Abstract
Background
Understanding the drivers of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission is crucial for control policies, but evidence of transmission rates in different settings remains limited.
Methods
We conducted a systematic review to estimate secondary attack rates (SARs) and observed reproduction numbers (Robs) in different settings exploring differences by age, symptom status, and duration of exposure. To account for additional study heterogeneity, we employed a beta-binomial model to pool SARs across studies and a negative-binomial model to estimate Robs.
Results
Households showed the highest transmission rates, with a pooled SAR of 21.1% (95% confidence interval [CI]:17.4–24.8). SARs were significantly higher where the duration of household exposure exceeded 5 days compared with exposure of ≤5 days. SARs related to contacts at social events with family and friends were higher than those for low-risk casual contacts (5.9% vs 1.2%). Estimates of SARs and Robs for asymptomatic index cases were approximately one-seventh, and for presymptomatic two-thirds of those for symptomatic index cases. We found some evidence for reduced transmission potential both from and to individuals younger than 20 years of age in the household context, which is more limited when examining all settings.
Conclusions
Our results suggest that exposure in settings with familiar contacts increases SARS-CoV-2 transmission potential. Additionally, the differences observed in transmissibility by index case symptom status and duration of exposure have important implications for control strategies, such as contact tracing, testing, and rapid isolation of cases. There were limited data to explore transmission patterns in workplaces, schools, and care homes, highlighting the need for further research in such settings.
Keywords: SARS-CoV-2, COVID-19, transmission, secondary attack rate, contact tracing
In this review, we systematically explore factors that affect transmission of SARS-CoV-2, such as age, symptom status, setting, and duration of contact. Our results highlight the importance of targeting interventions in settings where close and prolonged contacts occur.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus that emerged in China in late 2019 has since spread rapidly around the world, with more than 90 million confirmed cases and more than 2 million deaths reported globally by January 2021 [1]. The severity of the infection, particularly in the oldest age groups [2, 3], has resulted in many countries implementing socially disruptive interventions to prevent onward spread. Early interventions focused on case isolation alongside identification of close contacts. In countries where these measures were insufficient to contain the virus, other nonpharmaceutical interventions (NPIs) were introduced, including “stay-at-home” recommendations, closing schools, working from home, and wearing face masks. Governments are continually faced with the challenge of balancing social and economic harms caused by these NPIs against the resurgence of cases. It is therefore critical to improve understanding of where transmission is taking place so that public health interventions can be better targeted.
To date, there have been relatively few detailed systematic epidemiological studies on transmission of the SARS-CoV-2 virus, with most published studies from the early epidemic in China and reviews focusing on household transmission [4, 5]. These studies provide valuable information on key epidemiological statistics: the secondary attack rate (SAR), defined as the probability of onward infection from an index case among a defined group of close contacts and the observed reproduction numbers (Robs), defined as the observed average number of secondary cases per index case. Quantifying these parameters can help us understand the relative role that different settings play in sustaining transmission through identifying the location and types of contacts that constitute higher transmission potential.
Here, we present a systematic review to estimate SAR and Robs of SARS-CoV-2 in households, schools, workplaces, healthcare facilities, and social settings. In addition, we examine differences in these parameters by age of index cases and their contacts, duration of household exposure to the index case, household size, and symptom status of index cases.
METHODS
Systematic Review
Data Source and Search Strategy
We searched MEDLINE, Embase, MedRxiv, BioRxiv, arXiv, and Wellcome Open Research with no language restrictions up to July 6, 2020, using the search strategy: (“COVID-19” OR “Coronavirus” OR “SARS-CoV-2” OR “2019-nCoV”) AND (“attack rate*” OR “contact*” “OR “cluster*”), adapted for the preprint servers by removing Boolean operators and testing all possible search-term combinations. Studies were screened according to titles and abstracts, and then by review of full texts and their bibliographies. Two reviewers (H. A. T. and A. M.) screened the studies independently using predetermined criteria. Differences were resolved through consensus and discussion with a third reviewer (A. D.). The study protocol can be accessed through PROSPERO (registration number: CRD42020200177).
Inclusion Criteria
Eligible studies for review met the following criteria: (1) provided a definition of the case-contact setting and (2) reported the number of index cases (defined as the first identified case), the number of secondary cases and the total number of contacts, or a SAR and total number of contacts. Studies were included in the meta-analysis if they met 2 additional criteria (1) tested all contacts for SARS-CoV-2 infection regardless of symptom status and (2) reported on more than 1 index case (to minimize publication bias in single-case studies toward reporting larger outbreaks).
Data Extraction
We extracted summary data on study design, contact definition, testing method (reverse transcriptase-polymerase chain reaction/serology), testing strategy (all contacts irrespective of symptoms, symptomatic contacts only, or a subset of contacts), and the number of index cases, contacts identified, contacts tested, and secondary cases. Where available, we additionally extracted the following: age of index case, age of contacts, household size, duration of household exposure to an index case, and symptom status of the index case. Data were obtained directly from the reports, but when not explicitly stated, we obtained additional data from study authors. Studies were assessed for risk of bias using a critical appraisal tool checklist for prevalence studies, adapted to this study [6]. Articles were given a quality score to reflect methodological rigor, clarity, and transparency in reporting relevant to this study’s outcomes.
Statistical Analysis
Articles eligible for meta-analysis were stratified into the following settings: households, schools, workplaces, healthcare, group living, and social contacts. Within household contacts, we undertook a subgroup analysis stratifying by household size and the duration between symptom onset/confirmation and isolation or hospitalization of the index case. We explored age-dependent differences in infectivity and susceptibility across all contact locations and household contacts (stratified by ages 0–19 years and 20+ years). Finally, we examined differences in transmissibility by symptom status of index cases across all exposure locations because of limited studies stratifying by symptom status and exposure location. Stratifications were chosen to maximize the available data across studies.
Because of potential within-study correlation (eg, individuals in the same locations experiencing the same public health interventions and country-specific home, travel, and work practices) and between-study heterogeneity from study and population differences, we employed a beta-binomial model to pool SARs and a Poisson-gamma mixture model to pool Robs across studies (Supplementary Methods) [7–9].
RESULTS
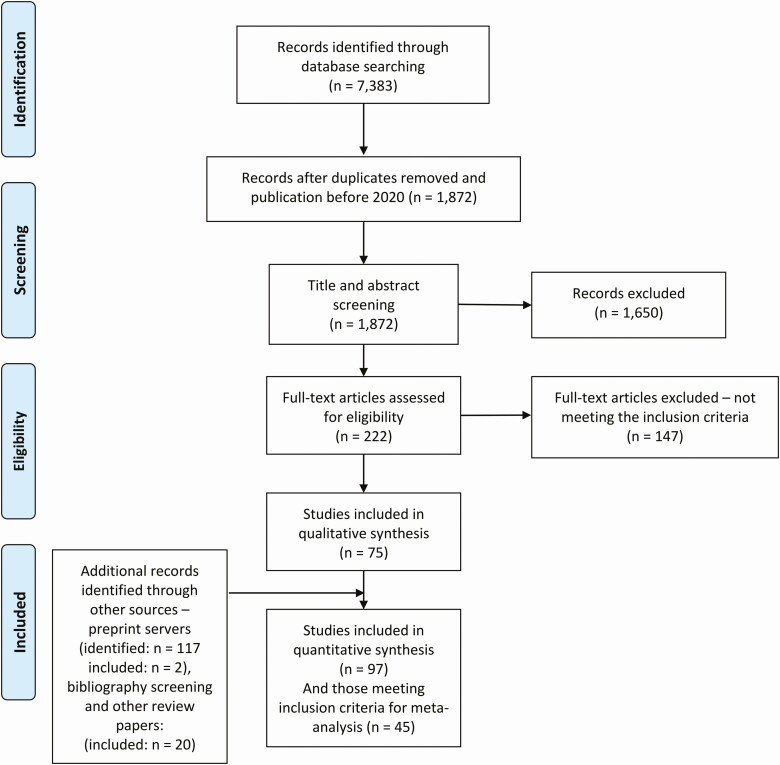
We identified 1872 published studies, 75 of which were included after full-text screening (Figure 1). A further 22 eligible studies were identified through preprint servers and bibliography screening. Of these 97 studies, summarized in Supplementary Tables 1–7, 67 tested all contacts regardless of symptom status. Among those, 45 reported data from >1 index case and were included in the meta-analysis.
Figure 1.
PRISMA flow diagram of study selection.
Household
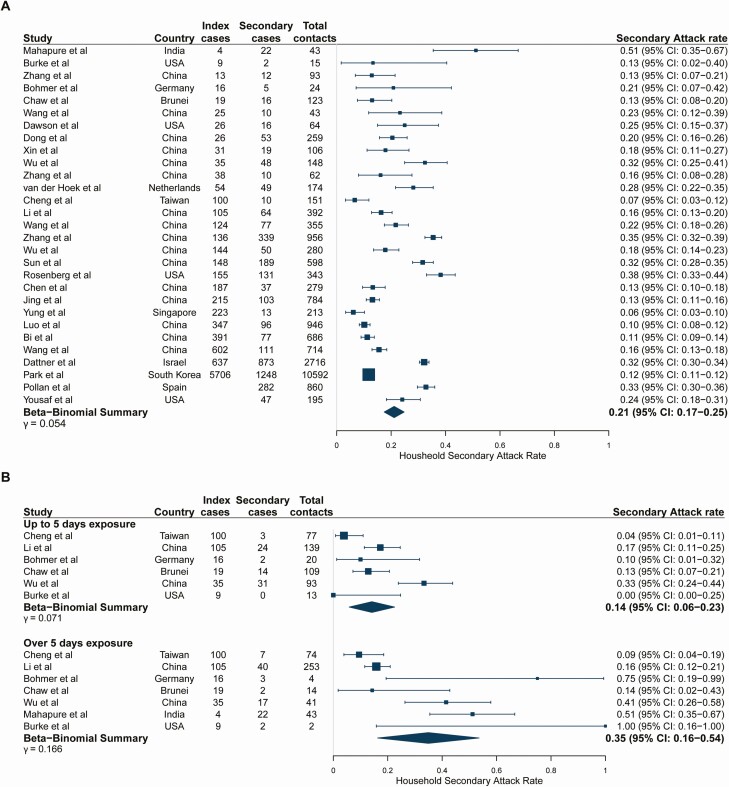
We identified 29 eligible studies reporting household contacts [10–38], with more than one-half carried out in China (Supplementary Table 1). Household definitions were broadly consistent across studies, requiring contacts to be living in the same residence as the index case. One study additionally included nonresident contacts who spent ≤24 hours in the same residence as the index case [22] (Supplementary Table 1), but its inclusion had no significant impact on the pooled SAR estimate.
Study estimates of household SARs ranged from 6.1% [25] to 51.2% [38] with a pooled estimate of 21.1% (95% confidence interval [CI]: 17.4–24.8) (Figure 2A). The relationship between the number of secondary cases and the number of index cases is shown in Supplementary Figure 1. Robs varied across studies from 0.05 [25] to 5.5 [38], with a pooled household Robs of 0.96 (95% CI: 0.67–1.32) (Supplementary Table 8). The SAR increased with longer durations of exposure (14.2% [95% CI: 5.8–22.5] with ≤5 days of exposure to an index case vs 34.9% [95% CI: 16.3–53.6] with >5 days of exposure; P = .05; Figure 2B). Longer durations of exposure were similarly associated with an increased pooled Robs (0.40 [95% CI: 0.21–0.72] with ≤5 days to 1.91 [95% CI: 0.86–3.55] with >5 days, P < .001) (Supplementary Table 9). There was a trend for decreasing transmission with increasing household size, but this was not statistically significant (P = .29) (Supplementary Figure 2). Study-level estimates for the small subset of studies reporting household sizes were highly variable.
Figure 2.
(A) Pooled overall household secondary attack rates. Studies are ordered by the number of index cases reported in the study. Studies of large household contact tracing investigations were included regardless of whether the number of index cases was reported in the study. (B) Stratified by duration of household exposure to symptomatic index case. Studies are ordered by the number of index cases reported in the study because this information was not given by exposure duration. Study-level point estimates and binomial confidence intervals are shown along with the pooled beta-binomial summary across studies. Exposure duration to the index case before isolation or hospitalization categories (≤5 days and >5 days) were selected to maximize usage of data.
Workplaces
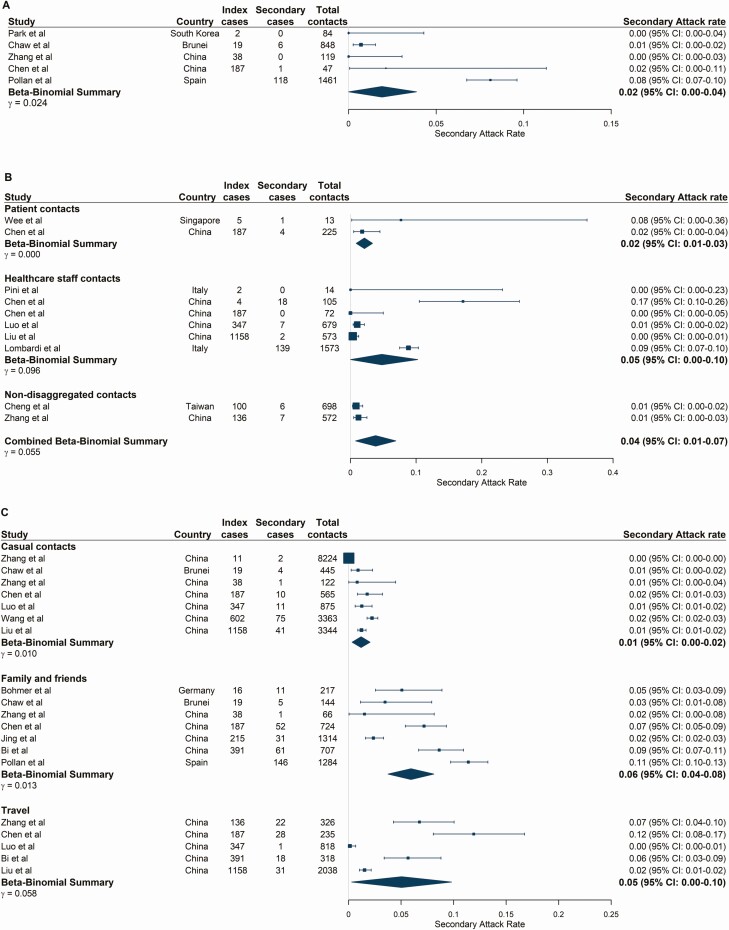
Seven studies reported workplace contacts (Supplementary Table 3) [13, 27, 28, 31, 39–41]. Three of these studies were cluster investigations from a single index case, including a boardroom meeting in Germany (SAR: 91.7% [95% CI: 61.5–99.8], 11/12) [41], supermarket employees in China (SAR: 9.2% [95% CI: 4.7–15.8], 10/120) [28] and call center colleagues in South Korea (SAR: 43.5% [95% CI: 36.8–50.4], 94/216) [40]. Excluding those, the pooled SAR reduced from 12.3% (95% CI: 1.3–22.5) (Supplementary Figure 3) to 1.9% (95% CI: 0.0–3.9) (Figure 3A). Workplace contacts likely represented a variety of sectors across studies, and only 1 study provided a detailed definition [40].
Figure 3.
Secondary attack rates stratified by exposure locations. (A) Workplace-based contacts. (B) Healthcare-based contacts. Beta-binomial summary estimates are presented for patient and healthcare staff contacts of index cases and a combined (nondisaggregated) contacts category. This “combined contacts” summary estimate was pooled across all studies in the healthcare setting and includes 2 studies in which disaggregated contact groups were not reported. (C) Social contact environments. Studies are ordered by the number of index cases reported at the study level. Large population-level studies were included irrespective of whether the number of index cases was reported by the study. Abbreviation: CI, confidence interval.
Healthcare Facilities
Twenty-eight studies reported healthcare-based contacts, 10 of which met our inclusion criteria for meta-analysis (Supplementary Table 4) [30, 33, 39, 42–46], including index case populations of patients [30, 33, 39, 42, 44, 45], healthcare workers [43], or both [21, 46]. Study-level SARs varied from 0.0% to 17.1%, resulting in a pooled estimate among any healthcare contacts of 3.6% (95% CI: 1.0–6.9) (Figure 3B). There was no significant difference in the pooled SAR between patient or healthcare staff contact subgroups (P = .64; Figure 3B). The estimated Robs in healthcare settings was 1.18 (95% CI: 0.65–2.04) but ranged between 0.0 and 4.5 (Supplementary Table 10, Supplementary Figure 1).
Social Settings
Twenty-two studies were identified with social contact settings including travelling, religious events, fitness classes, shopping, entertainment venues, and events with family and friends (Supplementary Table 6). Of these, 13 fulfilled the criteria for meta-analysis [10, 11, 13, 17, 26–28, 30, 31, 37, 39, 45, 47]. We estimate low SARs in low-contact events with casual contacts or strangers, with a pooled SAR of 1.2% (95% CI: 0.3–2.1) (Figure 3C). In contrast, in settings with more familiar and prolonged contact such as events with family and friends, the pooled SAR was 5.9% (95% CI: 3.8–8.1) and Robs was 0.38 (95% CI: 0.18–0.64) (Supplementary Table 11, Supplementary Figure 1). Travel-related contacts had an estimated SAR of 5.0% (95% CI: 0.3–9.8), similar to that in social events with family and friends (Figure 3C, Supplementary Table 6). Robs could not be estimated for any other contact groups because of insufficient data. Several other social settings with high levels of transmission were identified but could not be pooled (Supplementary Results).
Exposure Location Summary
Table 1 summarizes the pooled estimates of SAR and Robs across different exposure locations. Although the highest SARs were estimated for household contacts and in familial settings, the highest Robs was estimated for healthcare settings. Pooled estimates for schools and care homes were not possible because of scarcity of studies (Supplementary Results).
Table 1.
Summary Table of the Pooled SAR and Robs for the Exposure Locations Considered in this Study
| Setting | Pooled SAR (%) | 95% CI | Pooled Robs | 95% CI |
|---|---|---|---|---|
| Households | 21.1 | 17.4–24.8 | 0.96 | 0.67–1.32 |
| Social gatherings with family and friends | 5.9 | 3.8–8.1 | 0.38 | 0.18–0.64 |
| Travel | 5.0 | 0.3–9.8 | … | … |
| Healthcare | 3.6 | 1.0–6.9 | 1.18 | 0.65–2.04 |
| Workplace | 1.9 | 0.0–3.9 | … | … |
| Casual close contacts | 1.2 | 0.3–2.1 | … | … |
Where values are missing, there were not enough data available to estimate a pooled value.
Abbreviations: CI, confidence interval; Robs, observed reproduction numbers; SAR, secondary attack rate.
Age Effects
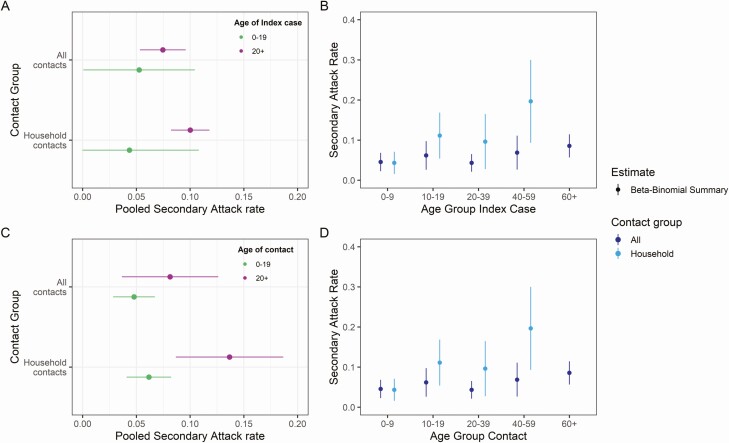
Ten studies provided age breakdowns of index cases and contacts [10, 12, 16, 22, 25, 31, 33, 37, 45, 48] meeting the inclusion criteria. Pooling across all exposure locations, we found no significant differences in transmissibility or susceptibility by age of index cases or contacts (Figure 4 and Supplementary Table 12, Supplementary Figures 4–5).
Figure 4.
Pooled estimates of secondary attack rates by age of the index case and contacts stratified by contact location. (A) Index cases stratified by 0–19 and 20+ year age brackets and exposure location to the index case. (B) Index cases stratified by 10-, 20-, and 40-year age brackets. (C) Contacts stratified by 0–19 and 20+ year age brackets and (D) exposure location contacts stratified by 10-, 20-, and 40-year age brackets. Point estimates were obtained from fitting a beta-binomial model to pooled study data, with 95% confidence intervals shown by horizontal and vertical bars. All contacts combine studies regardless of exposure locations, and household only those studies relating to household transmission. The pooled household secondary attack rates for ages 60+ is not shown because there were insufficient data for this age group.
However, we observed weak evidence for a difference in transmissibility and a significant difference in susceptibility by age in household studies (index 0–19 years: 4.4% [95% CI: 0.0–10.8] vs index ≥20 years: 10.0% [95% CI: 8.2–11.8], P = .07 and contact 0–19 years: 6.16% [95% CI: 4.1–8.2% vs contact ≥20 years: 13.7% [95% CI: 8.7–18.7], P = .01) (Figure 4A, Figure 4C). When further disaggregating age groups, the trend for SARs to increase with increasing age of index cases and contacts was maintained (Figure 4B, Figure 4D). We note that, across contact age groups, the SARs increased at a higher rate for household contacts (Figure 4D).
Symptom Status of Index Case
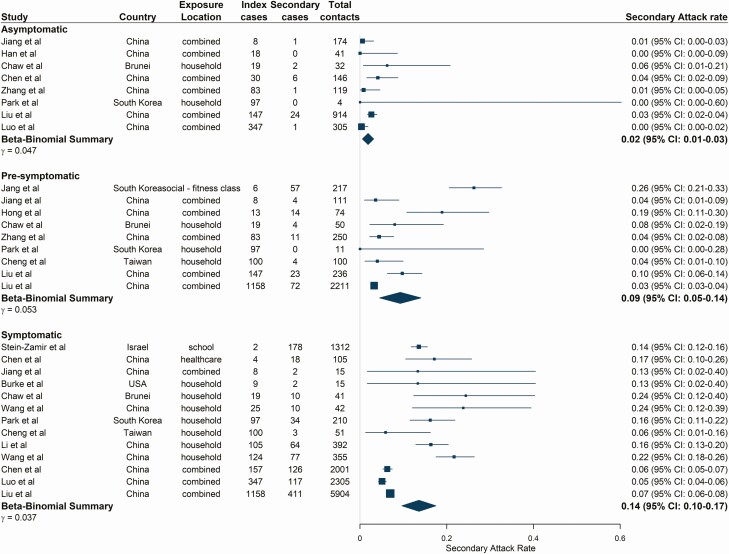
From 18 studies [18, 19, 22, 27, 29–31, 33, 39, 40, 44, 45, 47, 49–53], SAR was lowest for asymptomatic index cases at 1.9% (95% CI: 0.5–3.1) (Figure 5) (asymptomatic definitions and follow-up durations for included studies shown in Supplementary Table 13). SARs were significantly higher for presymptomatic and symptomatic index cases, estimated at 9.3% (95% CI: 4.5–14.0, P = .01) and 13.6% (95% CI: 9.7–17.5, P < .001), respectively (Figure 5), with similar patterns observed for pooled Robs estimates. Asymptomatic index cases resulted in the lowest Robs of 0.17 (95% CI: 0.04–0.45), followed by presymptomatic index cases (Robs = 0.78 [95% CI: 0.36–1.44], P = .004) and symptomatic index cases (Robs = 1.01 [95% CI: 0.57–1.61], P < .001) (Supplementary Table 14, Supplementary Figure 1).
Figure 5.
Estimated secondary attack rates from asymptomatic, presymptomatic, and symptomatic index cases. Studies are ordered by the number of index cases reported in the study as shown in the figure. Pooled estimates combine all exposure locations listed. Combined exposure locations relate to contact tracing studies where close contacts were not disaggregated by exposure location. Asymptomatic index cases were defined as those with a positive severe acute respiratory syndrome coronavirus 2 reverse transcriptase-polymerase chain reaction test and no reported clinical symptoms up to discharge or end of follow-up (at least 14 days). Presymptomatic index cases were defined as those not reporting symptoms at the time of testing or during exposure but later developed symptoms. Symptomatic index cases reported coronavirus disease 2019–associated symptoms at the time of sampling and/or during exposure. Abbreviation: CI, confidence interval.
DISCUSSION
This systematic review provides an indication of the types and places of contacts and index case/contact characteristics that facilitate SARS-CoV-2 transmission. We found evidence to suggest that more familiar prolonged contact increases the potential for transmission, as does the presence or potential for symptoms with moderate age-dependent effects observed at the household level. However, there were limited data to allow exploration of transmission patterns in workplaces, schools, and care homes.
Sustained daily contact within households may explain the high SAR (21.1%), which is comparable to the pooled estimate of an earlier systematic review (18.1%) [5]. Our estimate hides substantial heterogeneity between studies, with reported SARs ranging between 0% and 51%. This is similar to influenza household transmission, where SARs ranged from 1% to 38% [54, 55], but higher than those for SARS-1 (6%–8%) [56,57]. We found evidence of reduced SARs and Robs when index cases were isolated within 5 days of symptom onset, suggesting that household transmission characteristics are influenced by contact tracing and isolation policies. Although SARs could not be estimated in other residential settings, it is not unexpected that these locations report high study-specific attack rates. Elderly residents living in care homes are particularly vulnerable populations, with high risk of severe outcomes and coronavirus disease 2019 (COVID-19)-related mortality [2, 3], and. Understanding dynamics of transmission in these environments is vital to prevent further outbreaks. With many countries continuing to recommend “stay-at-home” measures, with cases isolating inside households, it is likely that this location will continue to be important in sustaining transmission.
We estimated a relatively low SAR (3.6%) in healthcare settings, which is substantially lower than that observed during the early stages of the SARS-1 epidemic [58–60]. This is potentially driven by the large number of identified and tested contacts per index case and stricter measures such as mask mandates and austere visitor policies. Although the probability of onward infection per individual contact is low in healthcare settings, the high number of contacts can lead to more opportunities for infection, which may explain the higher Robs (1.18) in this setting. It is plausible that sufficient infection control and personal protective equipment (PPE) limited transmission potential in these settings but could not be explored because of insufficient data. Notably, all studies took place in high- and middle-income countries where PPE is likely more widely available which, in addition to the diversity of these healthcare systems, make generalizations about SARS-CoV-2 transmission rates in healthcare facilities difficult.
Despite a relatively low transmission potential in index cases who do not develop symptoms (SAR 1.9%), our findings suggest that SAR from exposure to presymptomatic index cases is similar to that of symptomatic cases (9.3% vs 13.6%, P = .08). The difficulty in identifying presymptomatic cases, who have been estimated to account for 47% of onward transmission [61], is particularly challenging for control policies. Our results highlight the continued need for physical distancing policies and widescale testing and contact tracing to identify and isolate those cases not yet showing symptoms. With asymptomatic transmission rates estimated to be significantly lower, this could have positive implications for vaccines that protect against symptomatic disease, potentially reducing the risk of transmission also.
Although studies reporting on the age breakdown of index cases and contacts were limited, we found evidence for age dependence in transmissibility and susceptibility in the household context, which was more limited when pooling across all settings. There is significant uncertainty around these estimates because of the sparsity of data. Therefore, understanding potential age-dependent effects in transmissibility and susceptibility remains challenging. Observed age differences could relate to behavioral, contextual, or biological factors that are not yet fully understood [62]. There is growing evidence to suggest that children tend to experience mild or asymptomatic infections [63–67], which may explain the potential differences observed in SAR from patients younger than 20 years of age. Further to this, symptomatic testing policies may fail to detect childhood infections or identify children as index cases, which could bias estimates.
In contrast to influenza, in which schools are clearly important contributors to transmission [68, 69], school-based studies on SARS-CoV-2 are limited because of reactive school closures. In addition to schools closing, many countries have and continue to encourage work-from-home practices. Furthermore, workplace settings vary greatly not just by country, but also between sectors, with some more able to facilitate COVID-19 safety measures, highlighting the difficulty in ascertaining a universal workplace SAR. Currently, there is insufficient information to explore SARs in different types of schools, workplaces, or sectors.
We estimate relatively low Robs across all settings, suggesting that the use of contact tracing and isolation activities in these studies is an effective measure at reducing onward transmission. However, low numbers of studies were pooled and therefore there was considerable uncertainty in these estimates. Additionally, large cluster outbreaks can occur, as evidenced in several studies [28, 31, 38, 40, 41, 47, 70–73] and often, these events have the potential to overwhelm surveillance systems. The aggregate nature of the data used for our Robs estimates likely hides substantial individual-level heterogeneity in transmission potential, hindering estimation of the overdispersion factor relating to SARS-CoV-2 transmission. Characterizing this potential for “super-spreading events” will be critical for further informing where to direct contact tracing efforts. Early evidence suggests that transmission is overdispersed with around 19% of cases resulting in 80% of transmission in Hong Kong [74] and modeling studies based on reported global cluster sizes that suggested that as few as 10% of cases could account for 80% of all SARS-CoV-2 transmission [75]. The large cluster outbreaks identified in this review tend to be reported in indoor social or workplace settings, potentially highlighting these locations as facilitating super-spreading events [76].
There are several limitations to this study. As in any active outbreak response, data collected from multiple teams under different country guidelines are subject to variations in definitions, follow-up time, and testing protocols, limiting interpretation of pooled data. Furthermore, individual studies suffer from reporting and recall bias in identifying all contacts of a confirmed case. Across settings, index cases were commonly enrolled after presenting for medical attention and therefore can be biased toward infections that result in symptomatic or more serious illness, potentially distorting transmission pathways [54]. Without phylogenetic sequencing, it is difficult to confidently resolve these pathways which could bias SARs estimates; for example, if the index case was asymptomatic and is instead identified as a secondary case of the symptomatic case that first presented for testing or if transmission occurs outside the household but is attributed to a household index case. Globally, there is still a lack of detailed data from contact tracing studies to fully explore differing transmission potential by more precise exposure locations or individual characteristics. In this meta-analysis, the majority of studies came from China, where strict control policies were implemented, possibly limiting the generalizability of estimates. In addition, these studies were performed before the identification of new virus variants with potentially enhanced transmission potential [77]. It will be important to contrast our estimates with studies distinguishing between variants when available. Finally, evidence is continually emerging from newspaper articles, government reports, and press conferences that report on large outbreaks in care homes, schools, workplaces, and hospitals. Such sources cannot be systematically searched or reliably cited, and this information is not translated into published contact tracing studies because of limited public health resources and the prioritization of the ongoing emergency response.
In conclusion, early data suggest that SARS-CoV-2 transmission is highest in locations in which sustained and prolonged contacts are made, including households and other residential locations. With many countries continuing to issue stay-at-home measures, ensuring sufficient and early isolation of cases from other household members will be crucial to prevent spread. The identified similar transmission potential from presymptomatic and symptomatic cases is also challenging for control policies and highlights the importance of enhanced contact testing, that is not reliant on symptom status, rapid isolation, and physical distancing of both symptomatic and asymptomatic cases, as well as the continued use of appropriate PPE. Further research on transmission in different social settings, as well as in schools and workplaces, in which there are limited data to date, is required to continue to inform transmission reduction strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the following authors for providing information on primary studies included in this review and extracting additional relevant data: Drs. Rachel Burke, Hao-Yuan Cheng, Tasuku Hara, and Hsien-Ho Lin, and all of their colleagues involved in the original publications. The authors acknowledge all members of the Imperial College COVID-19 Response Team.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed are those of the authors and not necessarily those of the United Kingdom (UK) Department of Health and Social Care, the National Health Service, the National Institute for Health Research (NIHR), Public Health England (PHE), UK MRC, UKRI, or European Union.
Financial support. This work was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Modelling and Health Economics, a partnership between Public Health England, Imperial College London and the London School of Hygiene and Tropical Medicine (grant number: NIHR200908). We acknowledge funding from the Medical Research Council (MRC) Centre for Global Infectious Disease Analysis (grant number: MR/R015600/1), jointly funded by the UK Medical Research Council and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 program supported by the European Union. In addition, H. A. T. and A. M. are supported by a UK Medical Research Council Studentship (grant number: G98669).
Potential conflicts of interest. A. C. G. reports grants from Medical Research Council during the conduct of the study; grants from Bill and Melinda Gates Foundation, Wellcome Trust, National Institutes of Health, PATH, Medicines for Malaria Venture, and Integrated Vector Control Consortium and personal fees from Malaria programming from The Global Fund, outside the submitted work. N. M. F. reports serving as principal investigator of a grant for the MRC Centre for Global Infection Disease Analysis (UK Medical Research Council), a grant funding the NIHR Health Protection Research Unit for Mathematical Modelling and Health Economics (UK National Institute of Health Research), and a grant funding the Jameel Institute of Disease and Emergency Analytics at Imperial College (Community Jameel), during the conduct of the study. N. M. F. also reports serving as principal investigator of a National Institutes of Health U01 grant under the NIGMS MIDAS program (NIH NIGMS), as the co-investigator on a grant modeling antivirals against dengue (Janssen Pharmaceuticals), as the principal investigator of 2 grants from the Bill and Melinda Gates Foundation, including one funding the Vaccine Impact Modelling Consortium (Bill and Melinda Gates Foundation), and a grant from Gavi, funding the Vaccine Impact Modelling Consortium (Gavi, the Vaccine Alliance), outside the submitted work. All other authors declare no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. 2020. Available at: https://covid19.who.int/. Accessed 25 January 2021.
- 2. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lance t Infect Dis 2020;20:669-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salje H, Tran Kiem C, Lefrancq N, et al. Estimating the burden of SARS-CoV-2 in France. Science 2020; 369:208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madewell ZJ, Yang Y, Longini IM, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis of secondary attack rate. medRxiv Prepr Serv Heal Sci 2020. Available at: http://www.ncbi.nlm.nih.gov/pubmed/32766596. Accessed 17 November 2020. [DOI] [PMC free article] [PubMed]
- 5. Koh WC, Naing L, Chaw L, et al. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PLoS One 2020; 15:e0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute JB. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews: checklist for prevalence studies. Crit Apprais Checkl Preval Stud 2017; 7:147–53. [Google Scholar]
- 7. Williams DA. The analysis of binary responses from toxicological experiments involving reproduction and teratogenicity. Biometrics 1975; 31:949–52. [PubMed] [Google Scholar]
- 8. Pham TV, Piersma SR, Warmoes M, Jimenez CR. On the beta-binomial model for analysis of spectral count data in label-free tandem mass spectrometry-based proteomics. Bioinformatics 2010; 26:363–9. [DOI] [PubMed] [Google Scholar]
- 9. Young-Xu Y, Chan KA. Pooling overdispersed binomial data to estimate event rate. BMC Med Res Methodol 2008; 8:58. Available at: https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-8-58. Accessed 13 November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:911–9. Available at: https://pubmed.ncbi.nlm.nih.gov/32353347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Böhmer MM, Buchholz U, Corman VM, et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis 2020; 20:920–8. Available at: https://pubmed.ncbi.nlm.nih.gov/32422201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis 2020; 26:2465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenberg ES, Dufort EM, Blog DS, et al. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State-March 2020. Clin Infect Dis 2020; 71:1953-9. Available at: https://pubmed.ncbi.nlm.nih.gov/32382743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun WW, Ling F, Pan JR, et al. Epidemiological characteristics of 2019 novel coronavirus family clustering in Zhejiang Province. Zhonghua Yu Fang Yi Xue Za Zhi 2020; 54:E027. [DOI] [PubMed] [Google Scholar]
- 16. van der Hoek W, Backer JA, Bodewes R, et al. The role of children in the transmission of SARS-CoV-2. Ned Tijdschr Geneeskd 2020; 164:D5140. [PubMed] [Google Scholar]
- 17. Wang X, Pan Y, Zhang D, et al. Basic epidemiological parameter values from data of real-world in mega-cities: the characteristics of COVID-19 in Beijing, China. BMC Infect Dis 2020; 20:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Heal 2020; 5:e002794. Available at: https://pubmed.ncbi.nlm.nih.gov/32467353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Zhou Q, He Y, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J 2020; 55:2000544. Available at: https://pubmed.ncbi.nlm.nih.gov/32366488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu J, Huang Y, Tu C, et al. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis 2020; 71:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu Y, Song S, Kao Q, Kong Q, Sun Z, Wang B. Risk of SARS-CoV-2 infection among contacts of individuals with COVID-19 in Hangzhou, China. Public Health 2020; 185:57–9. Available at: http://www.sciencedirect.com/science/article/pii/S0033350620301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burke RM, Balter S, Barnes E, et al. Enhanced contact investigations for nine early travel-related cases of SARS-CoV-2 in the United States. PLoS One 2020; 15:e0238342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xin H, Jiang F, Xue A, et al. Risk factors associated with occurrence of COVID-19 among household persons exposed to patients with confirmed COVID-19 in Qingdao Municipal, China. Transbound Emerg Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yousaf AR, Duca LM, Chu V, et al. A prospective cohort study in non-hospitalized household contacts with SARS-CoV-2 infection: symptom profiles and symptom change over time. Clin Infect Dis 2020:ciaa1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yung CF, Kam KQ, Chong CY, et al. Household transmission of severe acute respiratory syndrome coronavirus 2 from adults to children. J Pediatr 2020; 225:249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang J, Litvinova M, Liang Y, et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science 2020; 368:1481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang W, Cheng W, Luo L, et al. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg Infect Dis 2020; 26:1924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang JZ, Zhou P, Han DB, et al. Investigation on a cluster epidemic of COVID-19 in a supermarket in Liaocheng, Shandong province. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41:E055. Available at: http://europepmc.org/abstract/MED/32340093. [DOI] [PubMed] [Google Scholar]
- 29. Li W, Zhang B, Lu J, et al. Characteristics of household transmission of COVID-19. Clin Infect Dis 2020; 71:1943–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo L, Liu D, Liao X, et al. Modes of contact and risk of transmission in COVID-19 among close contacts. medRxiv 2020. doi: 2020.03.24.20042606. Available at: https://www.medrxiv.org/content/medrxiv/early/2020/03/26/2020.03.24.20042606.full.pdf. [Google Scholar]
- 31. Chaw L, Koh WC, Jamaludin SA, Naing L, Alikhan MF, Wong J. Analysis of SARS-CoV-2 transmission in different settings, Brunei. Emerg Infect Dis 2020; 26:2598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J, Gong X, Wang Z, et al. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res 2020; 286:198043. Available at: https://pubmed.ncbi.nlm.nih.gov/32502551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng H-Y, Jian S-W, Liu D-P, et al. Contact tracing assessment of COVID-19 transmission dynamics in taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. medRxiv 2020. doi:2020.06.03.20121145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dawson P, Rabold EM, Laws RL, et al. Loss of taste and smell as distinguishing symptoms of coronavirus disease 2019. Clin Infect Dis 2021; 72:682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong XC, Li JM, Bai JY, et al. Epidemiological characteristics of confirmed COVID-19 cases in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41:638–41. Available at: http://europepmc.org/abstract/MED/32164400. [DOI] [PubMed] [Google Scholar]
- 37. Jing QL, Liu MJ, Zhang ZB, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahapure KS, Kulkarni NS. Asymptomatic transmission of severe acute respiratory syndrome-coronavirus 2 within a family cluster of 26 cases: why quarantine is important? J Glob Infect Dis 2020; 12:115–6. Available at: https://pubmed.ncbi.nlm.nih.gov/32774003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Y, Wang A, Yi B, et al. Epidemiological analysis of infection among close contacts of novel coronavirus pneumonia in Ningbo. Chinese J Epidemiol 2020; 41:667-71. [Google Scholar]
- 40. Park SY, Kim YM, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis 2020; 26:1666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hijnen D, Marzano AV, Eyerich K, et al. SARS-CoV-2 transmission from presymptomatic meeting attendee, Germany. Emerg Infect Dis 2020; 26:1935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wee LE, Hsieh JYC, Phua GC, et al. Respiratory surveillance wards as a strategy to reduce nosocomial transmission of COVID-19 through early detection: the experience of a tertiary-care hospital in Singapore. Infect Control Hosp Epidemiol 2020; 41:1–6. Available at: https://pubmed.ncbi.nlm.nih.gov/32381147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pini R, Faggioli G, Vacirca A, et al. Is it possible to safely maintain a regular vascular practice during the COVID-19 pandemic? Eur J Vasc Endovasc Surg 2020; 60:127–34. Available at: https://pubmed.ncbi.nlm.nih.gov/32499169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Tong X, Wang J, et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect 2020; 81:420–426. Available at: https://pubmed.ncbi.nlm.nih.gov/32504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu T, Liang W, Zhong H, et al. Risk factors associated with COVID-19 infection: a retrospective cohort study based on contacts tracing. Emerg Microbes Infect 2020; 9:1546–53. Available at: 10.1080/22221751.2020.1787799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lombardi A, Consonni D, Carugno M, et al. Characteristics of 1,573 healthcare workers who underwent nasopharyngeal swab for SARS-CoV-2 in Milano, Lombardy, Italy. Clin Microbiol Infect 2020; 26:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jang S, Han SH, Rhee JY. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg Infect Dis 2020; 26:1917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwok KO, Wong VWY, Wei WI, Wong SYS, Tang JW-T. Epidemiological characteristics of the first 53 laboratory-confirmed cases of COVID-19 epidemic in Hong Kong, 13 February 2020. Eurosurveillance 2020; 25:2000155. Available at: https://www.eurosurveillance.org/content/10.2807/1560–7917.ES.2020.25.16.2000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han X, Wei X, Alwalid O, et al. Severe acute respiratory syndrome coronavirus 2 among asymptomatic workers screened for work resumption, China. Emerg Infect Dis 2020; 26:2265–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hong L-X, Lin A, He Z-B, et al. Mask wearing in pre-symptomatic patients prevents SARS-CoV-2 transmission: an epidemiological analysis. Travel Med Infect Dis 2020; 36:101803. Available at: http://www.sciencedirect.com/science/article/pii/S1477893920302994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang XL, Zhang XL, Zhao XN, et al. Transmission potential of asymptomatic and paucisymptomatic severe acute respiratory syndrome coronavirus 2 infections: a 3-family cluster study in China. J Infect Dis 2020; 221:1948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Z, Chu R, Gong L, Su B, Wu J. The assessment of transmission efficiency and latent infection period on asymptomatic carriers of SARS-CoV-2 infection. Int J Infect Dis 2020; 99:325–7. Available at: http://www.sciencedirect.com/science/article/pii/S1201971220304719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stein-Zamir C, Abramson N, Shoob H, et al. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Euro Surveill 2020; 25:2001352. Available at: https://pubmed.ncbi.nlm.nih.gov/32720636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsang TK, Lau LLH, Cauchemez S, Cowling BJ. Household transmission of influenza virus. Trends Microbiol 2016; 24:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lau LLH, Nishiura H, Kelly H, Ip DKM, Leung GM, Cowling BJ. Household transmission of 2009 pandemic influenza A (H1N1): a systematic review and meta-analysis. Epidemiol 2012; 23:531–42. Available at: /pmc/articles/PMC3367058/?report=abstract. Accessed 19 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lau JTF, Lau M, Kim JH, et al. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis 2004; 10:235–43. Available at: /pmc/articles/PMC3322902/?report=abstract. Accessed 19 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goh DLM, Lee BW, Chia KS, et al. Secondary household transmission of SARS, Singapore. Emerg Infect Dis 2004; 10:232–34. Available at: /pmc/articles/PMC3322927/?report=abstract. Accessed 19 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ho AS, Sung JJY, Chan-Yeung M. An outbreak of severe acute respiratory syndrome among hospital workers in a community hospital in Hong Kong. Ann Intern Med 2003; 139:564–7. Available at: https://pubmed.ncbi.nlm.nih.gov/14530227/. Accessed 19 October 2020. [DOI] [PubMed] [Google Scholar]
- 59. Varia M, Wilson S, Sarwal S, et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ 2003; 169:285–92. Available at: www.cmaj.ca. Accessed 19 October 2020. [PMC free article] [PubMed] [Google Scholar]
- 60. World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). 2003. Available at: https://apps.who.int/iris/handle/10665/70863. Accessed 19 October 2020.
- 61. Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 2020; 17:e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moghadas SM, Fitzpatrick MC, Sah P, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A 2020; 117:17513–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ecdc. COVID-19 in childern and the role of school settings in COVID-19 transmission. 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-schools-transmission-August2020.pdf. Accessed 19 October 2020.
- 64. Patel NA. Pediatric COVID-19: systematic review of the literature. Am J Otolaryngol 2020; 41:102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr 2020; 175:73–80. Available at: https://jamanetwork.com/. Accessed 19 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Raba AA, Abobaker A, Elgenaidi IS, Daoud A. Novel coronavirus infection (COVID-19) in children younger than one year: a systematic review of symptoms, management and outcomes. Acta Paediatr 2020; 109:1948–1955. Available at: https://onlinelibrary.wiley.com/doi/10.1111/apa.15422. Accessed 19 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Heal 2020; 4:653–61. Available at: www.thelancet.com/child-adolescent. Accessed 19 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang L, Peres TG, Silva MVF, Camargos P. What we know so far about coronavirus disease 2019 in children: a meta-analysis of 551 laboratory-confirmed cases. Pediatr Pulmonol 2020; 55:2115–2127. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/ppul.24869. Accessed 19 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhao H, Joseph CA, Phin N. Outbreaks of influenza and influenza-like illness in schools in England and Wales, 2005/06. Eurosurveillance 2007; 12:3–4. Available at: http://www.eurosurveillance.org/content/10.2807/esm.12.05.00705-en. Accessed 19 October 2020. [DOI] [PubMed] [Google Scholar]
- 70. Kar-Purkayastha I, Ingram C, Maguire H, Roche A. The importance of school and social activities in the transmission of influenza A(H1N1)v: England, April - June 2009. Euro Surveill 2009; 14:19311. Available at: https://pubmed.ncbi.nlm.nih.gov/19712642/. Accessed 19 October 2020. [DOI] [PubMed] [Google Scholar]
- 71. Danis K, Epaulard O, Bénet T, et al. Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clin Infect Dis 2020; 71:825–32. doi 10.1093/cid/ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pavli A, Smeti P, Papadima K, et al. A cluster of COVID-19 in pilgrims to Israel. J Travel Med 2020. doi: 10.1093/jtm/taaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yusef D, Hayajneh W, Awad S, et al. Large outbreak of coronavirus disease among wedding attendees, Jordan. Emerg Infect Dis 2020; 26:2165–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang L, Zhang X, Zhang X, et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect 2020; 80:e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Adam DC, Wu P, Wong JY, et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med 2020. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 76. Endo A, Abbott S, Kucharski AJ, Funk S. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res 2020; 5:67. Available at: /pmc/articles/PMC7338915/?report=abstract. Accessed 11 November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qian H, Miao T, Liu L, Zheng X, Luo D, Li Y. Indoor transmission of SARS-CoV-2. Indoor Air 2020; ina.12766. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ina.12766. Accessed 17 November 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.