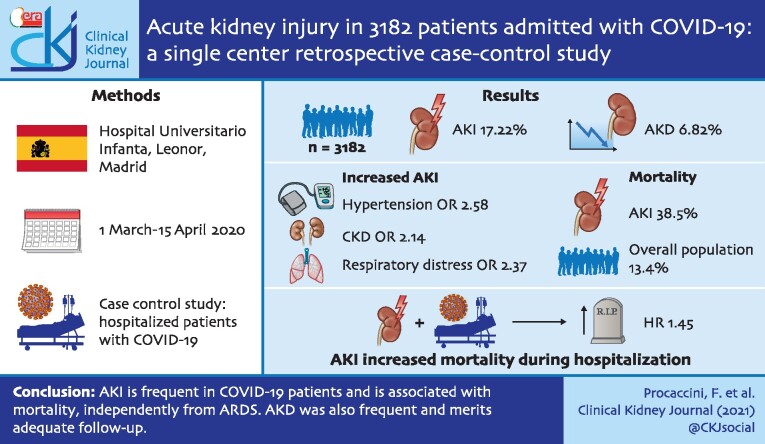
Abstract
Background
Acute kidney injury (AKI) may develop in coronavirus disease 2019 (COVID-19) patients and may be associated with a worse outcome. The aim of this study is to describe AKI incidence during the first 45 days of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in Spain, its reversibility and the association with mortality.
Methods
This was an observational retrospective case–control study based on patients hospitalized between 1 March and 15 April 2020 with SARS-CoV-2 infection and AKI. Confirmed AKI cases were compared with stable kidney function patients for baseline characteristics, analytical data, treatment and renal outcome. Patients with end-stage kidney disease were excluded.
Results
AKI incidence was 17.22% among 3182 admitted COVID-19 patients and acute kidney disease (AKD) incidence was 6.82%. The most frequent causes of AKI were prerenal (68.8%) and sepsis (21.9%). Odds ratio (OR) for AKI was increased in patients with pre-existent hypertension [OR 2.58, 95% confidence interval (CI) 1.71–3.89] and chronic kidney disease (CKD) (OR 2.14, 95% CI 1.33–3.42) and in those with respiratory distress (OR 2.37, 95% CI 1.52–3.70). Low arterial pressure at admission increased the risk for Stage 3 AKI (OR 1.65, 95% CI 1.09–2.50). Baseline kidney function was not recovered in 45.73% of overall AKI cases and in 52.75% of AKI patients with prior CKD. Mortality was 38.5% compared with 13.4% of the overall sample population. AKI increased mortality risk at any time of hospitalization (hazard ratio 1.45, 95% CI 1.09–1.93).
Conclusions
AKI is frequent in COVID-19 patients and is associated with mortality, independently of acute respiratory distress syndrome. AKD was also frequent and merits adequate follow-up.
Keywords: acute kidney injury, acute kidney disease, case–control, chronic kidney disease, COVID-19, mortality
Graphical Abstract
INTRODUCTION
Since the first case reports were published describing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in China there were suggestions that pulmonary tissue was not the only SARS-CoV-2 target, and nephrologists focused on acute kidney injury (AKI) as an important complication of hospitalized patients with acute respiratory distress syndrome (ARDS) [1, 2]. Reports from around the world underlined a high rate of AKI among coronavirus disease 2019 (COVID-19) patients and the need for renal replacement therapy (RRT).
Current knowledge on COVID-19 aetiopathogeneses indicates that the virus uses human angiotensin-converting enzyme 2 (ACE2) and the transmembrane serine protease 2 (TMPRSS2) as entrance to human cells, and these proteins locate in multiple organs like brain, oral mucosa, small intestine and kidney [3, 4].
Abundant ACE2 staining was observed in the brush border of the kidney proximal tubular cells and weak cytoplasmic staining in distal tubules and collecting ducts [3]. ACE2 and TMPRSS2 genes are co-expressed in proximal tubule cells and podocytes [5]. Series of kidney histology from China describe acute tubular necrosis as the main finding and identify viral particles in kidney tissue [6].
Clinical presentation of kidney injury ranged from mild microhaematuria and proteinuria to severe AKI, but information about the real magnitude of the problem is scarce, and the exact physiopathology of kidney injury is yet to be determined [7].
The aim of this study was to determine the incidence of AKI in patients with acute SARS-CoV-2 infection in the first 45 days of pandemic in a hospital located in one of the most affected areas of Spain and Madrid, where almost 60 000 people were diagnosed between March and May 2020 and seroprevalence of SARS-CoV-2 in the population was 11.7% [8, 9]. Moreover, we try to identify risk factors for AKI and mortality risk outcomes of COVID-19 patients with AKI.
MATERIALS AND METHODS
Study design
This is an observational case–control study analysing AKI incidence in hospitalized patients with SARS-CoV-2 infection at Hospital Universitario Infanta Leonor (HUIL) between 1 March and 15 April 2020. This is a secondary hospital attending a population of 345 206 inhabitants, with 269 beds for hospitalization and 8 intensive care unit (ICU) beds. During the pandemic, the hospital was reconverted to a COVID-19 hospital with 720 beds and 34 ICU beds. The study was approved by the scientific ethical committee of HUIL.
COVID-19 infection was defined based on positive polymerase chain reaction (PCR) for SARS-CoV-2 virus or on high clinical suspicion based on epidemiological data (direct contact with a positive case), clinical status, blood parameters [lymphopenia, increase in d-dimer, liver enzymes, fibrinogen and C-reactive protein (CRP)] and X-ray or computed tomography imaging [10–12].
Patients were considered for in-hospital care when they developed hypoxemic respiratory failure, sepsis, septic shock or extensive viral pneumonia in radiological findings (alveolar consolidation with peripheral distribution, round-glass opacities, interlobular septal thickening, pleural thickening, crazy-paving pattern) [13].
Main data about hospitalization such as sex, age, date of admission, death or discharge were collected from official hospital registries. Multiple hospitalizations due to the same clinical condition were considered as a unique admission time. Follow-up ended with death or with last analytical data available from primary care within 60 days after first admission. Patients with chronic kidney disease (CKD) Stage 5 [estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2] or in RRT were excluded.
AKI patients
As most patients with renal impairment were not directly referred to nephrological assessment, all patients with serum creatinine (sCr) ≥1.3 mg/dL during the hospitalization were retrospectively identified from the central laboratory system. After that, renal function of every patient was revised whether they met AKI criteria. AKI was defined as an increase in sCr ≥0.3 mg/dL over baseline sCr. Urine output was not considered due to lack of reliable data. The highest sCr value within a retrospective span of 1 year was considered as the baseline kidney function, and these data were collected from primary care electronical records (HORUS healthcare software) and from nephrology department registries when available. AKI aetiology was categorized as sepsis, prerenal, intrinsic renal, post-renal and unknown. Aetiology was reported in the official clinical registries. In most cases, it was not reported, so aetiology was defined retrospectively by a team of eight expert nephrologists based on analytical data, vital signs and clinical evolution. Every nephrologist assessed different patients and only in case of unclear aetiology was the case discussed with a senior member, and both had to agree on the final assignment. AKI secondary to severe sepsis included patients with at least two of these conditions: evidence of hypotension refractory to fluid repletion, tissue hypoperfusion, organ dysfunction, multiple organ failure or a Quick Sequential Organ Failure Assessment (qSOFA) score >1.
Haemodynamic prerenal state was considered in case of reported clinical sign of hypovolaemia caused by dehydration, haemorrhage, diuretics, gastrointestinal fluid loss, use of drug that affects glomerular haemodynamic and hypotension with response to fluid administration and fall of sCr within 72 h from peak. Intrinsic renal damage was considered when sCr decline started at over 72 h or was induced by nephrotoxic drugs (antibiotics, antineoplastic drugs, contrast media, anaesthetic drugs). All patients with glomerular disease were diagnosed before the start of this study and directly assessed by a nephrologist during the hospitalization. No kidney biopsies were performed during the study period. Post-renal disease was considered when a urinary tract obstruction was described by ultrasonography or resolution after bladder catheterization. AKI was classified as unknown when it was impossible to determine a clear aetiology. AKI was classified inside the main categories as drug-related only if the drug was considered to have a main role in the development of AKI and in two prescription situations: if a new drug was identified before the development of AKI, and it was chronologically compatible; if a drug was not discontinued within a new clinical situation. AKI was staged according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [14]. Acute kidney disease (AKD) was defined according to consensus from Acute Disease Quality Initiative (ADQI) as a loss of kidney function for a duration >7 days after exposure to an AKI initiating event [15].
AKI resolution was defined as return to baseline value of sCr. For CKD patients a final value >25% from baseline was considered as no resolution.
Control patients
After establishing the case cohort, a control group of the same size was generated from the main pool of patients admitted to the hospital in the same period and with sCr <1.3 mg/dL. A numeric identifier was assigned to any eligible patient, then STATA 16 (StataCorp LLC, Cary, NC, USA) was used to shuffle data randomly and obtain a sample without replacement. Controls were not matched using any variable.
ICU and RRT
During the period of the study, COVID-19 patients were divided into four categories of priority for ICU admission due to bed shortage, according to guidelines by national society of critically ill professionals [16]. Priority 1 category included critical and unstable patients requiring invasive mechanical ventilation whose monitoring and intensive treatment could not be provided outside the ICU. Patients with PaO2/FiO2 ratio <200 or <300 and organ failure but with non-invasive ventilation (NIV) were directed towards an intermediate care unit (Priority 2). Patients with terminal chronic disease, cognitive impairment or degenerative disease were not admitted (Priorities 3 and 4). Mechanical ventilation was not recommended in patients over 80 years of age. Patients between 70 and 80 years with moderate–severe comorbidities such as chronic heart failure, dilated cardiomyopathy, chronic obstructive pulmonary disease, cirrhosis and CKD were assessed on a case-by-case basis, with preference for NIV. Patients with the longest life expectancy were prioritized.
RRT was started in AKI characterized by oliguria (urine output <0.3 mL/kg/h for 24 h), refractory fluid overload, severe hyperkalaemia (K >6.5 mEq/L), rapidly rising urea levels (if otherwise unexplained) or severe metabolic acidosis (pH <7.1). RRT was not considered in patients with refractory multiple organ failure.
Data collection
Clinical data were collected from electronical health record system (SELENE—CERNER Corp.). All collected data were anonymized and subsequently analysed. Demographic data, baseline risk factors, including CKD, and treatment were recorded, including angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEi), systemic corticosteroids, statins, chemotherapy and biological treatments. Main clinical complications such as heart failure, ARDS, pulmonary embolism and disseminated intravascular coagulopathy (DIC) were collected along with experimental COVID-19 treatments. Data from AKI patients included baseline treatment with diuretics and antidiabetic agents and AKI characteristics. Analytical data were recorded only for cases and included lymphocyte counts, haemoglobin, platelet counts, d-dimer, urea, creatinine and CRP at admission, AKI onset and at Days 3 and 7, plus last available control; albumin, sodium, potassium, calcium and bicarbonate levels were recorded at AKI onset. The highest level of lactate dehydrogenase and interleukin-6 was also recorded. Urinary levels of creatinine, sodium and potassium and data concerning proteinuria and haematuria were collected for cases at AKI onset. eGFR was estimated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [17]. AKI management and renal outcome were also recorded.
Statistical analysis
Statistical analysis was performed with STATA 16 (StataCorp LLC). Normal distribution of variable was initially tested with box plot and cumulative density function of the normal distribution. Simple association of variables was measured with Student's t-test, chi-squared, Fisher's exact test and pairwise correlations. Variables with P > 0.3 in univariate analysis were not considered in multivariate logistic and Cox models. In predictive models backward stepwise selection was performed with 0.10 significance level for removal and 0.05 for addition. In logistic and Cox regression models, chunk test and likelihood-ratio test were performed to evaluate real variable effects. Best models were then selected after confounders were analysed (practical difference >10% from reference model). Missing data were not imputed and listwise method was used in the analysis. For Cox regressions, proportion hazard assumption was verified for variables in the final best model.
RESULTS
Incidence and timing of AKI
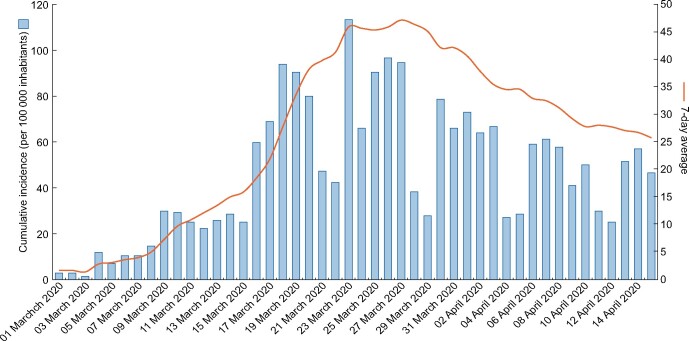
About 3182 patients were admitted for SARS-CoV-2 infection during the study period (Figure 1). Of these, 691 presented sCr ≥1.3 mg/dL, from which a total of 548 subjects defined the AKI group after excluding dialysis patients, unconfirmed SARS-CoV-2 infection and subjects not fulfilling AKI criteria (Figure 2). The overall incidence of AKI among patients hospitalized with COVID-19 was 17.22%. Of these, 389 (12.22% of all COVID-19 patients, 70.99% of AKI patients) presented AKI upon admission, while 159 (5.00% of all COVID-19 patients, 29.01% of AKI patients) developed in-hospital AKI with a median onset time of 3 days [interquartile range (IQR) 2–6].
FIGURE 1:
Cumulative incidence and 7 days average of patients admitted at Hospital Universitario Infanta Leonor with suspected COVID-19 between 1 March and 15 April 2020.
FIGURE 2:
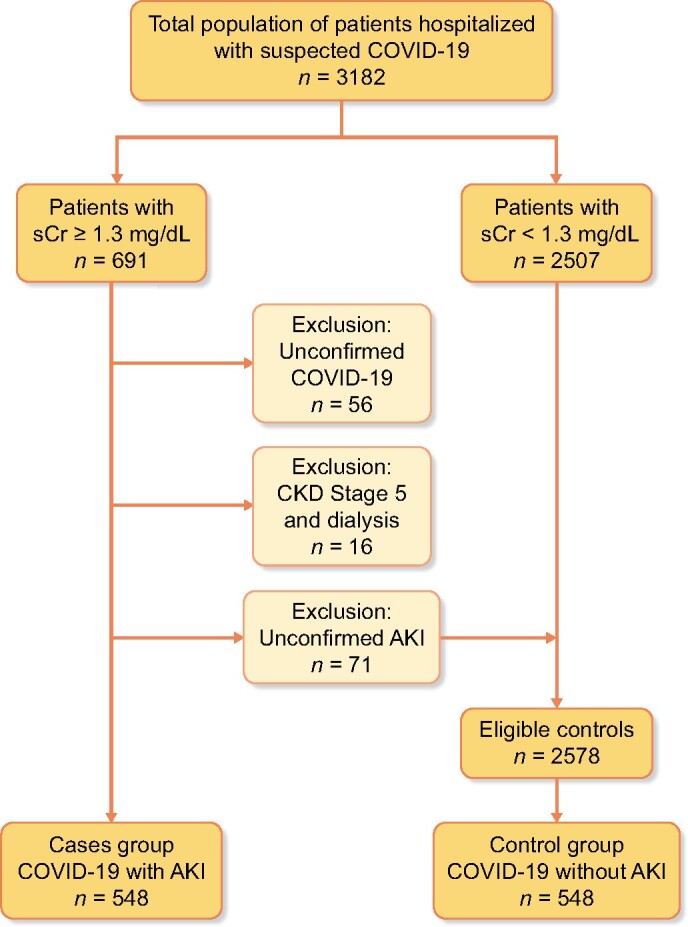
Flowchart of cases and controls selection. Patients with sCr <1.3 mg/dL were not actively revised to confirm COVID-19 criteria. Eligible controls were randomly sampled to obtain a control group of the same size of cases group. All patients in case and control group were actively revised to confirm COVID-19 criteria.
Main characteristics of AKI versus control patients
The control group was formed by 548 subjects without AKI from the main pool of 2578 subjects with confirmed COVID-19. A total of 83.21% of patients from both groups presented at least one chronic clinical condition at admission. Overall median hospitalization time was 9 days (IQR 5–14) and it was longer in AKI than in non-AKI patients (11 versus 8 days, P < 0.001). Median follow-up time of AKI patients was 11 days (IQR 5–21).
Baseline characteristics of AKI and control groups are shown in Table 1. AKI patients were older and mostly males, and had a higher prevalence of hypertension, cardiopathy and diabetes. ARDS developed more frequently in the AKI group (32.7 versus 15.3%, P < 0.001) as did heart failure, but there were no differences in baseline pulmonary diseases. CKD was more frequent in AKI patients and the most common causes were nephroangiosclerosis (37.61%) and diabetic nephropathy (19.91%; Table 2).
Table 1.
Baseline characteristics of case and control group patients
| Variables | Patients with AKI (n = 548) | Patients without AKI (n = 548) | P-value |
|---|---|---|---|
| Sex, n (%) | 0.02 | ||
| Females | 176 (32.12) | 225 (41.06) | |
| Males | 372 (67.88) | 323 (58.94) | |
| Age in years, mean (SD) | 75.09 (13.65) | 68.93 (14.51) | <0.001 |
| Females | 78.79 (11.50) | 73.56 (12.73) | |
| Males | 73.35 (S14.25) | 65.70 (14.81) | |
| Cardiopathy, n (%) | 198 (36.2) | 115 (21.06) | <0.001 |
| Females | 66 (37.5) | 56 (25.00) | |
| Males | 132 (35.58) | 59 (18.32) | |
| Hypertension, n (%) | 426 (78.02) | 275 (50.18) | <0.001 |
| Females | 148 (84.09) | 142 (63.11) | |
| Males | 278 (75.14) | 133 (41.18) | |
| Diabetes, n (%) | 217 (39.67) | 111 (20.26) | <0.001 |
| Females | 70 (39.77) | 48 (21.33) | |
| Males | 147 (39.62) | 63 (19.50) | |
| Chronic lung disease, n (%) | 176 (32.3) | 135 (24.64) | 0.09 |
| Asthma | 45 (8.26) | 39 (7.12) | |
| COPD | 92 (16.88) | 79 (14.42) | |
| OSA | 39 (7.16) | 17 (3.10) | |
| Smokers, n (%) | <0.001 | ||
| Active smokers | 33 (6.35) | 19 (3.79) | |
| Former smokers | 182 (35.00) | 102 (20.36) | |
| CKD, n (%) | 225 (41.05) | 45 (15.36) | <0.001 |
| Stage 2 | 25 (11.11) | 6 (13.34) | |
| Stage 3a | 101 (44.89) | 23 (51.11) | |
| Stage 3b | 74 (32.89) | 12 (26.67) | |
| Stage 4 | 25 (11.11) | 4 (8.89) | |
| Chronic liver disease, n (%) | 41 (7.51) | 12 (2.20) | <0.001 |
| Cancer, n (%) | 74 (13.5) | 51 (9.31) | 0.09 |
| Solid neoplasm | 54 (9.87) | 39 (7.14) | |
| Haematologic | 20 (3.66) | 12 (2.20) | |
| Obesity, n (%) | 182 (34.67) | 91 (18.96) | <0.001 |
| Female | 56 (33.53) | 39 (20.42) | |
| Male | 126 (35.20) | 52 (17.99) | |
| Immunologic/inflammatory disease, n (%) | 41 (7.50) | 46 (8.39) | 0.58 |
| ACEi, n (%) | 180 (32.91) | 116 (23.02) | <0.001 |
| ARBs, n (%) | 153 (27.97) | 65 (12.90) | <0.001 |
| Corticosteroids, n (%) | 22 (4.02) | 18 (3.58) | 0.71 |
| Statins, n (%) | 266 (48.63) | 103 (28.93) | <0.001 |
| Chemotherapy, n (%) | 22 (4.02) | 14 (2.78) | 0.27 |
| Immunotherapy, n (%) | 3 (0.55) | 4 (0.79) | 0.65a |
| PCR SARS-CoV-2+, n (%) | 412 (75.60) | 476 (84.7) | <0.05 |
| At admission | |||
| Fever, n (%) | 352 (60.58) | 410 (74.14) | <0.001 |
| Gastrointestinal losses, n (%) | 106 (19.34) | 123 (22.4) | 0.21 |
| Mean (SD) systolic pressure, mmHg | 125.88 (24.57) | 133.01 (22.15) | <0.001 |
| Mean (SD) diastolic pressure, mmHg | 70.18 (15.19) | 77.30 (13.90) | <0.001 |
| COVID-19 complications, n (%) | |||
| ARDS | 179 (32.66) | 81 (15.31) | <0.001 |
| Heart failure | 59 (10.77) | 17 (3.23) | <0.001 |
| DIC | 15 (2.74) | 6 (1.14) | 0.08 |
| PTE | 15 (2.74) | 15 (2.74) | 1.00 |
Obesity was defined as a body mass index >30. Immunologic/inflammatory diseases included rheumatologic diseases, chronic inflammatory bowel diseases, multiple sclerosis, miastenia gravis and thyroid autoimmune diseases.
Fisher’s exact test.
COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea; PTE, pulmonary thromboembolism.
Table 2.
Baseline characteristics of CKD in patients developing AKI
| CKD cause, n (%) | |
| Nephroangiosclerosis | 85 (37.61) |
| Diabetic nephropathy | 45 (19.91) |
| Chronic tubulointerstitial nephropathy | 24 (10.62) |
| Glomerulopathy | 15 (6.64) |
| Solitary kidney | 14 (6.19) |
| Cardiorenal syndrome | 11 (4.87) |
| Vascular | 7 (3.10) |
| Other (multiple myeloma, polycystic disease) | 2 (0.88) |
| Unknown | 23 (10.18) |
| Dipstick proteinuriaa (mg/dL), n/n (%) | |
| <30 | 114/162 (70.37) |
| 30–100 | 36/162 (22.22) |
| 100–300 | 8/162 (4.94) |
| 300–1000 | 4/162 (2.47) |
| Microhaematuriaa (>10 RBC/hpf), n/n (%) | 23/160 (14.38) |
| Albuminuriaa (mg/g), median (IQR) | 37.44 (30.01–227.94) |
| Ureaa (mg/dL), mean (SD) | 69.31 (25.32) |
| sCrb (mg/dL), mean (SD) | 1.40 (0.41) |
| Estimated filtration rate CKD-EPIb (mL/min/1.73 m2), mean (SD) | 46.63 (13.24) |
Last available control from nephrology department or primary care registries up to 1 year before COVID-19 hospitalization.
Highest values registered from nephrology department or primary care up to 1 year before COVID-19 hospitalization.
RBC/hpf, red blood cells per high power field.
AKI characterization
AKI Stage 1 was predominant with 70.07% (n = 384) of cases compared with Stage 2 19.34% (n = 106) and Stage 3 10.58% (n = 58). Key analytical data and baseline characteristics of AKI patients are listed in Table 3.
Table 3.
Analytical data at AKI diagnosis according to resolution of AKI
| Characteristic | All | AKI resolution | AKI no resolution | P-value |
|---|---|---|---|---|
| Haemoglobin (g/L) | 13.10 (2.14) | 13.45 (2.00) | 12.61 (2.14) | <0.001 |
| Lymphocytes (103/µL) | 1.00 (0.65) | 1.02 (0.41) | 0.97 (0.04) | 0.38 |
| Platelets (103/µL) | 223.20 (103.85) | 220.14 (96.09) | 230.26 (114.189) | 0.27 |
| Urea (mg/dL) | 75 (53–103)a | 65 (50–88)a | 86 (63–120)a | <0.001b |
| Creatinine (mg/dL) | 1.86 (0.75) | 1.72 (0.65) | 2.03 (0.83) | <0.001 |
| eGFR CKD-EPI (mL/min/1.73m2) | 36.53 (13.48) | 39.20 (13.23) | 32.94 (12.64) | <0.001 |
| Albumin (g/dL) | 2.70 (0.58) | 2.79 (0.55) | 2.61 (0.58) | 0.01 |
| Calcium (mg/dL) | 8.30 (0.83) | 8.48 (0.80) | 8.11 (0.82) | <0.001 |
| Calcium (albumin correction, mg/dL) | 9.39 (0.82) | 9.50 (0.80) | 9.42 (0.80) | 0.05 |
| Serum sodium (mmol/L) | 139.18 (6.48) | 138.24 (5.68) | 140.31 (7.16) | <0.001 |
| Serum potassium (mmol/L) | 4.49 (0.78) | 4.39 (0.72) | 4.59 (0.84) | 0.03 |
| Bicarbonate (mmol/L) | 23.26 (4.68) | 23.58 (4.76) | 22.87 (4.56) | 0.12 |
| CRP (mg/L) | 97.9 (43.6–187.3)a | 87.7 (41.7–162.9)a | 1157 (46.1–212)a | 0.01b |
| d-dimer (ng/mL) | 1270 (630–3960)a | 1020 (550–2050)a | 2060 (830–5120)a | <0.001b |
Data expressed as mean (SD), unless stated otherwise. AKI resolution was defined as return to baseline value of sCr or eGFR. For CKD patients a final value >25% from baseline was considered as no resolution.
Median with IQR.
Mann–Whitney test.
The most frequent cause of AKI was prerenal/haemodynamic (68.8%), followed by sepsis (21.9%; Table 4). Drugs had a role in AKI development in 42.86% of intrinsic renal and 19.62% of haemodynamic cases.
Table 4.
Causes of AKI
| Cause | All AKI | AKI (duration 0–7 days) | AKD (duration >7 days) |
|---|---|---|---|
| Haemodynamic | 377 (68.80) | 222 (67.07) | 155 (71.43) |
| No drug-related | 303 (80.37) | 187 (84.23) | 116 (74.83) |
| Drug-related (diuretics, ACE, ARB) | 74 (19.62) | 35 (15.77) | 39 (25.16) |
| Sepsis | 120 (21.90) | 88 (26.59) | 32 (14.75) |
| Intrinsic renal | 14 (2.55) | 6 (1.81) | 8 (3.69) |
| Not drug-related | 8 (57.14) | 4 (66.67) | 4 (50.00) |
| Drug-related (contrast, CTX, NSAID) | 6 (42.86) | 2 (33.33) | 4 (50.00) |
| Post-renal | 5 (0.91) | 1 (0.30) | 4 (1.84) |
| Unknown | 32 (5.84) | 14 (4.23) | 18 (8.29) |
Data expressed as n (%). Drug-related AKI is presented with frequency in their main aetiologic categories.
CTX, chemotherapy; NSAID, non-steroidal anti-inflammatory drugs.
Dipstick urinalysis was performed in 125 (22.81%) patients with AKI. Among these, 59 patients presented microhaematuria, but in only 26 (44.08%) of them was it new-onset microhaematuria. There was no difference in new-onset microhaematuria between patients with previous normal kidney function and CKD patients (38.46 versus 61.54%, P = 0.62). Moreover, 65 patients presented proteinuria above 100 mg/dL, but in only 25 (38.46%) of them was it new-onset proteinuria, and in just 6 patients (24%) proteinuria was above 300 mg/dL.
AKD
A total of 217 (6.82% of all COVID-19 patients, 39.60% of AKI) patients met AKD criteria with characteristics described in Table 5. In this subgroup, sepsis and ARDS were less frequent than in AKI non-AKD patients (Tables 4 and 5). Median hospitalization time was 15 days (IQR 11–21) versus 7 (IQR 4–13) in the no AKD group.
Table 5.
Baseline characteristics by AKI duration
| Variables | AKI (duration 0–7 days) (n = 331) | AKD (duration >7 days) (n = 217) | P-value |
|---|---|---|---|
| Sex, n (%) | 0.53 | ||
| Females | 109 (32.93) | 67 (30.88) | |
| Males | 222 (67.07) | 150 (69.12) | |
| Age in years, mean (SD) | 73.96 (14.06) | 76.82 (12.85) | 0.99 |
| Females | 78.91 (11.73) | 78.60 (11.19) | |
| Males | 71.53 (14.50) | 76.02 (13.48) | |
| Cardiopathy, n (%) | 110 (33.33) | 88 (40.55) | 0.09 |
| Females | 39 (35.78) | 27 (40.30) | |
| Males | 71 (32.13) | 61 (40.67) | |
| Hypertension | 254 (77.20) | 172 (79.26) | 0.57 |
| Females | 90 (82.57) | 58 (86.57) | |
| Males | 164 (74.55) | 114 (76.00) | |
| Diabetes | 131 (39.70) | 86 (39.63) | 0.98 |
| Females | 47 (43.12) | 23 (34.33) | |
| Males | 84 (38.01) | 63 (42) | |
| Chronic lung disease | 108 (32.93) | 68 (31.34) | 0.39 |
| Asthma | 30 (9.15) | 15 (6.91) | |
| COPD | 51 (15.55) | 41 (18.89) | |
| OSA | 27 (8.23) | 12 (5.53) | |
| Smokers | 0.93 | ||
| Active smokers | 19 (6.03) | 14 (6.83) | |
| Former smokers | 111 (35.24) | 71 (34.63) | |
| CKD | 114 (34.44) | 112 (51.61) | <0.001 |
| Stage 2 | 14 (12.28) | 12 (10.71) | |
| Stage 3a | 50 (44.25) | 51 (45.54) | |
| Stage 3b | 40 (35.40) | 34 (30.36) | |
| Stage 4 | 10 (8.85) | 15 (13.39) | |
| Chronic liver disease | 27 (8.21) | 14 (6.45) | 0.45 |
| Cancer | 41 (12.97) | 33 (15.21) | 0.35 |
| Solid neoplasm | 32 (10.13) | 22 (10.14) | |
| Haematologic | 9 (2.85) | 11 (5.07) | |
| Obesity | 112 (35.00) | 70 (34.15) | 0.84 |
| Female | 36 (34.29) | 20 (32.26) | |
| Male | 76 (35.35) | 50 (34.97) | |
| Immunologic/inflammatory disease | 22 (6.67) | 19 (8.76) | 0.36 |
| ACEi | 107 (32.42) | 73 (33.64) | 0.77 |
| ARBs | 94 (28.48) | 59 (27.19) | 0.74 |
| Corticosteroids | 15 (4.55) | 7 (3.23) | 0.44 |
| Statins | 159 (48.18) | 107 (49.31) | 0.80 |
| Chemotherapy | 13 (3.94) | 9 (4.15) | 0.99 |
| Immunotherapy | 2 (0.61) | 1 (0.46) | 0.79a |
| PCR SARS-CoV-2+ | 234 (71.12) | 178 (82.41) | <0.05 |
| At admission | |||
| Fever | 198 (59.82) | 134 (61.75) | 0.65 |
| Gastrointestinal losses | 64 (19.34) | 42 (19.35) | 0.99 |
| Mean (SD) systolic pressure, mmHg | 118.95 (23.63) | 121.88 (24.38) | 0.91 |
| Mean (SD) diastolic pressure, mmHg | 66.51 (15.27) | 67.83 (15.83) | 0.83 |
| COVID-19 complications | |||
| ARDS | 126 (38.07) | 53 (24.42) | <0.001 |
| Heart failure | 31 (9.37) | 28 (12.9) | 0.19 |
| DIC | 7 (2.11) | 7 (3.23) | 0.42 |
| PTE | 9 (2.72) | 6 (2.76) | 0.96 |
| Mortality | 138 (41.69) | 73 (33.64) | 0.05 |
Data expressed as mean (SD), unless stated otherwise. aFisher’s exact test
COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea; PTE, pulmonary thromboembolism.
Variables associated with AKI
In the univariate analysis, hypertension, cardiopathy, diabetes, obesity, CKD, chronic treatment with ARBs, ACEi, statins, low arterial pressure at admission and onset of ARDS and heart failure were all significantly associated with AKI development (Supplementary data S1). In a predictive multiple regression model, hypertension and CKD patients showed an increased risk for AKI, with an odds ratio (OR) of 2.58 [95% confidence interval (CI) 1.71–3.89] and 2.14 (95% CI 1.33–3.42), respectively. ARDS (OR 2.37, 95% CI 1.52–3.70) and hypotension at admission (OR 1.96, 95% CI 1.18–3.26) also showed a higher risk for AKI when adjusted for age in the same model (Table 6). The same risk factors were investigated in the AKD subgroup versus non-AKD confirming CKD as a risk factor with an OR 1.82 (95% CI 1.25–2.65), while ARDS had an OR 0.55 (95% CI 0.37–0.81; Table 7).
Table 6.
Risk factors associated with AKI, logistic multiple regression
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Odds ratio | P-value | 95% CI | Odds ratio | P-value | 95% CI |
| Sex (male sex over female) | 1.47 | <0.001 | 1.14–1.89 | |||
| Age (years) | 1.03 | <0.001 | 1.02–1.04 | |||
| 0–50 (reference) | ||||||
| 50–60 | 1.05 | 0.86 | 0.60–1.82 | 1.01 | 0.97 | 0.51–2.01 |
| 60–70 | 1.34 | 0.26 | 0.81–2.25 | 1.61 | 0.17 | 0.81–3.21 |
| 70–80 | 2.05 | <0.001 | 1.25–3.37 | 3.74 | <0.001 | 1.75–7.96 |
| 80–90 | 3.41 | <0.001 | 2.09–5.59 | 1.89 | 0.07 | 0.94–3.79 |
| 90–100 | 3.03 | <0.001 | 1.61–5.69 | 2.92 | 0.03 | 1.10–7.78 |
| Hypertension | 3.52 | <0.001 | 2.70–4.58 | 2.58 | <0.001 | 1.71–3.89 |
| Cardiopathy | 2.12 | <0.001 | 1.62–2.78 | |||
| Diabetes | 2.58 | <0.001 | 1.97–3.39 | |||
| Obesity | 2.27 | <0.001 | 1.70–3.03 | |||
| CKD | 3.86 | <0.001 | 2.70–5.54 | 2.14 | <0.001 | 1.33–3.42 |
| Chronic hepatopathy | 3.60 | <0.001 | 1.87–6.94 | |||
| Statins | 2.33 | <0.001 | 1.75–3.08 | |||
| Corticosteroids (chronic treatment) | 1.13 | 0.71 | 0.59–2.10 | |||
| ARBs | 2.62 | <0.001 | 1.90–3.61 | |||
| ACEi | 1.64 | <0.001 | 1.25–2.15 | |||
| Metformin (versus no antidiabetic) | 2.50 | 0.01 | 1.21–5.17 | |||
| Metformin + SGTL2i (versus no antidiabetic) | 2.21 | 0.45 | 0.28–17.35 | |||
| Asthma (versus no lung disease) | 1.29 | 0.27 | 0.36–1.62 | |||
| COPD (versus no lung disease) | 1.30 | 0.12 | 0.94. –1.81 | |||
| OSA (versus no lung disease) | 2.56 | <0.001 | 1.42–4.61 | |||
| Active smokers (versus no smokers) | 2.16 | 0.10 | 1.21–3.88 | |||
| Former smokers (versus no smokers) | 2.22 | <0.001 | 1.67–2.95 | |||
| Immunologic/inflammatory disease | 0.88 | 0.58 | 0.57–1.37 | |||
| Hypotension at admission | 2.43 | <0.001 | 1.64–3.59 | 1.96 | <0.001 | 1.18–3.26 |
| Congestive cardiac failure | 3.60 | <0.001 | 2.07–6.27 | |||
| ARDS | 2.67 | <0.001 | 1.98–3.59 | 2.37 | <0.001 | 1.52–3.70 |
| DIC | 2.26 | 0.10 | 0.34–1.50 | |||
| Pulmonary thromboembolism | 0.72 | 0.39 | 0.34–1.50 | |||
Reference category is the absence of specific risk factor, if not otherwise specified. Variables with P < 0.05 in both univariate and multivariate analysis are highlighted in bold.
COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea.
Table 7.
Risk factors associated with AKD, logistic multiple regression
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Odds ratio | P-value | 95% CI | Odds ratio | P-value | 95% CI |
| Sex (male sex over female) | 1.10 | 0.61 | 0.76–1.59 | |||
| Age (years) | 1.03 | 0.05 | 1.02–1.04 | |||
| 0–50 (reference) | ||||||
| 50–60 | 1.71 | 0.29 | 0.64–4.56 | 1.68 | 0.31 | 0.62–4.57 |
| 60–70 | 1.13 | 0.80 | 0.45–2.87 | 0.95 | 0.92 | 0.37–2.45 |
| 70–80 | 2.35 | 0.06 | 0.98–5.60 | 1.93 | 0.15 | 0.80–4.69 |
| 80–90 | 2.02 | 0.11 | 0.86–4.75 | 1.55 | 0.33 | 0.64–3.75 |
| 90–100 | 2.76 | 0.04 | 1.03–7.39 | 1.96 | 0.19 | 0.71–5.40 |
| Hypertension | 1.13 | 0.57 | 0.74–1.71 | |||
| Cardiopathy | 1.36 | 0.09 | 0.96–1.95 | |||
| Diabetes | 1.00 | 0.99 | 0.70–1.41 | |||
| Obesity | 0.96 | 0.84 | 0.67–1.39 | |||
| CKD | 2.03 | <0.001 | 1.43–2.88 | 1.82 | 0.002 | 1.25–2.65 |
| Chronic hepatopathy | 0.77 | 0.45 | 0.39–1.50 | |||
| Statins | 1.05 | 0.80 | 0.74–1.47 | |||
| Corticosteroids (chronic treatment) | 0.70 | 0.44 | 0.28–1.75 | |||
| ARBs | 1.05 | 0.77 | 0.73–1.52 | |||
| ACEi | 0.94 | 0.74 | 0.64–1.37 | |||
| Metformin (versus no antidiabetic) | 1.14 | 0.53 | 0.75–1.73 | |||
| Metformin + SGTL2i (versus no antidiabetic) | 1.13 | 0.83 | 0.35–3.64 | |||
| Asthma (versus no lung disease) | 0.73 | 0.36 | 0.38–1.42 | |||
| COPD (versus no lung disease) | 1.19 | 0.47 | 0.75. –1.88 | |||
| OSA (versus no lung disease) | 0.66 | 0.25 | 0.32–1.37 | |||
| Active smokers (versus no smokers) | 1.14 | 0.73 | 0.55–2.35 | |||
| Former smokers (versus no smokers) | 0.99 | 0.94 | 0.67–1.44 | |||
| Immunologic/inflammatory disease | 1.34 | 0.37 | 0.71–2.55 | |||
| Hypotension at admission | 0.91 | 0.67 | 0.60–1.38 | |||
| Congestive cardiac failure | 1.43 | 0.19 | 0.83–2.47 | |||
| ARDS | 0.53 | <0.001 | 0.36–0.77 | 0.55 | 0.003 | 0.37–0.81 |
| DIC | 1.54 | 0.42 | 0.53–4.46 | |||
| Pulmonary thromboembolism | 1.01 | 0.97 | 0.36–2.90 | |||
Data refer to AKD patients versus non-AKD patients. Variables with P < 0.05 in both univariate and multivariate analysis are highlighted in bold.
COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea.
In addition, low arterial pressure at admission was associated with AKI severity with an OR 1.65 (95% CI 1.09–2.50) for Stage 3 AKI. No association was found with fever, gastrointestinal losses and baseline treatment with Type 2 sodium-glucose cotransporter inhibitors (SGLT2i).
Resolution of AKI and AKD
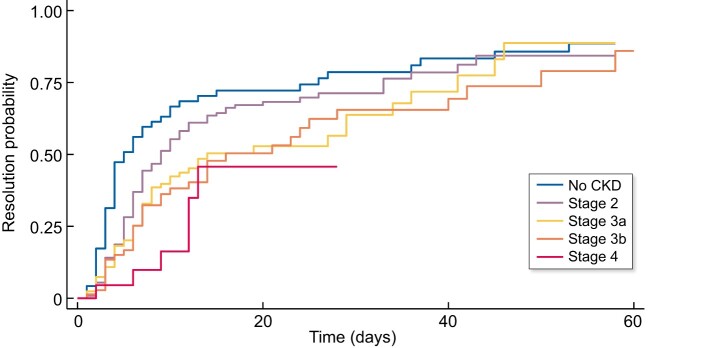
No resolution of AKI occurred in 241 (45.73%) patients and in 52.75% of CKD patients. AKI resolution was directly associated with aetiology and baseline kidney function (Figures 3 and 4), and recovery at 7 days was 33.94% (see Table 9).
FIGURE 3:
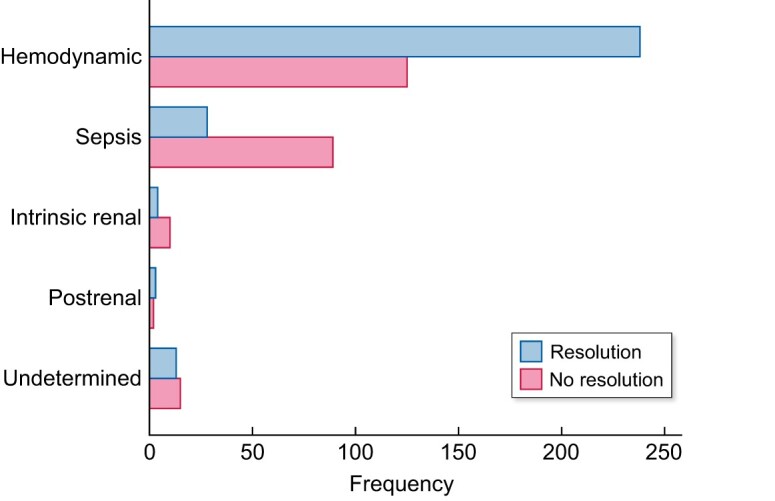
AKI resolution was defined as return to baseline value of sCr. For CKD patients, a value >25% from baseline at the end of follow-up was considered as no resolution.
FIGURE 4:
AKI resolution occurred with higher probability and earlier in patients with normal kidney function or low-stage CKD.
Table 9.
Renal outcome and survival of COVID-19 patients with AKI
| AKI resolution, n (cumulative %) | Mortality, n (cumulative %) | |
|---|---|---|
| 0–7 days | 186 (33.94) | 126 (23.00) |
| 7–14 days | 245 (44.70) | 173 (31.57) |
| 14–21 days | 254 (46.35) | 189 (34.49) |
| 21–28 days | 264 (48.18) | 194 (35.40) |
| 28–35 days | 270 (49.27) | 197 (35.95) |
| 35–60 days | 286 (52.19) | 211 (38.50) |
Median resolution time in patients with AKD was 13 days (IQR 10–23) versus 4 days (IQR 2–6) in the no AKD group. Acidosis, hypernatremia and a high serum level of d-dimer, CRP and lactate dehydrogenase showed association with no resolution of AKI in the univariate Cox regression. In a multivariate model adjusted for CKD and ARDS effect, hypernatremia and acidosis showed a hazard ratio (HR) of 2.28 (95% CI 1.43–3.60) and 1.54 (95% CI 1.13–2.10) respectively for no resolution of AKI (Supplementary data S2). Intravenous treatment with corticosteroids was associated with lower risk of AKI non-recovery (HR 0.71, 95% CI 0.51–0.99). Patients with AKD presented lower levels of CRP (Table 8) than others AKI patients and no resolution was significatively more frequent in this group compared with no-AKD (52.63 versus 41.19%, P = 0.01). New-onset microhaematuria or proteinuria was not associated with a worse renal outcome.
Table 8.
Comparative analytical data by AKI duration
| Characteristic | AKI (duration 0–7 days) | AKD (duration >7 days) | P-value |
|---|---|---|---|
| Haemoglobin (g/L) | 13.15 (2.14) | 13.02 (2.15) | 0.45 |
| Lymphocyte (103/µL) | 0.97 (0.62) | 1.04 (0.71) | 0.22 |
| Platelet (103/µL) | 226.52 (103.70) | 218.17 (104.10) | 0.36 |
| Urea (mg/dL) | 86.03 (48.50) | 88.55 (55.45) | 0.60 |
| 75 (53–106)a | 76 (I52.5–100.5)a | ||
| Creatinine (mg/dL) | 1.82 (0.65) | 1.93 (0.88) | 0.08 |
| GFR CKD-EPI (mL/min/1.73 m2) | 37.40 (13.71) | 35.21 (13.04) | 0.06 |
| Albumin (g/dL) | 2.66 (0.57) | 2.76 (0.59) | 0.15 |
| Calcium (mg/dL) | 8.34 (0.78) | 8.29 (0.91) | 0.67 |
| Calcium (albumin correction) (mg/dL) | 9.44 (0.72) | 9.31 (0.92) | 0.32 |
| Serum sodium (mmol/L) | 139.73 (6.81) | 138.63 (6.51) | 0.06 |
| Serum potassium (mmol/L) | 4.42 (0.77) | 4.56 (0.79) | 0.08 |
| Bicarbonate (mmol/L) | 23.16 (4.83) | 23.43 (4.45) | 0.56 |
| CRP (mg/L) | 115.65 (49.50–204.55) a | 76.5 (35.00–153.00) a | 0.001b |
| d dimer (ng/mL) | 1250 (650–3980)a | 1290 (620–3700)a | 0.76b |
Presented analytical are collected at AKI onset. Data are presented as mean (SD) unless otherwise indicated. Variables with statistical difference between groups are highlighted in bold.
Median with IQR.
Mann–Whitney test.
ICU and kidney replacement therapy
Forty-four patients (8.07%) with AKI were admitted to the ICU because of ARDS. Of these, 7/44 (15.90%) received RRT because of anuria and hyperkalaemia, 3 with intermittent haemodialysis and 4 with continuous RRT. Nine out of 44 patients (20.45%) presented criteria for RRT but only after limitation of life support. Of patients with AKI not admitted to the ICU, 36 (6,56%) presented criteria for RRT but did not have access to the treatment because the nephrology department was not consulted.
Variables associated with mortality
Mortality of all patients admitted to the hospital with COVID-19 during the period of the study was 13.42% (427 registered deaths). Mortality in the AKI group was higher than in the control group (38.50 versus 13.05%, P < 0.001). Mortality among CKD patients with AKI was 41.15%. Median time between AKI and death was 6 days (IQR 2–12), and was 13 days (IQR 10–18) in the AKD subgroup. Mortality was lower in the AKD subgroup than in the AKI no-AKD subgroup (33.64 versus 41.69%, P = 0.05), with a 7-day mortality of 31.57% (Table 9). Patients with no resolution of AKI registered more deaths compared with those that recovered from AKI (69.29 versus 12.59%, P < 0.001).
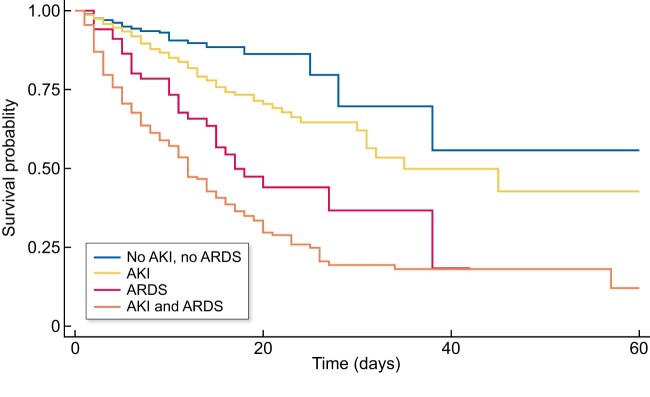
Survival analysis with Kaplan–Meier showed worse survival in patients who developed AKI independently of concomitant ARDS (Figure 5). In a Cox regression model, AKI showed an HR of 1.41 (95% CI 1.06–1.87) for mortality risk at any time of admission after adjusting for age, sex, COVID-19 complications analysed and possible confounders (Supplementary data S3). Mortality risk was also increased by ARDS with an HR of 4.06 (95% CI 3.17–5.18). Corticosteroids and hydroxychloroquine were associated with lower mortality rate (HR 0.68, 95% CI 0.48–0.95 and HR 0.56, 95% CI 0.31–0.99, respectively). Other experimental treatments (tocilizumab, remdesivir, lopinavir/ritonavir, azithromycin) showed no benefit.
FIGURE 5:
Kaplan–Meier survival curve. Patients with concomitant AKI and ARDS are less likely to survive than patients who presented only one of these conditions. AKI demonstrates its role as a major risk factor for mortality.
DISCUSSION
This study is the first to analyse the AKI incidence in a single secondary COVID-19 hospital of a highly affected area in Spain during the peak of the pandemic, when healthcare was overwhelmed. The main findings are the negative impact of both non-dialysis CKD and AKI on survival, independently of ARDS and persistent AKD for a sizable group of AKI patients.
On 25 February 2020, the first case of COVID-19 was reported in Madrid and the rapidly evolving spread of the disease disrupted clinical workflows in both primary care and in every hospital department. Since the first patients were admitted, a high AKI incidence and the need for direct nephrological care were observed.
Epidemiological data set AKI incidence in hospitalized patients in the pre-COVID-19 era at 10–15% with a 10% mortality, although AKI definition often varies from the KDIGO guidelines in published literature [18, 19]. The overall incidence of AKI in this study was 17.22% of hospitalized SARS-CoV-2-infected patients. This is higher than early reports from China [20] but lower than published data from the New York City area (22.2%) [21, 22]. In-hospital AKI development of 5% is comparable to results by Cheng et al. [2].
SARS-CoV-2 primarily affected elderly people, who are more prone to AKI due to their comorbidities and their lower renal functional reserve [23]. In the present study, 83% of subjects had at least one chronic condition at admission and 41% of AKI cases had CKD. CKD and hypertension were independent risk factors for AKI along with ARDS and low arterial pressure at admission. ACEi and ARBs were associated with AKI but only in univariate analysis, likely representing the coexistence of conditions that sensitize to AKI development. Our data suggest that antihypertensive treatment together with febrile syndrome and limited access to the healthcare system may contribute to AKI development facilitating hypotension at admission. The higher risk of no resolution of AKI in patients with hypernatremia and acidosis probably defines elderly patients with prerenal state secondary to insufficient water consumption or severe sepsis. Hypernatremia emergency patients with AKI were already reported to have poor outcomes [24, 25]. In this context, CKD patients are a vulnerable population and COVID-19 may aggravate their evolution. CKD increased the risk of both AKI and AKD, and, remarkably, more than half of CKD patients failed to recover baseline renal function, similar to data published by Chan et al. [26].
It is less clear whether SARS-CoV-2 may directly injure the nephrons. In this regard, some patients without prior CKD developed new-onset microhaematuria and proteinuria. This subgroup did not show a worse outcome, but the low number of collected urine specimens limits the interpretation of these findings.
The rate of continuous RRT (CRRT) was lower than in prior reports and we identified several potential contributors [27]. First, CRRT was only possible in the ICU setting, and ICU capacity in our hospital was insufficient to deliver proper medical attention to the whole population at the peak of pandemic. Transfer of ICU patients to other hospitals was not possible until a later stage. Moreover, AKI patients’ eligibility was limited by comorbidities and older age. Given the supplies shortage, care for younger patients was prioritized. Anyway, the nephrology department was not consulted for many patients achieving RRT criteria, probably because of rapidly worsening of respiratory conditions and staff oversaturation. Mortality in AKI patients in the present study was in line with COVID-19 literature reports (8.5–35%) [21, 28]. High mortality in AKI patients may be partly explained by a higher rate of comorbidities, ARDS and older age. However, AKI was an independent risk factor for in-hospital mortality in COVID-19 patients apart from ARDS. The additive effect on mortality is more evident in short-lasting AKI when patients present a more severe inflammatory profile and early death precluded them from reaching AKD condition. This result should concern nephrologists and all specialists attending COVID-19 patients, to optimize treatment based on renal function (Figure 6).
FIGURE 6:
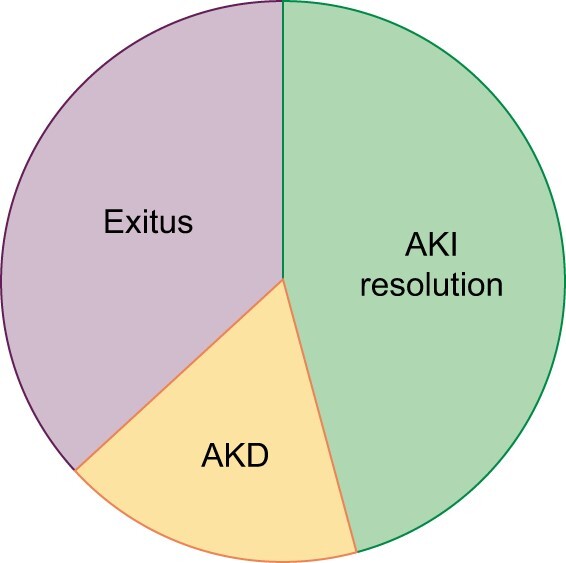
Distribution of AKI resolution, AKD and exitus at the end of follow-up.
When experimental treatments for COVID-19 were analysed, only intravenous corticosteroid administration was associated with higher AKI resolution and lower mortality rates. Hydroxychloroquine showed a similar result, but it may suffer from selection bias because 94% of patients were treated with this drug and untreated patients were often older or at high life risk at admission.
LIMITATIONS
This study has potential limitations. AKI may have been underestimated as patients with baseline sCr <1.00 mg/dL were missed by the strategy to identify AKI. Moreover, these patients may have been labelled as controls and some eligible controls could present unconfirmed SARS-CoV-2 infection.
CONCLUSIONS
In conclusion, AKI is a complication in hospitalized patients with SARS-CoV-2 infection associated with increased mortality. Patients with CKD and hypertension are at higher risk to develop AKI, and renal function can be compromised after discharge. We hypothesize that continuous antihypertensive drug use without adequate medical supervision in symptomatic COVID-19 patients may have contributed to hypotension and the onset of AKI.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
A special thanks to all healthcare personnel who participated in patients care during the COVID-19 pandemic.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDINGS
The authors received no specific funding for this work
REFERENCES
- 1. Guan W-J, Ni Z-y, Hu Y. et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamming I, Timens W, Bulthuis ML. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine-Weber H, Schroeder S. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan XW, Xu D, Zhang H. et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 2020; 46: 1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perico L, Benigni A, Remuzzi G.. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron 2020; 144: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Official epidemiologic reports from Dirección General de Salud Pública, Servicio Madrileño de Salud y hospitales privados – Consejería de Sanidad Comunidad de Madrid 2020. https://www.comunidad.madrid/servicios/salud/2019-nuevo-coronavirus (19 October 2020, date last accessed)
- 9. Levey AS, Stevens LA, Schmid CH. et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 (erratum in: Ann Intern Med 2011; 155: 408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Global Surveillance for human infection with coronavirus disease (COVID-19) Interim guidance 2020. https://www.who.int/publications/i/item/global-surveillance-for-human-infection-with-novel-coronavirus-(2019-ncov) (31 August 2020, date last accessed)
- 11.World Health Organization. Laboratory testing strategy recommendations for COVID-19: interim guidance 2020. https://www.who.int/publications/i/item/laboratory-testing-strategy-recommendations-for-covid-19-interim-guidance (31 August 2020, date last accessed)
- 12. Wu J, Pan J, Teng D. et al. Interpretation of CT signs of 2019 novel coronavirus (COVID-19) pneumonia. Eur Radiol 2020; 30: 5455–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kooraki S, Hosseiny M, Myers L. et al. Coronavirus (COVID-19) outbreak: What the department of radiology should know. J Am Coll Radiol 2020; 17: 447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–c184 [DOI] [PubMed] [Google Scholar]
- 15. Chawla L, Bellomo R, Bihorac A. et al. ; on behalf of the Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 16. Rubio O, Estella A, Cabre L. et al. Recomendaciones éticas para la toma de decisiones difíciles en las unidades de cuidados intensivos ante la situación excepcional de crisis por la pandemia por COVID-19: revisión rápida y consenso de expertos [Ethical recommendations for a difficult decision-making in intensive care units due to the exceptional situation of crisis by the COVID-19 pandemia: a rapid review and consensus of experts]. Med Intensiva 2020; 44: 439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preliminary official reports from ENE-COVID19 study: national study of sero-epidemiology for SARS-CoV2 infection in Spain 2020. https://www.mscbs.gob.es/ciudadanos/ene-covid/docs/ESTUDIO_ENE-COVID19_PRIMERA_RONDA_INFORME_PRELIMINAR.pdf (19 October 2020, date last accessed)
- 18. Al-Jaghbeer M, Dealmeida D, Bilderback A. et al. Clinical decision support for in-hospital AKI. J Am Soc Nephrol 2018; 29: 654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet 2019; 394: 1949–1964 [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Li X, Chen H. et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 2020; 51: 343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson S, Hirsch JS, Narasimhan M. et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, McGoogan JM.. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239. [DOI] [PubMed] [Google Scholar]
- 24. Gao XP, Zheng CF, Liao MQ. et al. Admission serum sodium and potassium levels predict survival among critically ill patients with acute kidney injury: a cohort study. BMC Nephrol 2019; 20: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woitok BK, Funk GC, Walter P. et al. Dysnatremias in emergency patients with acute kidney injury: a cross-sectional analysis. Am J Emerg Med 2020; 38: 2602–2606 [DOI] [PubMed] [Google Scholar]
- 26. Chan L, Chaudhary K, Saha A. et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2021; 32: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang X, Jin Y, Li R. et al. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Version 2. Crit Care 2020; 24: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pei G, Zhang Z, Peng J. et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 2020; 31: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.