dL) upon discharge.
CG is a histopathological feature that has been associated with other viral infections; most characteristically seen in HIV-associated nephropathy, but also seen in Epstein–Barr virus, cytomegalovirus and parvovirus B19 infections [36]. APOL1 expression is upregulated by viral infections and other inflammatory diseases that activate interferons and toll-like receptor-3 [37]. Thus, in individuals with high-risk APOL1 genotypes (which are most common in persons of West African ancestry), SARS-CoV-2 infection and the resulting hyper-inflammation may act as a ‘second hit’ that leads to podocyte dysregulation, injury and development of CG [38]. Several investigators have now termed this association of CG with SARS-CoV-2 as COVID-19-associated nephropathy, or COVAN [39, 40].
Other podocytopathies
Other podocytopathies including minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) have been reported in patients with COVID-19 and nephrotic syndrome (NS). Kudose et al. reported an AA patient presenting with AKI and NS [9]. Pathology revealed diffuse effacement of the foot processes consistent with MCD along with ATI and TRIs. Interestingly, this patient was found to be homozygous for the high-risk APOL1 genotype (G1/G1). Another case of MCD was described in a 52-year-old woman presenting with NS [12]. It is possible that the viral infection triggers podocytopathy in some patients. Non-collapsing FSGS has also been described along with other pathologic findings likely representing underlying chronic disease (Supplementary data, Table S1 and Table 3).
Vasculitis
Glomerulonephritis such as anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis [10, 41, 42], anti-glomerular basement membrane (GBM) disease [43] and immunoglobulin A (IgA) vasculitis with nephritis [44] have been reported in patients with COVID-19. Three cases of ANCA vasculitis and six cases of anti-GBM disease have been reported so far. Most patients had severe kidney impairment with five out of nine patients requiring dialysis. All nine patients received treatment with various immunosuppressive therapies and were alive, with just one patient requiring dialysis at the time of discharge (Table 1).
Membranous nephropathy
Two cases of membranous nephropathy (MN) have been described, of which one was phospholipase A2 receptor antibody (PLA2R) positive by serum and another was PLA2R negative [9]. The authors postulated that since PLA2R is also present in the respiratory tract, it could be a potential source for antigen presentation to incite or potentiate anti-PLA2R autoimmune responses. This association could also be co-incidental.
Lupus nephritis
Crescentic transformation of long-standing pre-existing lupus nephritis presenting as AKI and NS in a patient with severe COVID-19, was reported by Kudose et al. [9]. This may highlight the role of an inflammatory milieu to exacerbate pre-existing immune-mediated diseases in patients with COVID-19.
Thrombotic microangiopathy
TMA has been described in patients with COVID-19 either as the primary finding or in combination with other pathologic features like acute tubular necrosis (ATN) and CG (Figure 4). There has been recent evidence suggesting that the signs and symptoms of severe COVID-19 resemble more the pathophysiology and phenotype of complement-mediated TMA [10, 45, 46] rather than sepsis-induced coagulopathy. In the study by Akilesh et al. a total of six patients with evidence of kidney-related TMA were described. Two patients had a history of prior gemcitabine exposure (one of whom was an active cocaine user) while another patient was noted to have low C3 level suggestive of activation of alternative complement pathway [12]. Four patients also had evidence of CG in addition to the TMA. Overall acute TMA and more subtle ultrastructural evidence of acute endothelial cell injury were present in 35% of the cases. Jhaveri et al. described a patient with diffuse cortical necrosis and widespread glomerular microthrombi, related to defects in alternate complement pathways [45]. Another case of TMA was demonstrated on the kidney biopsy in a patient with recent gemcitabine exposure [10, 47]. Of the patients with isolated TMA (three patients), two of them died and all three required dialyses [10, 45]. Endothelial dysfunction has been suggested as one of the main mechanisms of COVID-19-associated organ damage including AKI [13, 45, 48] The authors also postulate that COVID-19 can predispose patients to conditions that make them prone to endothelial injury like hypertension, prothrombotic states and antibody-mediated rejection (AMR) [12] Although intriguing, the relationship of TMA to COVID-19 remains speculative. Kudose et al. did not report any cases of TMA in their large cohort. CG and TMA can co-exist as stated by prior studies in relation to glomerular ischemia [49]. This poses an interesting cause and effect dilemma about TMA in COVID-19. In addition, some authors have proposed that the TMA seen with COVID-19 could be related to hydroxychloroquine [50, 51]. To our knowledge and at the time of this writing, no cases have been reported of kidney vein thrombosis associated with COVID-19, although kidney arterial thrombosis leading to kidney infarction or kidney infarctions has been reported [52–55].
FIGURE 4.
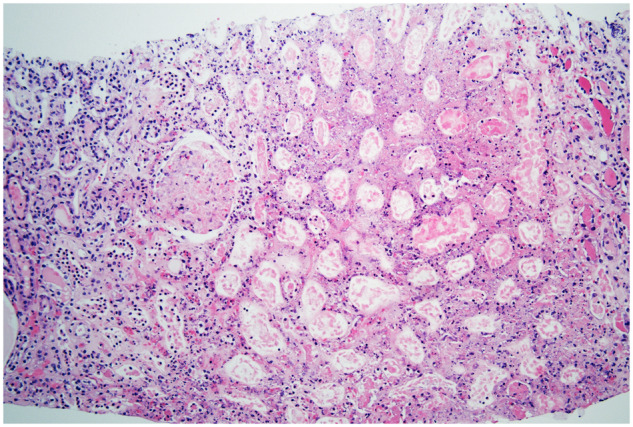
A 67-year-old female presents with a cough and fevers. SARS-CoV-2 pneumonia is confirmed. In the next few days, her laboratory data showed worsening anemia and thrombocytopenia. Her clinical course deteriorates where she requires intubation and pressor support. Her kidney function worsens requiring kidney replacement therapy. After platelet transfusion, a kidney biopsy is performed. The biopsy confirms cortical necrosis with acute TMA. Renal cortical necrosis is the most severe expression of TMA and is characterized by coagulative necrosis with degenerative changes of all cell types and involved structures, including tubules, glomeruli and the vasculature. Adjacent parenchyma may exhibit reactive changes (hematoxylin and eosin stain, ×100).
A post-mortem case-series (with a mean hypoxic delay of 33 h) demonstrated diffuse ATI [56], with 3/18 patients showing fibrin thrombi in glomerular capillaries. Thrombosis of the kidney microvasculature has also been highlighted in other post-mortem kidney samples from patients who received cardiopulmonary resuscitation before death [57]. The autopsy series from New York University found that in addition to findings of ATI, platelet-rich fibrin microthrombi were present in peritubular capillaries and venules in several cases [57]. In one case, there was TMA within the glomeruli with large platelet-rich microthrombi, red cell fragmentation and mesangiolysis. All of the cases exhibited mild to moderate arteriolosclerosis (AS), and one demonstrated arteriolar hyalinosis. Another autopsy series from the UK evaluated 10 patients. Interestingly, thrombotic features were observed in at least one major organ in all full autopsies, predominantly in the lung [8/9 (89%) patients], heart [5/9 (56%)] and kidney [4/9 (44%)]. Evidence of ATI has been noted in all nine patients examined [23] (Table 2).
Mixed pathology lesions
Several cases were reported where the kidney biopsy showed mixed lesions (Table 3) that are consistent with TMA along with CG and ATI. Several had other findings such as diabetic nephropathy (DN) and FSGS, along with post-infectious glomerulonephritis in one as well. Of the nine patients described in the literature with combined lesions that include, five of them are on dialysis and all are alive at the time of this writing [12] (Tables 1 and 3).
Interstitial nephritis
Acute interstitial nephritis (AIN) as a secondary finding in association with CG, FSGS and ATI has been reported in COVID-19 as well [12]. Other than that, AIN has not been reported with COVID-19.
Treatment-related AKI
Certain treatment-related causes of AKI in patients with COVID-19 such as the use of antiviral agents leading to tubulointerstitial diseases [58, 59], and two cases of biopsy-proven vitamin C-related oxalate nephropathy [60] have also been reported. In addition, kidney infarction has been postulated as a cause of AKI [9, 55]. As suggested earlier, some authors have postulated that the TMA seen with COVID-19 could have been treatment related [50, 51].
KIDNEY PATHOLOGY IN THE TRANSPLANTED PATIENT
AKI in the kidney transplant patient with COVID-19 has been described in eight patients in the literature (Table 4). Akilesh et al. describe three patients with a kidney transplant who had COVID-19 and were biopsied for a rising creatinine [12]. Two of these patients had microvascular inflammation and positive C4d staining in peritubular capillaries in association with elevated donor-specific antibodies , meeting Banff criteria for active antibody-mediated rejection. One patient also had IgA deposits (1–2+) on IF and a single arterial thrombus suggestive of TMA. The third patient had features of ATI on the biopsy and had rapid clinical resolution of AKI. Kudose et al. described three patients with kidney transplants and COVID-19, two of whom were biopsied for AKI [9]. One patient had Grade 2A cellular rejection and the other had ATI. The third patient who underwent a nephrectomy was found to have cortical infarction with severe interstitial fibrosis and tubular atrophy. CG has been described in an allograft recipient presenting with NS and AKI whose donor was found to have low-risk G0/G2 phenotype [61]. Yamada et al. reported a patient with NS, who had MCD on the kidney biopsy whose donor was found to be positive for high-risk APOL1 genotype G1/G1 [62]. In additon, studies have shown that CG can be present in TMA in the kidney allograft, possibly secondary to glomerular ischemia [49]. These pathologic findings represent the complex interplay of hemodynamic factors, cytokine storm, endothelial injury and development of donor-specific antibodies, all of which contribute to AKI in kidney transplant recipients. Similar to native kidneys, viral infections can precipitate CG in transplant recipients, even with low-risk APOL1 status of the donor.
Table 4.
Summary of kidney biopsies done in kidney transplant patients with COVID-19
| Pathology | Age, years | Sex | Race/ ethnicitya | Presentation | SCr, mg/dLb | Proteinuria, g/g | KRT | Kidney follow-upc | Patient outcome | Other | Country | Reference # | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ATI | 54 | Female | Hispanic | AKI | 2.9 | 0.2 | No | 2.2 | Alive | – | USA | Kudose (JASN) [9] |
| 2 | AMR | 47 | Female | Black | AKI | 1.63 | NA | No | NA | Alive | – | USA | Akilesh (AJKD) [12] |
| 3 |
IgA + FSGS+ Chronic AMR |
54 | Male | AA | AKI | 1.9 | 3.0 | No | 2.7 | Alive | – | USA | Akilesh (AJKD) [12] |
| 4 |
IgA + FSGS + TMA + chronic AMR |
42 | Male | Hispanic | AKI | 1.2 | 0.1 | No | 1.3 | Alive | – | USA | Akilesh (AJKD) [12] |
| 5 | Cellular rejection | 54 | Female | White | AKI | 2.6 | 0.2 | No | 2.1 | Alive | Received steroid, thymoglobulin and IVIG | USA | Kudose (JASN) [9] |
| 6 | Infarction | 22 | Male | Black | ESKD | 9.5 | NA | Yes | On dialysis | Alive | Nephrectomy sample | USA | Kudose (JASN) [9] |
| 7 | CG + ATI | 29 | Male | Sub-Saharan | AKI | 5.3 | 0.8 | No | 3.2 | Alive |
APOL1+ G1/G0 |
France | Lazareth (AJKD) [61] |
| 8 | MCD + ATI | 49 | Female | Black | AKI, NS | 3.5 | 6.3 | No | 1.3 and proteinuria resolved | Alive | Steroid | USA |
Yamada (Trans Proc) [62] |
AA, African American; AKI, acute kidney injury; APOL1, Apolipoprotein L1; ATI. Acute tubular injury; CG, collapsing glomerulopathy; KRT, kidney replacement therapy; MCD, minimal change disease; MN, membranous nephropathy; MCD, minimal change disease; NA, not available; NS, nephrotic syndrome; SCr, serum creatinine; TMA, thrombotic microangiopathy; FSGS focal segmental glomerulosclerosis; AIN, acute interstitial nephritis; DN, diabetic nephropathy; AMR, antibody mediated rejection; JASN, Journal of American Society of Nephrology; AJKD, American Journal of Kidney Diseases; Trans Proc, Transplantation Proceedings; IVIG, intravenous immunoglobulin.
Race/ethnicity are cited directly from the source article.
Values refer to the peak SCr (mg/dL).
Values refer to SCr (mg/dL) upon discharge.
CONCLUSION
In summary, SARS-Cov-2-infected patients with AKI can present with diverse pathological findings. Although direct viral infection of the kidney is possible, it is not a common or widespread finding at the time of this writing. ATI is the most common clinical and pathologic finding in patients with AKI, both in biopsy and autopsy tissue. CG is the most common glomerular disease often in association with high-risk APOL1 genotypes. The varied pathologic findings in the glomeruli may be a result of a heightened immune response in COVID-19 or may be coincidental. TMA is the third most common pathology noted. Of the three pathologies described, TMA has the worst prognosis, with all patients requiring dialysis. As our experience with COVID-19 grows, the kidney pathology findings will be better elucidated.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This article is part of a supplement supported by Fresenius Medical Care without any influence on its content.
CONFLICT OF INTEREST STATEMENT
K.D.J. is a consultant for Astex and Natera and is a paid contributor to Uptodate.com, and receives honorarium from the International Society of Nephrology and the American Society of Nephrology. V.B. receives honorarium from the International Society of Nephrology. None of the authors has conflicts of interests to declare.
Supplementary Material
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan L, Chaudhary K, Saha A. et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2020; 31: 2145–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robbins-Juarez SY, Qian L, King KL. et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep 2020; 5: 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng JH, Hirsch JS, Hazzan A. et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 2020. 10.1053/j.ajkd.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta S, Coca SG, Chan L. et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol 2020; 32: 161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradley BT, Maioli H, Johnston R. et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020; 396: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golmai P, Larsen CP, DeVita MV. et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol 2020; 31: 1944–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kudose S, Batal I, Santoriello D. et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma P, Uppal NN, Wanchoo R. et al. ; on behalf of Northwell Nephrology COVID-19 Research Consortium. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol 2020; 31: 1948–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santoriello D, Khairallah P, Bomback AS. et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 2158–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akilesh S, Nast CC, Yamashita M. et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis 2021; 77: 82–93.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farkash EA, Wilson AM, Jentzen JM.. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol 2020; 31: 1683–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kissling S, Rotman S, Gerber C. et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 2020; 98: 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puelles VG, Lütgehetmann M, Lindenmeyer MT. et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383: 590–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braun F, Huber TB, Puelles VG.. Proximal tubular dysfunction in patients with COVID-19: what have we learnt so far? Kidney Int 2020; 98: 1092–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Remmelink M, De Mendonça R, D’Haene N. et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care 2020; 24: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller SE, Brealey JK.. Visualization of putative coronavirus in kidney. Kidney Int 2020; 98: 231–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frelih M, Erman A, Wechtersbach K. et al. SARS-CoV-2 virions or ubiquitous cell structures? Actual dilemma in COVID-19 era. Kidney Int Rep 2020; 5: 1608–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dittmayer C, Meinhardt J, Radbruch H. et al. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet 2020; 396: e64–e65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng JH, Bijol V, Sparks MA. et al. Pathophysiology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis 2020; 27: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanley B, Naresh KN, Roufosse C. et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 2020; 1: e245–e253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohamed MMB, Lukitsch I, Torres-Ortiz AE. et al. Acute kidney injury associated with coronavirus disease 2019 in urban new Orleans. Kidney360 2020; 1: 614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Werion A, Belkhir L, Perrot M. et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int 2020; 98: 1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xia P, Wen Y, Duan Y. et al. Clinicopathological features and outcomes of acute kidney injury in critically Ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol 2020; 31: 2205–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaillard F, Ismael S, Sannier A. et al. Tubuloreticular inclusions in COVID-19–related collapsing glomerulopathy. Kidney Int 2020; 98: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu H, Larsen CP, Hernandez-Arroyo CF. et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol 2020; 31: 1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magoon S, Bichu P, Malhotra V. et al. COVID-19-related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med 2020; 2: 488–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsen CP, Bourne TD, Wilson JD. et al. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep 2020; 5: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma Y, Nasr SH, Larsen CP. et al. COVID-19-associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med 2020; 2: 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta RK, Bhargava R, Shaukat AA. et al. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol 2020; 21: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Couturier A, Ferlicot S, Chevalier K. et al. Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J 2020; 13: 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peleg Y, Kudose S, D’Agati V. et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep 2020; 5: 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Izzedine H, Brocheriou I, Arzouk N. et al. COVID-19 associated collapsing glomerulopathy: a report of two cases and a literature review. Int Med J 2020; 50: 1551–1558 [DOI] [PubMed] [Google Scholar]
- 36. Chandra P, Kopp JB.. Viruses and collapsing glomerulopathy: a brief critical review. Clin Kidney J 2013; 6: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nichols B, Jog P, Lee JH. et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 2015; 87: 332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedman DJ, Pollak MR.. Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab 2016; 27: 204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Velez JCQ, Caza T, Larsen CP.. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol 2020; 16: 565–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nasr SH, Kopp JB.. COVID-19-associated collapsing glomerulopathy: an emerging entity. Kidney Int Rep 2020; 5: 759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moeinzadeh F, Dezfouli M, Naimi A. et al. Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy, a case report. Iran J Kidney Dis 2020; 14: 239–242 [PubMed] [Google Scholar]
- 42. Uppal NN, Kello N, Shah HH. et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep 2020; 5: 2079–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prendecki M, Clarke C, Cairns T. et al. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int 2020; 98: 780–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suso AS, Mon C, Oñate Alonso I. et al. IgA vasculitis with nephritis (Henoch-Schönlein purpura) in a COVID-19 patient. Kidney Int Rep 2020; 5: 2074–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jhaveri KD, Meir LR, Flores Chang BS. et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int 2020; 98: 509–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Izzedine H, Jhaveri KD, Perazella MA.. Vascular injury and COVID-19-related mortality: what lies below the tip of the iceberg? Clin Nephrol 2020; 94: 11–13 [DOI] [PubMed] [Google Scholar]
- 47. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teuwen LA, Geldhof V, Pasut A. et al. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020; 20: 389–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nadasdy T, Allen C, Zand MS.. Zonal distribution of glomerular collapse in renal allografts: possible role of vascular changes. Hum Pathol 2002; 33: 437–441 [DOI] [PubMed] [Google Scholar]
- 50. Wanchoo R, Barilla-LaBarca ML. et al. Response to thrombotic microangiopathy: COVID-19 or hydroxychloroquine? Kidney Int 2020; 98: 162032976849 [Google Scholar]
- 51. Hasbal NB. Thrombotic microangiopathy: COVID-19 or hydroxychloroquine? Kidney Int 2020; 98: 1619–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kashi M, Jacquin A, Dakhil B. et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res 2020; 192: 75–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Philipponnet C, Aniort J, Chabrot P. et al. Renal artery thrombosis induced by coronavirus disease 2019. Clin Kidney J 2020; 13: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ammous A, Ghaffar MA, El-Charabaty E. et al. Renal infarction in COVID-19 patient. J Nephrol 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Post A, den Deurwaarder ESG, Bakker SJL. et al. Kidney infarction in patients with COVID-19. Am J Kidney Dis 2020; 76: 431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Menter T, Haslbauer JD, Nienhold R. et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020; 77: 198–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rapkiewicz AV, Mai X, Carsons SE. et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine 2020; 24: 100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Binois Y, Hachad H, Salem JE. et al. Acute kidney injury associated with lopinavir/ritonavir combined therapy in patients with Covid-19. Kidney Int Rep 2020; 5: 1787–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arrestier R, Stehle T, Letavernier E. et al. Lopinavir-ritonavir associated acute kidney injury is not related to crystalluria in critically-ill COVID-19 patients. Kidney Int Rep 2020; 5: 2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fontana F, Cazzato S, Giovanella S. et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients With COVID-19. Kidney Int Rep 2020; 5: 1815–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lazareth H, Péré H, Binois Y. et al. COVID-19-related collapsing glomerulopathy in a kidney transplant recipient. Am J Kidney Dis 2020; 76: 590–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamada M, Rastogi P, Ince D. et al. A case report: minimal change disease with nephrotic syndrome associated with COVID-19 after APOL1 risk variant kidney transplantation. Transplant Proc 2020; 52: 2693–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.