Abstract
There are an estimated 1.8 billion Muslims worldwide, with the majority of them choosing to fast during the month of Ramadan. Fasting, which requires abstinence from food and drink from dawn to sunset can be up to 20 h per day during the summer months in temperate regions. Fasting can be especially challenging in patients on haemodialysis and peritoneal dialysis. Moreover, there is concern that those with chronic kidney disease (CKD) can experience electrolyte imbalance and worsening of renal function. In this article, current literature is reviewed and a decision-making management tool has been developed to assist clinicians in discussing the risks of fasting in patients with CKD, with consideration also given to circumstances such as the coronavirus disease 2019 pandemic. Our review highlights that patients with CKD wishing to fast should undergo a thorough risk assessment ideally within a month before Ramadan, as they may require medication changes and a plan for regular monitoring of renal function and electrolytes in order to fast safely. Recommendations have been based on risk tiers (very high risk, high risk and low–moderate risk) established by the International Diabetes Federation and the Diabetes and Ramadan International Alliance. Patients in the very high risk and high risk categories should be encouraged to explore alternative options to fasting, while those in the low–moderate category may be able to fast safely with guidance from their clinician. Prior to the commencement of Ramadan, all patients must receive up-to-date education on sick-day rules and instructions on when to terminate their fast or abstain from fasting.
Keywords: chronic kidney disease, COVID-19, dialysis, fasting, pandemic, Ramadan
INTRODUCTION
There are an estimated 1.8 billion Muslims worldwide [1]. These include large minority populations in many Western countries—there are approximately 2.7 million Muslims in the UK constituting 4.8% of the British population [1]. With the increasing prevalence of chronic kidney disease (CKD) in the UK and Europe [2] combined with globalization, nephrologists are likely to have patients asking for their opinion on the suitability of fasting.
The fast of Ramadan is observed by abstaining from food and drink, including water, from sunrise to sunset [3]. However, those who are ill or have underlying health conditions can be religiously exempt. The dates for Ramadan are based on a 12-month lunar calendar, thus Ramadan falls 11 days earlier annually and over an ∼33-year period passes through all four seasons, leading to shorter fasts in the winter months and longer fasts in the summer months of the northern hemisphere. Fasting during the summer months can be up to 20 h in temperate regions [4].
Medical research on Ramadan is a nascent field and many studies are observational in nature and findings not generalizable. Given the lack of formal guidance and expert consensus, the aim of this review is to provide an up-to-date appraisal of current literature and to subsequently provide healthcare professionals (HCPs) with an easy decision-making tool that can facilitate and guide discussions relating to the risks of fasting with their CKD patients. Although fasting is an obligation for Muslims, it remains a personal choice. Many Muslims, especially with chronic diseases, tend to fast against medical advice [5, 6], and these guidelines would aid clinicians and patients alike in supporting them in the decision-making process.
METHODS
We undertook a narrative review of current literature on Ramadan fasting in patients with CKD. The PubMed and Google Scholar databases were searched for studies using the terms ‘Ramadan’, ‘fasting’, ‘kidney’, ‘dialysis’ and ‘chronic kidney disease’ in various combinations. All retrieved articles were considered for inclusion and existing reviews of literature on these topics were also reviewed for references where appropriate. No language or study restrictions were applied but, for practical considerations, only English-language articles were reviewed. Retrieved articles included systematic reviews, observational studies and narrative reviews. While the focus in this review relates to the effect of fasting in CKD, fasting during the coronavirus disease 2019 (COVID-19) pandemic is briefly discussed.
Recommendations on risk stratification and management of patients were made through the consensus of authors based on their clinical experience of managing Muslim patients fasting during Ramadan. They are meant to be informative and do not form a directive; treating physicians need to take into consideration the patient’s wishes and individual circumstances. We utilized a three-tiered risk assessment based on the widely used criteria established by the International Diabetes Federation and the Diabetes and Ramadan International Alliance (IDF-DAR) to form recommendations [2]. Recommendations in Table 5 can be used by HCPs to assign a risk level and provide fasting advice accordingly [7].
Table 5.
Categorizing risk based on IDF-DAR risk categories
| Risk level | Low–moderate risk | High risk | Very high risk |
|---|---|---|---|
| Advice | Listen to medical advice | Should not fast | Must not fast |
| CKD stage | Stages 1–3 with stable kidney function | Stages 1–3 with unstable kidney functiona |
Stages 4–5 (non-dialysis)b Patients on all forms of HD and PD Stages 3–5 patients with a history of pre-existing cardiovascular disease |
| Other groups | CKD patients prone to urinary tract infections or stone formation |
CKD patients with known electrolyte abnormalities. Patients at risk of dehydration due to fluid restriction requirements or need for diuretics. Patients on ACE-Is/ARBs, SGLT2 inhibitors and mineralocorticoid receptor antagonists |
Patients on tolvaptan Pregnant CKD patients |
Unstable patients includes those with rapidly declining GFR, history of fluid overload and frailty.
Although HD and PD patients would be considered very high risk, a select group may be able to fast following risk stratification and counselling. Factors to consider include residual renal function, fluid balance, potassium >6.0 mmol/L, motivation, compliance with medical advice, considered alternatives to fasting and winter fasting.
RESULTS
No randomized controlled trials (RCTs) were identified; all studies were observational in nature. The majority of the studies were carried out in Middle Eastern countries during the winter season where the fasting duration ranged from 12 to 14 h. In contrast, the fasting duration in temperate regions during the summer months can be up to 20 h and therefore findings are not generalizable (Table 1). Although there are data on hospital attendance due to renal colic during Ramadan, no data are available on the rate of hospitalization due to acute kidney injury (AKI) or hyperkalaemia [8]. Reported differences in studies refer to statistically significant differences. A review of the literature is presented below.
Table 1.
Approximate start dates for Ramadan and duration of fasts for 2021–51
| Year | Approximate start date of Ramadana | Duration of first fast in Londonb, h | Mean daytime temperature in London, °C | Duration of first fast in Riyadh, h | Mean daytime temperature in Riyadh, °C |
|---|---|---|---|---|---|
| 2021 | 13 April | ∼15.5–16.5 | 13 | ∼13–14 | 33 |
| 2031 | 16 December | ∼9.5–10 | 6 | ∼11.5–12.5 | 22 |
| 2041 | 28 August | ∼15.5–16.5 | 19 | ∼13–14 | 43 |
| 2051 | 12 May | ∼17.5–18.5 | 16 | ∼14–15 | 39 |
Dates may differ by a day either side depending on methodology used to determine the new moon.
Scholarly difference of opinion exists in relation to the onset of dawn in temporate regions in the summer.
Dates for Ramadan are based on a 12-month lunar calendar, thus Ramadan falls 11 days earlier annually and over a ∼33-year period passes through all four seasons.
Data were taken from www.islamicfinder.org.
CKD
A systematic review by Bragazzi [9] identified 26 studies, of which 5 concerned CKD non-dialysis (CKD-ND) and dialysis (CKD-D) patients. Only 11/26 were done in cold seasons and 3/26 were in hot seasons; there were incomplete data for the remainder. These studies reported fasting overall is well tolerated in patients with CKD, with some caveats. Bragazzi [10] also conducted a meta-analysis from six studies with 350 CKD patients during Ramadan and monitored changes in estimated glomerular filtration rate (eGFR). Of these studies, only two were done in CKD-D and CKD-ND patients, the rest were in transplant recipients. Only one study included patients with CKD-ND Stage 5 (total of five patients; Table 2) [13]. Pooled results from all six studies did not show significant differences in the change in GFR {standardized mean difference ± SD of 0.00 ± 0.098 [95% confidence interval (CI) −0.19–0.19]; P = 0.99}.
Table 2.
Published studies in non-dialysis CKD patients
| Author | Year | CKD stages (non-dialysis) | No. of patients | Outcome measure | Result |
|---|---|---|---|---|---|
| Al Muhanna [11] | 1998 | Moderate to severe CKD, CrCl <35 mL/min | 36–18 males and 18 females | Change in renal function (CrCl) | CrCl pre-Ramadan 17.2 ± 3.5 mL/min, end of Ramadan 13.2 ± 2.2 mL/min and 2 weeks later 13.7 ± 3.2 mL/min |
| El-Wakil et al. [12] | 2007 | Mean GFR for study group 33.3 ± 21.1 mL/min; for controls 111.6 ± 21.3 mL/min | 12 (40% males) and 6 controls (100% males) | Change in GFR measured by technetium-99m DTPA and NAG | Change in GFR not statistically significant with −6.56 ± 31.1% change in CKD patients compared with 9.58 ± 30.1% in controls (p < 0.43). Although NAG was different between CKD and control group, there was no statistically significant difference in NAG within the CKD group pre- and post-Ramadan |
| Bernieh et al. [13] | 2010 | CKD Stages 3–5 | 31 (61.3% males) | CrCl (Cockcroft Gault), albumin, lipids, weight | CrCl increased post-Ramadan compared with pre-Ramadan. This could be explained by observed decease in body weight |
| Al-Wakeel [14] | 2014 | CKD Stages 3 and 4 (dialysis cohort excluded in this table) | 39 (23.1% males) | Change in renal function (CrCl) | No significant change noted. Potassium pre-Ramadan 4.8 ± 0.6 mmol/L, post-Ramadan 4.7 ± 0.5 mmol/L. CrCl pre-Ramadan 40.8 ± 25.4 mL/min and post-Ramadan 44 ± 29.3 mL/min |
| NasrAllah and Osman [15] | 2014 | CKD Stages 3–5 | 106: 52 fasting (32% males), 54 non-fasting (27% males) | Cardiovascular outcomes | In the fasting group, 6 adverse cardiovascular events occurred compared with 1 in the control group. All of those affected in the fasting group had an associated decrease in eGFR. The mean deviation in eGFR in the fasting group was −3% (SD 17.8) compared with ±1.3% (SD 24.5) in the non-fasting group |
| Mbarki et al. [16] | 2015 |
Mean CrCl 72.85 ± 40 mL/min Group 1: <60 mL/min (20 patients), Group 2: 30–59 mL/min (26 patients), Group 3: 15–29 mL/min (5 patients) |
60 (41.6% males) | Development of AKI (as defined by KDIGO criteria) | Seven patients met the criteria for AKI. In five there was full recovery and in two there was partial. Follow-up was 1 week post-Ramadan and findings were not statistically significant |
| AA Bakhit et al. [17] | 2017 |
CKD Stages 3–5 (36 CKD Stage 3, 24 CKD Stage 4, and 5 CKD Stage 5) |
65 (61.5% males) |
Change in renal function (eGFR by CKD-EPI) pre- and 3 months post-Ramadan |
Mean eGFR 31.1 ± 13.3 mL/min and SCr 206 ± 88 μmol/L, mean increase during Ramadan to 214 μmol/L and a decrease to 209 μmol/L RR of worsening of renal function: CKD Stage 3B 1.6 (95% CI 0.5–5.4), CKD Stage 4 3.6 (95% CI 1–13.9), CKD Stage 5 2.2 (95% CI 0.7–6.5) |
| Kara et al. [18] | 2017 | CKD Stages 3–4 | 45 fasting (31% male) and 49 non-fasting (25% male) | Change in renal function (eGFR) | No difference within group or between groups |
| Ekinci et al. [19] | 2018 | CKD Stages 1–2 with ADPKD | 23 fasting (17.4% males) and 31 non-fasting (41.9% males) | Change in eGFR, electrolytes, KIM-1 and NGAL | No statistically significant difference in any of the observed measures |
| Hassan et al. [20] | 2018 | CKD Stages 2–4 | 31 fasting (54.8% males) and 26 non-fasting (53.8% males) | Change in eGFR | No significant difference found |
| Alawadi et al. [21] | 2019 | CKD Stage 3 | 19 (57.8% males) | Glucose level, change in blood pressure, HbA1c, renal function (eGFR) and BMI | No significant change found |
| Chowdhury et al. [22] | 2019 | CKD Stage 3 | 68 fasting (51.4% males) and 71 non-fasting (49.2% males) | Change in renal function (eGFR by MDRD) and urine PCR | No significant differences in biochemical parameters |
| Mahmoud and Barakat [23] | 2019 | CKD Stages 3–4 | 20 (60% females) | Renal function (eGFR by CKD- EPI) fatigue, mood and cognition | No change in renal function. However, fatigue, mood and cognition were worse when measured after Ramadan |
| Baloglu et al. [24] | 2020 | CKD Stages 2–3 | 117 (69.2% males) | Development of AKI (as defined by KDIGO criteria) | 27 developed AKI, history of hypertension was associated with AKI, unclear if AKI resolved and whether patients were on RAAS inhibitors or diuretics |
| Eldeeb et al. [25] | 2020 | CKD Stages 3–4 | 34 (58.8% females) and 37 controls (59.5% females) | Renal function (eGFR by CKD- EPI) central and brachial blood pressures | Improved central and brachial blood pressures, weight and creatinine were lower post-Ramadan |
ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CrCl, creatinine clearance; DTPA, diethylenetriaminepentaacetic acid; HbA1c, haemoglobin A1c; KDIGO, Kidney Diease: Improving Global Outcomes; KIM-1, kidney injury molecule 1; MDRD, Modification of Diet in Renal Disease; NAG, N-acetyl-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; PCR, protein:creatinine ratio; RAAS, renin–angiotensin–aldosterone system.
In a prospective cohort study from Saudi Arabia that enrolled CKD-ND and HD patients, metabolic profile and renal function change were studied before, during and 3–4 weeks after Ramadan [14]. Of the 39 CKD patients, 10 were CKD-ND Stage 5 and 19 CKD-ND Stage 4. Only stable CKD patients were enrolled; those with unstable renal function, episodes of pulmonary oedema or poorly controlled diabetes were excluded. Patients were given low potassium diet advice and asked to restrict fluid intake to 1.5–2 L. Antihypertensives and diuretics were continued but modified to once-daily preparations. Those with a tendency for developing hyperkalaemia on angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs) were switched to alternatives and advised on calcium or sodium polystyrene sulphonate. No significant change in weight, blood pressure, creatinine clearance, Modification of Diet in Renal Disease eGFR or electrolyte measurements were seen. None reported symptoms of uraemia, eight had lower limb oedema and no patients needed hospital admission. There was no comparator group (Table 2).
In a UK observational study by Chowdhury et al.[22], stable patients with coexisting CKD-ND Stage 3 and type 2 diabetes were enrolled during a 19-h fast. Sixty-eight patients fasted and 71 did not. They found no significant differences in outcome measures or adverse events [22].
An interesting cohort study in CKD-ND Stages 3–5 patients that looked at major adverse cardiovascular events (MACEs) found that fasting patients with a history of cardiovascular disease had a higher risk of MACEs; the hypothesis being that dehydration causes hyperviscosity. In a total of 131 patients in two groups (fasting and non-fasting), baseline serum creatinine (SCr) in the fasting group was 247 ± 123.8 μmol/L, an SCr increase of >30% at Day 7, with a sensitivity of 66.6% and specificity of 89% in predicting cardiovascular events. A increase in SCr was noted in 60.4% of patients by Day 7 of fasting and was associated with the intake of renin–angiotensin–aldosterone system antagonists [relative risk (RR) 2; P = 0.002]. Adverse cardiovascular events were observed in six patients in the fasting cohort and were associated with an increase in SCr after 1 week of fasting (P = 0.009) and the presence of pre-existing cardiovascular disease (RR 15; P = 0.001). In comparison, only one event was reported in the non-fasting group. The regression model adjusted for baseline characteristics including the presence of pre-existing cardiovascular disease but did not adjust for antihypertensive medication use, which was associated with an increase in SCr [15].
Long-term follow-up data of the effects of fasting during the month of Ramadan on renal function in patients with CKD-ND and CKD-D are lacking. A study by Ghalib et al. [26] looked at the effects of repeated fasting in kidney transplant recipients. They studied the effect of fasting over 3 consecutive years and found no difference in eGFR between fasters (56.4 mL/min) and non-fasters (55.4 mL/min).
RENAL REPLACEMENT THERAPY
Haemodialysis (HD)
A cross-sectional study of 635 Saudi patients on HD during the hot season showed no difference in outcomes. The following parameters were collected in the month prior to and during Ramadan (following the longer, 2-day interdialytic periods): weight gain, pre- and post-dialysis blood pressure, serum potassium, phosphorus, albumin, hypotensive episodes and saline volume infused during the dialysis session. No statistically significant differences were found in the measured parameters. In the fasting group, 22% of patients were working compared with 14.6% in the non-fasting group. There were only two dialysis shifts during the day, and as performing dialysis during fasting in the daytime would invalidate the fast, it is likely that patients observed fasting during non-dialysis days. Fasting patients were younger, working, missed dialysis sessions and had higher phosphate levels compared with the non-fasting group. No serious adverse events were reported in the fasting group (Table 3) [29].
Table 3.
Published studies on HD patients
| Author | Year | No of patients | Outcome measure | Result |
|---|---|---|---|---|
| Al-Khader [27] | 1991 | 40 | IDWG and change in electrolytes | Mean IDWG pre-Ramadan 2.2 ± 0.3 kg, during Ramadan 2.84 ± 0.35 kg, none presented with pulmonary oedema, patients fasted on non-dialysis days. Potassium mean pre-Ramadan 5.05 ± 0.4 mmol/L, during Ramadan 5.76 ± 0.45 mmol/L |
| Adnan et al. [28] | 2014 | 35 | Dialysis and biochemical parameters | Weight reduction was seen in all patients, no difference in IDWG, number of hypotensive episodes was lower at the end of Ramadan compared with pre-Ramadan. No difference in potassium or significant elevations. No difference in urea reduction ratio, albumin level was high and phosphate was lower at the end of Ramadan |
| Alshamsi et al. [29] | 2016 | 635 | Biochemical and dialysis parameters | Other than phosphate level, which was higher in the fasting group, no other differences in dialysis or biochemical parameters were observed |
| Imtiaz et al. [30] | 2016 | 252 did not fast and 34 fasted | Biochemical parameters | Albumin was higher in the fasting group, no other significant differences between groups |
| Khazneh et al. [31] | 2019 |
269 There were three groups: non-fasting; fasting, who fasted every day including dialysis days; and partially fasting, who fasted on non-dialysis days |
IDWG and biochemical parameters | Higher IDWG in the fasting group, higher potassium by 0.48 mEq/L in the fasting group compared with non-fasting |
| Megahed [32] | 2019 | 965 in fasting group and 1090 non-fasters | Dialysis parameters and mortality | Potassium was <5 mmol/L in all groups, mortality was higher in non-fasting group, patients in the fasting group were younger and had fewer comorbidities |
| Al Wakeel et al. [14] | 2014 | 32 | Biochemical parameters | Significant increase in phosphate, hyperkalaemia in 15.6% and hyponatraemia in 28%. No hospital admissions were observed |
| Adanan et al. [33] | 2020 | 87 | BMI, interdialytic weight gain and dialysis parameters | Intermittent fasting during Ramadan led to reduced BMI, IDWG and other nutritional parameters. Improvement seen in phosphate, albumin and urea levels |
BMI, body mass index.
In a 12-week multicentre observational study in Malaysia of 87 patients where patients formed their own controls, nutritional and functional parameters compared 2 weeks before and 4 weeks after Ramadan did not show significant changes. Interestingly, handgrip strength improved post-Ramadan, along with phosphate control and interdialytic weight gain (IDWG) [33]. The time point of biochemical parameter measurements in relation to a patient’s weekly dialysis schedule is not known. Data on whether participants were working or whether they observed fasting on dialysis days was also not provided.
In a recent study from Palestine conducted during the summer, 269 HD patients were divided into three groups: those who fasted daily, those who partially fasted (observed fast on non-dialysis days) and those who did not fast. Biochemical parameters were measured 1 month prior, in the second week of and 2 weeks after Ramadan. The mean age was 57.5 years and the average fasting duration was 16 h. The reference non-fasting group mean IDWG was 3.2 kg, whereas the fast group was noted to have an additional IDWG of 0.62 kg (P = 0.001) and the partially fasting group was 0.23 kg (P = 0.005). Potassium levels in all groups were <6.0 mmol/L [31], although the fasting group had a higher mean potassium than those who did not fast (P < 0.001). Adverse events or hospitalizations were not recorded. There were no significant differences between the fasting groups in age (P = 0.064), gender (P = 0.202), dialysis vintage (P = 0.202) or hypertension status (P = 0.765), however, the non-fasting group had a higher proportion of diabetic patients (58.1%) than the fasting (38.7%) and partial fasting groups (31.6%) (P < 0.001). Biochemical parameters were tested before a dialysis session and it is unclear if any were done after a weekend off period.
With respect to mortality, retrospective data of 1841 patients from 1989 to 2012 in a single centre in Karachi, Pakistan, showed a higher mortality during Ramadan for patients on HD, but fasting status was not determined and other than a percentage, no other statistical analysis was performed to justify the observation [34]. In contrast, a recent observational study from Egypt compared 965 patients who fasted at least partially with 1090 who did not and found mortality to be higher in the non-fasting group. This could be explained by the fact that patients in the fasting group were younger and had fewer comorbidities; no regression analysis was done to adjust for confounders [32].
Peritoneal dialysis
Only one study was identified that reported outcomes of fasting in peritoneal dialysis (PD) patients. Stable patients were included and patients with uncontrolled diabetes, hypertension, angina, postural hypotension or a history of non-compliance with therapy were excluded (Table 4). Dialysis prescriptions were modified. Continuous ambulatory peritoneal dialysis (CAPD) patients performed three exchanges at night with either a 1.36% or 2.27% strength PD fluid (after dusk) and an icodextrin daytime fill. Similarly, continuous cycling peritoneal dialysis (CCPD) patients performed a rapid 6-h cycle starting at 2100 and ending at 0300 with an icodextrin daytime fill. Of the 31 patients in the study, less than a quarter (22.5%) developed complications but did not experience serious morbidity. Two patients developed peritonitis that required admission and discontinuation of modified therapy, one developed pleural effusion and three experienced hypotension, two in the CAPD group and one in the CCPD group. In the CCPD group, one patient developed lower limb oedema that was managed by adjusting the dialysate concentration and fluid restriction. Urine output, Kt/Vurea and creatinine clearance were not statistically different when compared pre- and post-Ramadan according to the authors, although no P-values were reported [35].
Table 4.
Published study on PD patients
| Author | Year | No. of patients | Outcome studied | Result |
|---|---|---|---|---|
| Al-Wakeel et al. [35] | 2013 | 31 | Safety and adequacy | No statistically significant difference in urine output or Kt/V urea |
Kt/Vurea as a measure of dialysis adequacy.
DISCUSSION OF EXISTING DATA
All identified studies were observational, which are prone to selection bias, and had small sample sizes. It is likely that the patients in the studies are more motivated and willing to adhere to dietary advice and fluid restriction than others. Other than in one study where patients with pre-existing cardiovascular disease were included, all other studies excluded unstable patients or patients with significant comorbidities. Treatment changes had to be made to medications and in some studies to dialysis therapy. It is also important to note that the majority of the literature in this area is from the Middle East and North Africa, where the duration of fasting is ~12–14 h, with reduced working hours, compared with European countries where fast durations can be as long as 20 h, with normal work hours. Hence the findings are not generalizable (Table 1).
CKD
The majority of the studies included stable CKD-ND Stage 3 patients and only a small number of CKD-ND Stage 4 and an even smaller number of CKD-ND Stage 5 patients. In the reported literature, despite study design flaws, it appears that stable CKD-ND Stage 3 patients are able to observe the fast without any ill consequences as long as they are closely monitored and adhere to medical advice. The same cannot be said about CKD-ND Stages 4 and 5 patients, who are at higher risk of renal function deterioration due to dehydration and life-threatening electrolyte abnormalities. The studies that included CKD-ND Stages 4–5 patients did not have a comparator group, therefore it is difficult to make an evidence-based conclusion in this group; patients would have to be motivated and compliant with advice in order to fast safely. In addition, patients with CKD and known cardiovascular disease should be discouraged from fasting given the concerns raised in one study about adverse cardiovascular outcomes [15].
Whether repeated fasting in subsequent years has long-term effects is also unknown, although studies on transplant recipients showed no long-term consequences. Some CKD patients may be prone to stone formation and urinary tract infections. Meeting their hydration requirements after breaking their fast is key if they want to fast [8, 36].
HD
In the reported literature, HD patients were younger. In contrast, the mean age of HD patients in the UK is 67 years [31, 37], with frailty as a common comorbidity [38]. Dialysis is a catabolic state and dialysis patients normally have to follow a restricted diet of low potassium and phosphate. While fasting, the difficulty imposed with dietary restrictions in such patients can make it difficult to meet their nutritional requirements, further increasing their frailty and adding to morbidity. Although published studies showed no significant adverse effects, electrolyte imbalances and higher IDWGs have been observed, as has increased mortality during Ramadan [14, 31, 34].
PD
In the single reported study of patients on PD, patients had to undergo modification of their treatment regime in order to fast [35].
RELIGIOUS CONSIDERATIONS ON FASTING AND RECOMMENDATIONS PERTAINING TO CKD-ND AND CKD-D PATIENTS
As a general rule, any form of nutrition and medication that involves administration via a mucosal route, i.e. oral, nasal or rectal, is not permitted for a person observing the fast; this includes any form of fluid including water. The use of topical, intramuscular or subcutaneous medications such as insulin is permitted and does not invalidate the fast [2]. While there are religious edicts (fatawaa) that state that fasting is NOT invalidated by HD [39], there is a contemporary scholarly view that such treatments while fasting are impermissible and invalidate the fast. However, patients may choose to fast on non-dialysis days or with modifications to the PD prescription, and this is the position adopted by the authors of this review [40, 41].
It is traditional practice to have two meals over a 24-h period: Suhoor (also known as Sehri), the pre-dawn meal, and Iftaar, the sunset meal. It is also worth noting that fasting [35] has been associated with beneficial changes in general well-being [3]. Ultimately the decision to fast or not rests with the individual concerned. Islam permits, and indeed supports, those with appropriate ailments to terminate the fast or be exempted from fasting, the two main options being:
Making up the missed fast when health permits them to do so, either when the illness is no longer present, such as in acute illness, or when health is not worsened by fasting at another point in time (e.g. in the winter) [3].
An exemption from fasting in those whose illness will not permit them to fast, this being replaced by a requirement to feed the poor, known as fidyah.
Appropriate ailments that can also include old age/frailty or a condition that, through fasting, can adversely affect the person’s health [1, 4]. This also includes abstaining from the use of medication that increases the risk of decompensation of chronic, but stable health conditions. Arriving at such decisions is based on the premise that the ailment will worsen or recovery will be delayed or impaired by fasting or a substantial fear that either may occur. This can be determined by prior experience of fasting with the ailment, common knowledge that this can happen or on the advice/opinion of an appropriate HCP [5]. Muslims are encouraged if they have any uncertainty regarding the various dispensations to seek counsel from a trusted religious authority.
Despite valid exemptions, there is an intense desire to fast during this month even among those who are considered high risk, e.g. the elderly and multimorbid, individuals with diabetes mellitus and patients with CKD [6, 15]. A more detailed exposition pertaining to the religious obligation of fasting and practical considerations in relation to health and illness with particular consideration to diabetes has been published elsewhere [3].
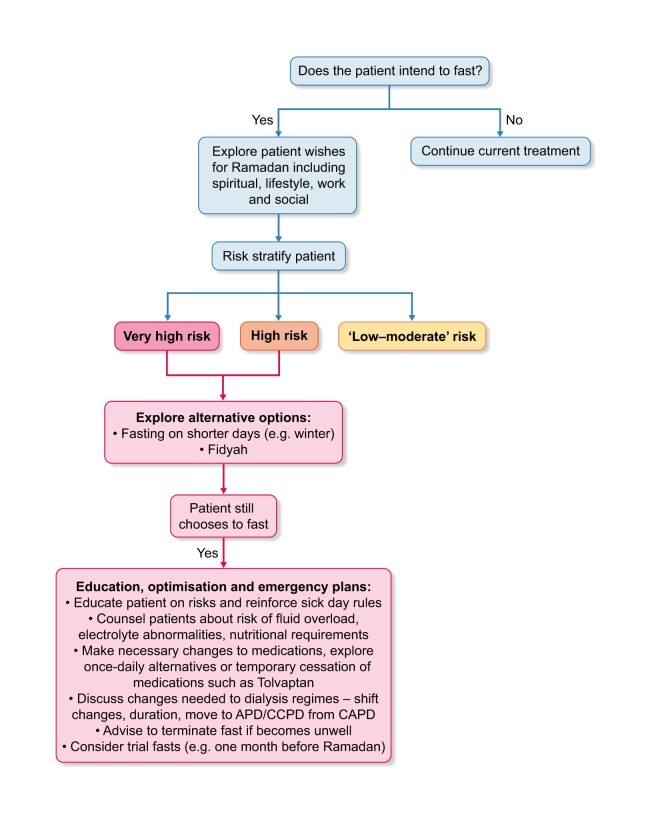
The IDF-DAR convened recognized experts to develop joint practical guidelines on the management of diabetes during Ramadan. The guidance is based on three risk categories: very high risk, where patients must not fast; high risk, where patients should not fast; and low–moderate risk, where patients should be guided by their clinician. The guidance was approved by religious scholars [2]. We have developed a decision-making tool based on the principles of the IDF-DAR risk tiers that HCPs can use when counselling patients regarding the risk of complications if they fast. Consensus was sought from renal physicians involved in the care of Muslim patients and a religious scholar while developing our guidance. Patients in the very high risk and high risk tiers should receive medical advice that they must not fast and should not fast, respectively. Those in the low–moderate risk category may be able to fast at the discretion of their physician as to the ability of the individual to tolerate the fast (Table 5). During counselling, patients and HCPs should consider the alternatives, which for some patients may be safer options (see Figure 1).
FIGURE 1.
Decision-making pathway when a patient wishes to fast during Ramadan.
Trial fasting
Following any necessary medication or dialysis treatment changes, patients should consider a trial of fasting for a few days prior to the start of Ramadan (we suggest within the month prior) with close monitoring to establish safety and tolerability.
Winter fasting
In temperate regions, the time period between dawn and dusk can be as short as 8–10 h in the winter, and for many patients this may be tolerable and safely achievable without changes in medication or dialysis regimes [4]. It is also important to reinforce sick day rules on which medications to stop during an acute illness; examples include ACE-Is, ARBs, tolvaptan, sodium–glucose cotransporter-2 inhibitors, mineralocorticoid receptor antagonists and diuretics.
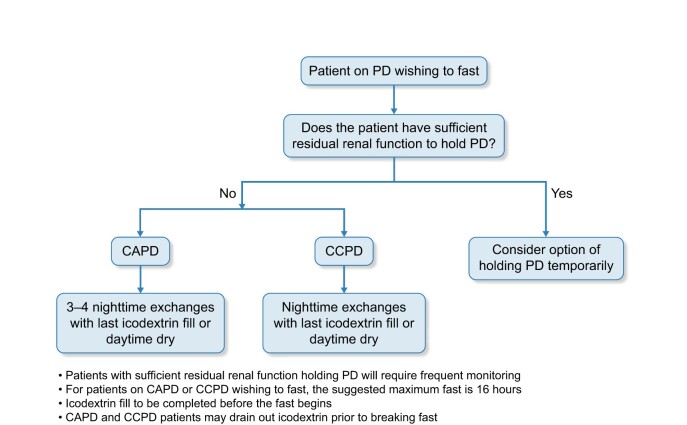
Options for PD patients
The decision-making tool in Figure 2 was developed specifically for PD patients wishing to fast.
FIGURE 2.
Decision-making pathway for PD patients wishing to fast.
For both modalities, volume, strength of fluid and treatment duration are as per the treating clinician’s prescription. If required, patients may be able to drain icodextrin before completion of the fast. Daytime dry may be an option for some patients. We would advise patients to fast when the total duration of the fast does not exceed 16 h in order for exchanges to be carried out adequately over a minimum 8-h period.
HD patients
Options for HD patients wishing to fast are limited. Some may be able to fast on non-dialysis days after individual risk stratification while others should consider the alternatives discussed above.
Specific considerations in the context of the COVID-19 pandemic
Although the world is in a different phase of the pandemic, it is far from over. Therefore patients and clinicians may want to consider local factors when counselling patients about the risks and feasibility of fasting during the pandemic.
CKD-ND
Logistical challenges of having blood tests during the pandemic may mean monitoring is not readily available. Patients who in normal circumstances may be able to observe the fast must consider alternatives to fasting.
HD and PD
Some dialysis units in the UK moved to twice-weekly sessions during the peak of the pandemic. Potassium levels were managed with binders along with dietary restrictions. Fasting when dialysis treatments had to be reduced to twice a week posed very significant risk of complications and death. Similarly, PD patients may not have access to monitoring or be able to modify their regime and hence must consider not fasting and explore alternatives.
CONCLUSION
Data regarding the safety and feasibility of patients with CKD observing the Ramadan fast are scarce. Stable CKD-ND (up to Stage 3) patients may be able to fast with close monitoring, whereas HD and PD patients are considered very high risk. We suggest risk-stratifying patients wishing to fast. Patients whose illnesses do not permit them to fast should explore alternatives. Well-designed observational studies with large sample sizes or RCTs are needed to address the gap in knowledge (Table 6). We propose a method of risk stratifying and managing patients that can be used to facilitate patient-centric conversations, aid decision-making, improve patient and clinician satisfaction and provide a safer Ramadan experience.
Table 6.
Research needs
| Category | Research gap |
|---|---|
| All | Capture fasting status on an annual basis prospectively including number of days |
| All | RCTs to assess safety and tolerability of fasting |
| All | Well-designed observational studies in temperate regions in summer and winter months |
| All | Incidence of hyperkalaemia in fasting individuals |
| All | Hospitalization due to AKI, fluid overload, electrolyte abnormalities |
| All | Capture information on lifestyle factors such as work conditions and working hours during Ramadan |
| CKD, non-dialysis |
High-risk patients to be included in future studies, e.g. patients with ADPKD on tolvaptan, patients with tendency for electrolyte imbalance Include patients with unknown causes of CKD Whether fasting can lead to progression of ADPKD |
| HD |
Include home HD patients Include patients wishing to fast on dialysis days with appropriate adjustment to dialysis treatment Include older patients |
| PD |
Studies with larger sample sizes Include patients with diabetes and hypertension Intermittent fasting (fasting on non-dialysis days) versus fasting on dialysis days |
ADPKD, autosomal dominant polycystic kidney disease.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES
- 1. Patel NR, Kennedy A, Blickem C. et al. Having diabetes and having to fast: a qualitative study of British Muslims with diabetes. Health Expect 2015; 18: 1698–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hassanein M, Al-Arouj M, Hamdy O. et al. Diabetes and Ramadan: practical guidelines. Diabetes Res Clin Pract 2017; 126: 303–316 [DOI] [PubMed] [Google Scholar]
- 3. Ghouri N, Hussain S, Mohammed R. et al. Diabetes, driving and fasting during Ramadan: the interplay between secular and religious law. BMJ Open Diab Res Care 2018; 6: e000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghouri N, Gatrad R, Sattar N. et al. Summer-winter switching of the Ramadan fasts in people with diabetes living in temperate regions. Diabet Med 2012; 29: 696–697 [DOI] [PubMed] [Google Scholar]
- 5. Abolaban H, Al-Moujahed A.. Muslim patients in Ramadan: a review for primary care physicians. Avicenna J Med 2017; 7: 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salti I, Bénard E, Detournay B. et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care 2004; 27: 2306–2311 [DOI] [PubMed] [Google Scholar]
- 7. Waqar S, Ghouri N.. Managing Ramadan queries in COVID-19. BJGP Open 2020; 4: bjgpopen20X101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emami-Naini A, Roomizadeh P, Baradaran A. et al. Ramadan fasting and patients with renal diseases: a mini review of the literature. J Res Med Sci 2013; 18: 711–716 [PMC free article] [PubMed] [Google Scholar]
- 9. Bragazzi NL. Ramadan fasting and chronic kidney disease: a systematic review. J Res Med Sci 2014; 19: 665–676 [PMC free article] [PubMed] [Google Scholar]
- 10. Bragazzi NL. Ramadan fasting and chronic kidney disease: does estimated glomerular filtration rate change after and before Ramadan? Insights from a mini meta-analysis. Int J Nephrol Renovasc Dis 2015; 8: 53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al Muhanna FA. Ramadan fasting and renal failure. Saudi Med J 1998; 19: 319–321 [PubMed] [Google Scholar]
- 12. El-Wakil HS, Desoky I, Lotfy N. et al. Fasting the month of Ramadan by Muslims: could it be injurious to their kidneys? Saudi J Kidney Dis Transpl 2007; 18: 349–354 [PubMed] [Google Scholar]
- 13. Bernieh B, Al HM, Boobes Y. et al. Fasting Ramadan in chronic kidney disease patients: clinical and biochemical effects. Saudi J Kidney Dis Transpl 2010; 21: 898–902 [PubMed] [Google Scholar]
- 14. Al Wakeel JS. Kidney function and metabolic profile of chronic kidney disease and hemodialysis patients during Ramadan fasting. Iran J Kidney Dis 2014; 8: 321–328 [PubMed] [Google Scholar]
- 15. NasrAllah MM, Osman NA.. Fasting during the month of Ramadan among patients with chronic kidney disease: renal and cardiovascular outcomes. Clin Kidney J 2014; 7: 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mbarki H, Tazi N, Najdi A. et al. Effects of fasting during Ramadan on renal function of patients with chronic kidney disease. Saudi J Kidney Dis Transpl 2015; 26: 320–324 [DOI] [PubMed] [Google Scholar]
- 17. Bakhit AA, Kurdi AM, Wadera JJ. et al. Effects of Ramadan fasting on moderate to severe chronic kidney disease. A prospective observational study. Saudi Med J 2017; 38: 48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kara E, Sahin OZ, Kizilkaya B. et al. Fasting in Ramadan is not associated with deterioration of chronic kidney disease: a prospective observational study. Saudi J Kidney Dis Transpl 2017; 28: 68–75 [DOI] [PubMed] [Google Scholar]
- 19. Ekinci I, Erkoc R, Gursu M. et al. Effects of fasting during the month of Ramadan on renal function in patients with autosomal dominant polycystic kidney disease. Clin Nephrol 2018; 89: 103–112 [DOI] [PubMed] [Google Scholar]
- 20. Hassan S, Hassan F, Abbas N. et al. Does Ramadan fasting affect hydration status and kidney function in CKD patients? Ann Nutr Metab 2018; 72: 241–247 [DOI] [PubMed] [Google Scholar]
- 21. Alawadi F, Rashid F, Bashier A. et al. The use of Free Style Libre Continues Glucose Monitoring (FSL-CGM) to monitor the impact of Ramadan fasting on glycemic changes and kidney function in high-risk patients with diabetes and chronic kidney disease stage 3 under optimal diabetes care. Diabetes Res Clin Pract 2019; 151: 305–312 [DOI] [PubMed] [Google Scholar]
- 22. Chowdhury A, Khan H, Lasker SS. et al. Fasting outcomes in people with diabetes and chronic kidney disease in East London during Ramadan 2018: the East London diabetes in Ramadan survey. Diabetes Res Clin Pract 2019; 152: 166–170 [DOI] [PubMed] [Google Scholar]
- 23. Mahmoud MA, Barakat EA.. Effect of Ramadan fasting on fatigue, mood, and cognition in old chronic kidney disease Egyptian patients: a pilot study. J Egypt Soc Nephrol Transplant 2019; 19: 63–67 [Google Scholar]
- 24. Baloglu I, Turkmen K, Kocyigit I. et al. The effect of Ramadan fasting on kidney function in patients with chronic kidney disease. Int Urol Nephrol 2020; 52: 1337–1343 [DOI] [PubMed] [Google Scholar]
- 25. Eldeeb AA, Mahmoud MA, Ibrahim AB. et al. Effect of Ramadan fasting on arterial stiffness parameters among Egyptian hypertensive patients with and without chronic kidney disease. Saudi J Kidney Dis Transpl 2020; 31: 582–588 [DOI] [PubMed] [Google Scholar]
- 26. Ghalib M, Qureshi J, Tamim H. et al. Does repeated Ramadan fasting adversely affect kidney function in renal transplant patients? Transplantation 2008; 85: 141–144 [DOI] [PubMed] [Google Scholar]
- 27. Khader AAA. Effect of diet during Ramadan on patients on chronic haemodialysis. Saudi Med J 1991; 12: 30–31 [Google Scholar]
- 28. Wan Md Adnan WA, Zaharan NL, Wong MH. et al. The effects of intermittent fasting during the month of Ramadan in chronic haemodialysis patients in a tropical climate country. PLoS One 2014; 9: e114262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alshamsi S, Binsaleh F, Hejaili F. et al. Changes in biochemical, hemodynamic, and dialysis adherence parameters in hemodialysis patients during Ramadan. Hemodial Int 2016; 20: 270–276 [DOI] [PubMed] [Google Scholar]
- 30. Imtiaz S, Salman B, Dhrolia MF. et al. Clinical and biochemical parameters of hemodialysis patients before and during Islamic month of Ramadan. Iran J Kidney Dis 2016; 10: 75–78 [PubMed] [Google Scholar]
- 31. Khazneh E, Qaddumi J, Hamdan Z. et al. The effects of Ramadan fasting on clinical and biochemical markers among hemodialysis patients: a prospective cohort study. PLoS One 2019; 14: e0218745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Megahed AF, El-Kannishy G, Sayed-Ahmed N.. Status of fasting in Ramadan of chronic hemodialysis patients all over Egypt: a multicenter observational study. Saudi J Kidney Dis Transpl 2019; 30: 339–349 [DOI] [PubMed] [Google Scholar]
- 33. Adanan NIH, Md Ali MS, Lim JH. et al. Investigating physical and nutritional changes during prolonged intermittent fasting in hemodialysis patients: a prospective cohort study. J Ren Nutr 2020; 30: e15–e26 [DOI] [PubMed] [Google Scholar]
- 34. Imtiaz S, Nasir K, Dhrolia MF. et al. Mortality trend among hemodialysis patients during the Islamic month of Ramadan: a 24 years retrospective study. J Coll Physicians Surg Pak 2015; 25: 189–192 [PubMed] [Google Scholar]
- 35. Al Wakeel J, Mitwalli AH, Alsuwaida A. et al. Recommendations for fasting in Ramadan for patients on peritoneal dialysis. Perit Dial Int 2013; 33: 86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al Mahayni AO, Alkhateeb SS, Abusaq IH. et al. Does fasting in Ramadan increase the risk of developing urinary stones? Saudi Med J 2018; 39: 481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.UK Renal Registry. UK Renal Registry 21st Annual Report – data to 31/12/2017, Bristol, UK.https://renal.org/sites/renal.org/ files/publication/file-attachments/21st_UKRR_ Annual_Report.pdf (29 December 2020, date last accessed)
- 38. Sy J, Johansen KL.. The impact of frailty on outcomes in dialysis. Curr Opin Nephrol Hypertens 2017; 26: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dar Al-Ifta Al-Missriyyah. Does Dialysis Invalidate Fasting?https://www.dar-alifta.org/Foreign/ViewFatwa.aspx?ID=6180 (29 December 2020, date last accessed)
- 40. Rashid R. Part 2–What Invalidates Fasting Related to Body Cavities. https://jknfatawa.co.uk/the-fiqh-of-fasting-pt1-the-cavity/ (26 February 2021, date last accessed)
- 41.Islam Quaetion & Answer. How Should a Person Who Suffers Kidney Failure and Who Has Dialysis Three Times a Week Fast?https://islamqa.info/en/answers/49987/how-should-a-person-who-suffers-kidney-failure-fast (29 December 2020, date last accessed)