Abstract
Background
Experiences from the first wave of the 2019 coronavirus disease (COVID-19) pandemic can aid in the development of future preventive strategies. To date, risk prediction models for COVID-19-related incidence and outcomes in hemodialysis (HD) patients are missing.
Methods
We developed risk prediction models for COVID-19 incidence and mortality among HD patients. We studied 38 256 HD patients from a multi-national dialysis cohort between 3 March and 3 July 2020. Risk prediction models were developed and validated, based on predictors readily available in outpatient HD units. We compared mortality among patients with and without COVID-19, matched for age, sex and diabetes.
Results
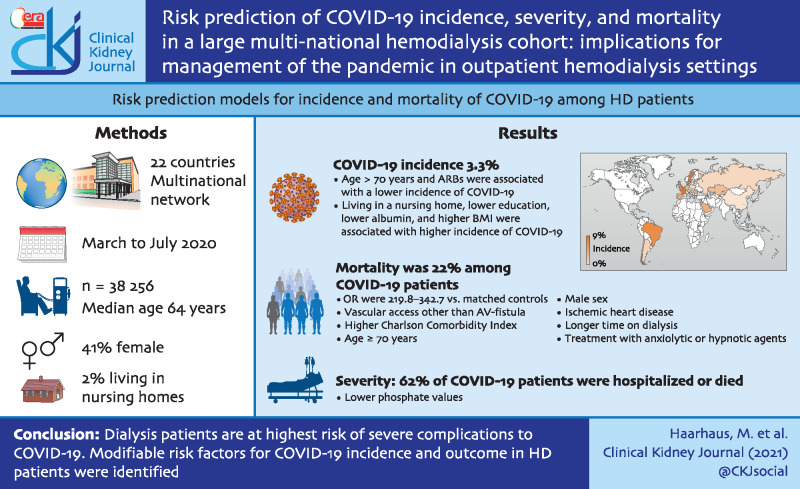
During the observational period, 1259 patients (3.3%) acquired COVID-19. Of these, 62% were hospitalized or died. Mortality was 22% among COVID-19 patients with odds ratios 219.8 [95% confidence interval (CI) 80.6–359] to 342.7 (95% CI 60.6–13 595.1), compared to matched patients without COVID-19. Since the first wave of the pandemic affected most European countries during the study, the risk prediction model for incidence of COVID-19 was developed and validated in European patients only [N = 22 826 area under the ROC curve(AUC)Dev 0.64, AUCVal 0.69]. The model for prediction of mortality was developed in all COVID-19 patients (AUCDev 0.71, AUCVal 0.78). Angiotensin receptor blockers were independently associated with a lower incidence of COVID-19 in European patients.
Conclusions
We identified modifiable risk factors for COVID-19 incidence and outcome in HD patients. Our risk prediction tools can be readily applied in clinical practice. This can aid in the development of preventive strategies for future waves of COVID-19.
Keywords: coronavirus, COVID-19, hemodialysis, mortality, SARS-CoV-2
Graphical Abstract
INTRODUCTION
The 2019 coronavirus disease (COVID-19) is a viral disease due to infection with the novel Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2). COVID-19 has rapidly spread in many countries and on 11 March 2020, the World Health Organization declared it a pandemic. Diagnosis is commonly based on a positive PCR sample for SARS-CoV-2. Reported incidence rates in general populations vary between and within countries, depending on sampling practices and local factors, thus, the actual spreading of COVID-19 is not well known. However, evolving serology data indicate a considerable proportion of asymptomatic individuals among those infected with SARS-CoV-2. As of 30 September 2020, >33 million cases were reported from 188 countries/regions, with 1 004 314 deaths attributed to COVID-19 (Johns Hopkins Coronavirus Resource Centre).
The clinical presentation of COVID-19 varies, ranging from asymptomatic to severe disease with high mortality. The respiratory tract, gastrointestinal tract and cardiovascular system are most frequently affected, but neurologic symptoms, coagulopathies and acute kidney failure among others have also been reported [1]. Elderly patients and those affected by arterial hypertension, diabetes mellitus, obesity, chronic pulmonary diseases and cardiovascular conditions appear to be more susceptible to acquire COVID-19 and to present more severe forms of the disease. These conditions are frequently present in chronic hemodialysis (HD) patients. End-stage kidney disease has been proposed as a model for premature ageing, rendering the study of dialysis patients a valuable instrument for the development of prevention strategies in the elderly population in general. Reports on incidence and the clinical picture of COVID-19 in HD patients are sparse and reflect mostly experiences from regional or national cohorts [2–5]. Incidence rates, based on PCR testing, vary between 2% and 20%, with considerable regional variation. Average cumulative incidences of ˂10% in surviving patients have been described in dialysis populations, based on IgG antibody testing [6, 7], whereas T-cell immunity indicates a higher cumulative incidence than reflected by antibody serology [8]. Case fatality rates in HD cohorts varied between 20% and 35% in previous reports, which was more than twice as high as for COVID-19 in the respective general populations, identifying HD patients as a high-risk population [4, 5, 9]. Our aim was to describe COVID-19 epidemiology and develop risk prediction models for incidence and outcomes of COVID-19 in a multinational HD cohort, comprising >38 000 HD patients from 22 countries.
MATERIALS AND METHODS
Study design and data source
This study is an observational cohort study, performed on the Renal Information Management System (iRIMS), which collects demographic and clinical data prospectively from all patients treated within a multi-national dialysis network. Routine laboratory parameters are collected monthly or at greater intervals for control of anemia, acid-base and electrolyte balance, nutrition, inflammation and chronic kidney disease (CKD)-mineral and bone disorder and dialysis efficacy.
For this study, laboratory data for the observational period between 3 March and 3 July 2020, and the three preceding months were retrieved. In addition, demographic and clinical data, dialysis-specific parameters and information about medications were obtained. Patients with a COVID-19 diagnosis were identified through an incidence reporting system, which is part of the iRIMS registry. A COVID-19 diagnosis was defined by a positive SARS-CoV-2 PCR sample.Severe COVID-19 was defined as a diagnosis of COVID-19 in combination with hospital admission or death. Primary outcome for this study was the incidence of COVID-19. Secondary outcomes were incidence of severe disease and death due to COVID-19. Any death or hospitalization that occurred during the observational period after diagnosis of COVID-19 was registered and considered to be associated with COVID-19. Causes of death are routinely registered in the iRIMS database by dialysis personnel at each dialysis facility, based on hospital records. The categories available in iRIMS are cardiac, cerebral, infection, liver disease, gastro-intestinal bleeding, metabolic, endocrinologic and other. Categorization is performed by health care personnel and treating physician at each dialysis facility. The study was performed in accordance with the Declaration of Helsinki and its amendments. All patients have given informed consent to collection of data in iRIMS. For the current registry study, data were extracted anonymously, therefore, no separate informed consent was required.
Study participants
All patients aged ≥18 years on HD for at least 3 months and admitted permanently to a clinic within the dialysis network between 3 March and 3 July 2020 were included in the study. The observational period started at inclusion and continued until 3 July, death or loss to follow-up. All patients underwent systematic screening for COVID-19 related symptoms at each visit to the dialysis facility, according to a uniform contingency plan during the entire observational period. According to the contingency plan, symptomatic patients underwent PCR testing for SARS-CoV-2, either in the dialysis facility or at the emergency room, if referred to a hospital. Implementation of the contingency plan was monitored during the observational period and no substantial differences between countries were detected with respect to screening and testing of patients. Patients diagnosed with COVID-19 at any time during the observational period were compared to patients without a COVID-19 diagnosis. As many European countries experienced the top of the pandemic during the observational period, whereas most non-European countries were in earlier phases of the pandemic, we analysed the incidence of COVID-19 separately in European and non-European populations.
Statistical analysis
Numeric and categorical data are presented as median with 25th to 75th percentiles and frequencies, respectively. Comparison of categorical data was performed by the chi-square test, or Fisher’s exact test as appropriate. We used Mann–Whitney test and the Kruskal–Wallis test to compare numerical data across COVID-19 status. We applied propensity score matching to compare mortality rates in patients with and without COVID-19 using a logistic regression model, in which COVID-19 status was regressed on observed baseline characteristics (age, sex and diabetes). Four different propensity score methods were used: propensity score matching (one-to-one), stratification (divide subjects into five equal-size groups using the quintiles of the estimated propensity score), inverse probability of treatment weighting using the propensity score and covariate adjustment using the propensity score [10].
Risk prediction models
We developed and validated risk prediction models for the incidence of COVID-19 in European patients and for mortality due to COVID-19 in patients from all countries. We used multivariable logistic regression models, including all variables with a P < 0.2 in the univariate analyses. In addition, the following variables, considered to be of clinical relevance for the predicted outcomes, were used in all multivariable analyses: age, gender, body mass index (BMI), diabetes mellitus, treatment with active vitamin D, hemoglobin, serum inorganic phosphorus, calcium and intact parathyroid hormone (PTH). To adjust for differences between countries, we generated a binary dummy variable for each country and calculated odds ratios (ORs) for each country with all other countries as reference. All countries were included in the multivariable analyses with the exception of countries that demonstrated collinearity. No imputation of missing data was performed, since missing data in predictor variables do not constitute any bias in analyses of complete cases, if the reasons for the missing data are unrelated to the predicted outcomes. Under such circumstances, methods to address missing data are not required to avoid bias [11, 12]. We used 8170 valid cases for the development of the prediction model of COVID-19 incidence in European patients and 453 valid cases for the development of the prediction model of COVID-19 associated mortality. We assessed internal validity with a bootstrapping (Boot) procedure (1000 replications), since Boot is the preferred method for internal validation when the development sample contains a large number of candidate predictors and results in accurate estimates of model performance [13, 14]. The model performance was evaluated by measuring the area under the ROC curve (AUC), sensitivity, specificity and accuracy [15].
RESULTS
Study population
Of the 38 236 HD patients included in the study, 22 826 were European and 15 410 non-European (Supplementary data, Figure S1). Baseline characteristics of the global study population are listed in Supplementary data, Tables S1 and S2. There were 40.8% females, median age was 64 (52–74) years and 1.8% was living in a nursing home. Almost all patients (89.9%) presented with arterial hypertension, 21.3% were treated with angiotensin-converting enzyme (ACE) inhibitors, 18.1% with angiotensin receptor blockers (ARBs), 36.7% had diabetes and 26.5% were obese. Baseline characteristics of the European study population are listed in Supplementary data, TablesS3 and S4. In summary, median age was 68 (57–77) years, there were 39.1% females and 2.6% were living in a nursing home.
Incidence of COVID-19 in all patients and in patients from European countries
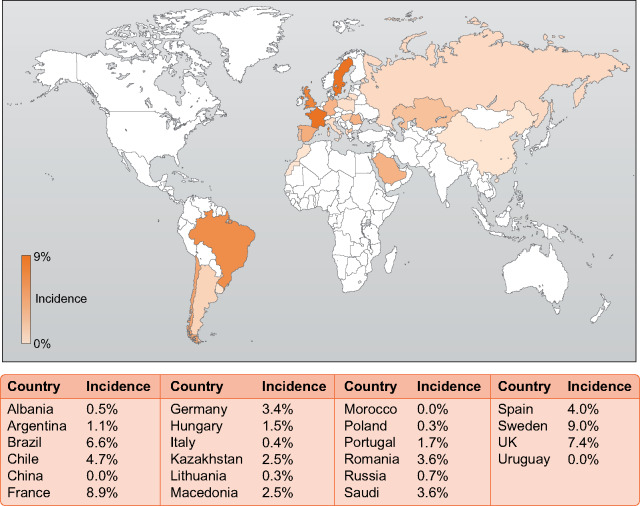
During the observational period, 1259 patients were diagnosed with COVID-19, corresponding to an incidence of 3.3%. Incidence rates per country ranged from 0% to 9% (Figure 1). Incidence rates in the general populations of the countries included in this study are listed in Supplementary data, Table S5. Cumulative incidence, hospitalization and mortality are shown in Figure 2, incidence on a daily basis is shown in Supplementary data, Figure S2. In the European sub-cohort 791 patients (3.5%) were diagnosed with COVID-19, while incidence in non-European patients was 3.0% (N = 468, P = 0.021). The strongest independent predictor of COVID-19 infection was living in a nursing home (Tables 1 and 2). Other common risk factors were a higher BMI, a lower educational level and a lower serum albumin concentration before COVID-19 diagnosis, whereas age >70 years was associated with a lower incidence of COVID-19 (Tables 1 and 2). Noteworthy, the use of ARBs was associated with a lower incidence of COVID-19 in European patients (Table 2).
FIGURE 1:
Incidence rates per country.
FIGURE 2:
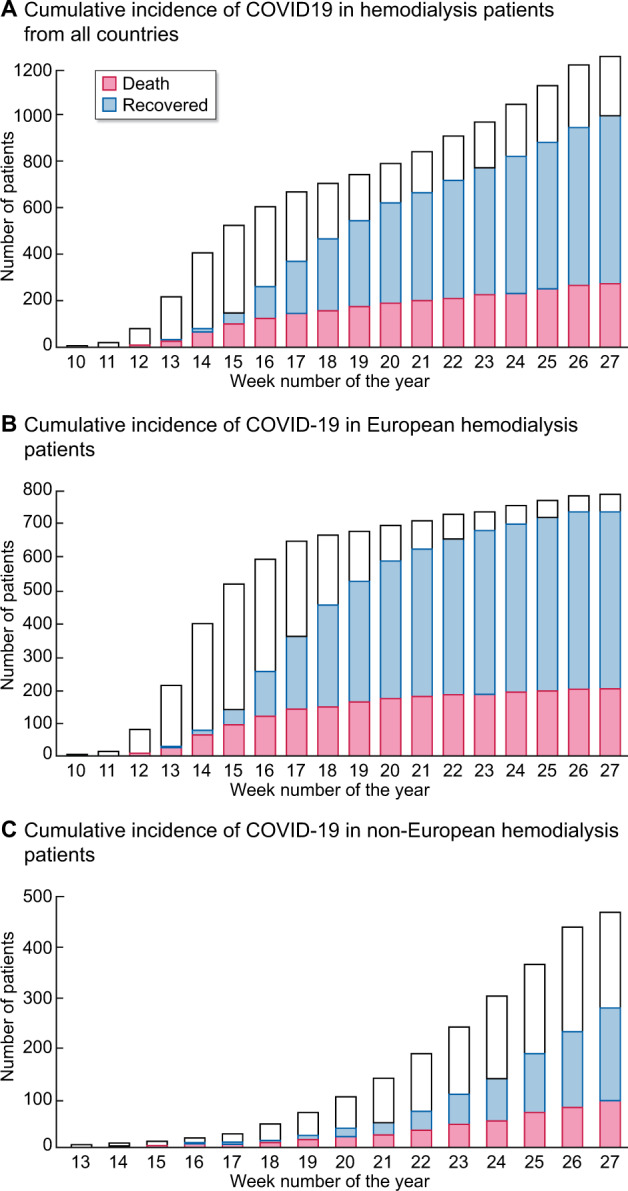
Cumulative incidence, hospitalization and mortality.
Table 1.
Independent risk factors for incidence of COVID-19 in all HD patients
| Independent risk factors for COVID-19 | OR (95% CI) | P-value |
|---|---|---|
| Age (≥70 years) | 0.79 (0.6–0.98) | 0.045 |
| Sex (male) | 0.88 (0.7–1.1) | 0.272 |
| BMI | 1.15 (1.01–1.31) | 0.035 |
| Diabetes mellitus | 1.25 (0.97–1.61) | 0.083 |
| Vitamin D supplementation | 0.96 (0.76–1.21) | 0.707 |
| Hemoglobin | 0.95 (0.85–1.05) | 0.285 |
| Albumin | 0.96 (0.93–1) | 0.032 |
| Inorganic phosphorus | 1.04 (0.96–1.12) | 0.330 |
| Calcium | 0.93 (0.8–1.09) | 0.385 |
| Intact PTH | 0.96 (0.79–1.17) | 0.682 |
| Living in a nursing home | 2.94 (1.52–5.71) | 0.001 |
| Education—primary school | 1.67 (0.48–0.93) | 0.017 |
| Glomerulonephritis | 1.15 (0.84–1.57) | 0.396 |
| Weight gain | 1.03 (0.95–1.11) | 0.540 |
| Cardiovascular disease | 1.04 (0.81–1.33) | 0.773 |
| Gastrointestinal diseases | 1.08 (0.83–1.41) | 0.579 |
| Mental and behavioural disorders | 1.3 (0.9–1.88) | 0.163 |
| HIV infection | 1.39 (0.19–10.2) | 0.743 |
| Parathyroidectomy | 0.55 (0.28–1.09) | 0.085 |
| ARBs | 0.72 (0.53–0.99) | 0.092 |
| ACE inhibitors | 1.18 (0.89–1.56) | 0.262 |
| Nitrates | 1.1 (0.79–1.53) | 0.579 |
| Antidepressants | 1.11 (0.7–1.75) | 0.664 |
| Phosphate binders | 1.09 (0.86–1.39) | 0.479 |
| Calcimimetics | 1.04 (0.72–1.5) | 0.828 |
| Fibrates | 1.24 (0.65–2.37) | 0.515 |
| Oral anticoagulants | 1.41 (0.98–2.03) | 0.061 |
| Statins | 1.13 (0.88–1.46) | 0.338 |
| Acetylic salicylic acid | 1.18 (0.93–1.49) | 0.178 |
ORs were calculated by logistic regression models, adjusted for all variables with a P < 0.2 in the univariate analyse, country and the following variables, considered to be of clinical relevance for the predicted outcomes: age, gender, BMI, diabetes mellitus, treatment with active vitamin D, hemoglobin, serum inorganic phosphorus, calcium and intact PTH. HIV, human immunodeficiency virus.
Table 2.
Independent risk factors for incidence of COVID-19 in European HD patients
| Independent risk factors for COVID-19 in European countries | OR (95% CI) | P-value |
|---|---|---|
| Age (≥70 years) | 0.64 (0.44–0.95) | 0.028 |
| Sex (male) | 1.23 (0.95–1.59) | 0.118 |
| BMI | 1.18 (1.01–1.37) | 0.032 |
| Diabetes mellitus | 0.97 (0.73–1.28) | 0.811 |
| Vitamin D supplementation | 1.13 (0.86–1.47) | 0.376 |
| Hemoglobin | 0.94 (0.79–1.07) | 0.216 |
| Albumin | 0.94 (0.9–0.97) | 0.000 |
| Inorganic phosphorus | 1.10 (1.01–1.23) | 0.032 |
| Calcium | 0.93 (0.78–1.1) | 0.395 |
| Intact PTH | 0.97 (0.79–1.19) | 0.783 |
| Living in a nursing home | 2.7 (1.48–4.91) | 0.001 |
| Education—secondary school | 1.98 (1.51–2.6) | 0.000 |
| Glomerulonephritis | 0.9 (0.64–1.25) | 0.516 |
| Adult polycystic kidney disease | 0.5 (0.26–0.95) | 0.135 |
| Arteriovenous fistula | 1.06 (0.55–2.05) | 0.865 |
| Interdialytic weight gain | 1.01 (0.93–1.11) | 0.764 |
| Gastrointestinal disease | 1.11 (0.86–1.45) | 0.424 |
| HIV virus | 1.31 (0.18–9.49) | 0.790 |
| ARBs | 0.7 (0.49–1.01) | 0.046 |
| ACE inhibitors | 1.03 (0.74–1.41) | 0.881 |
| Nitrates | 1.19 (0.85–1.68) | 0.309 |
| Antidepressants | 1.34 (0.91–1.97) | 0.133 |
| Phosphate binders | 0.94 (0.72–1.23) | 0.666 |
| Calcimimetics | 1.24 (0.82–1.89) | 0.311 |
| Fibrates | 1.07 (0.55–2.11) | 0.837 |
| Oral anticoagulants | 1.17 (0.82–1.69) | 0.386 |
| Statins | 1.23 (0.94–1.61) | 0.128 |
ORs were calculated by logistic regression models, adjusted for all variables with a P < 0.2 in the univariate analyse, country and the following variables, considered to be of clinical relevance for the predicted outcomes: age, gender, BMI, diabetes mellitus, treatment with active vitamin D, hemoglobin, serum inorganic phosphorus, calcium and intact PTH.
Risk factors for death in patients with COVID-19
At the end of the observational period, 979 of the 1259 patients with COVID-19 had survived, corresponding to a case fatality rate of 22%. Distributions of causes of death were similar in COVID-19 positive and negative patients (Supplementary data, Table S6). After propensity score matching, ORs for mortality in patients with COVID-19 were 219.8 [95% confidence interval (CI) 80.6–359] to 342.7 (95% CI 60.6–13 595.1) (Supplementary data, Table S7) compared to matched dialysis patients without COVID-19. COVID 19 patients who died demonstrated a higher Charlson comorbidity index (Supplementary data, Figure S3). Independent risk factors for mortality in patients with COVID-19 are listed in Table 3. Presence of an arteriovenous fistula was associated with improved survival, compared to other types of dialysis vascular access. We observed no association of cardiovascular pharmacotherapy, including ACE inhibitors or ARBs, with mortality.
Table 3.
Logistic regression analysis of risk factors for mortality in all HD patients
| OR (95% CI) | P-value | |
|---|---|---|
| Demographic parameters | ||
| Age ≥70 years | 1.55 (1.08–2.23) | 0.017 |
| Sex (male) | 1.44 (1.02–2.04) | 0.039 |
| BMI | 1.17 (0.97–1.42) | 0.106 |
| Diabetes mellitus | 1.18 (0.84–1.66) | 0.352 |
| Employed/working | 1.24 (0.9–1.71) | 0.189 |
| Separated, divorced or widow | 1.29 (0.82–2.01) | 0.268 |
| Laboratory parameters before infection | ||
| 25(OH) vitamin D (ng/mL) | 0.93 (0.68–1.27) | 0.648 |
| Hemoglobin (1 g/dL) | 0.91 (0.79–1.05) | 0.213 |
| Albumin (g/dL) | 0.97 (0.93–1.02) | 0.253 |
| Inorganic phosphorus (mg/dL) | 0.96 (0.86–1.07) | 0.453 |
| Calcium (mg/dL) | 1.02 (0.84–1.23) | 0.879 |
| Intact PTH (pg/mL) | 1.05 (0.81–1.36) | 0.732 |
| Comorbidities | ||
| Glomerulonephritis | 0.8 (0.48–1.31) | 0.367 |
| Ischemic heart diseases | 1.41 (0.99–2.00) | 0.048 |
| Pulmonary diseases | 0.74 (0.49–1.13) | 0.168 |
| Neoplasms | 1.27 (0.81–1.98) | 0.296 |
| Mental and behavioural disorders | 1.11 (0.71–1.74) | 0.648 |
| Connective tissue disease | 4.22 (0.74–23.97) | 0.105 |
| Other endocrine disease | 1.41 (0.89–2.23) | 0.143 |
| Dialysis related parameters | ||
| AV fistula | 0.6 (0.43–0.84) | 0.003 |
| Time on dialysis (month) | 1 (1–1.01) | 0.015 |
| Medication | ||
| ACE inhibitors | 0.86 (0.57–1.3) | 0.474 |
| Calcium antagonists | 1 (0.74–1.35) | 0.991 |
| Digitalis | 1.27 (0.36–4.43) | 0.708 |
| Nitrates | 1.18 (0.77–1.81) | 0.453 |
| Antidepressant | 1.12 (0.67–1.86) | 0.678 |
| Anxiolytic or hypnotic | 1.72 (1.12–2.64) | 0.014 |
| Statins | 1.08 (0.77–1.52) | 0.646 |
ORs were calculated by logistic regression models, adjusted for all variables with a P < 0.2 in the univariate analyse, country and the following variables, considered to be of clinical relevance for the predicted outcomes: age, gender, BMI, diabetes mellitus, treatment with active vitamin D, hemoglobin, serum inorganic phosphorus, calcium and intact PTH.
Risk factors for severe disease
Severe disease was identified in 775 patients (61.6%). These patients were significantly older (67 years versus 61 years, P < 0.001), had a higher prevalence of diabetes (51.7% versus 40%, P < 0.001), cardiovascular disease (49.5% versus 34.5%, P < 0.001) and a higher Charlson comorbidity index (6 versus 5, P < 0.001). Considering baseline analytical results, severe COVID-19 patients had significantly lower median serum albumin (38.3 mg/dL versus 38.7 mg/dL, P < 0.01) and higher median C-reactive protein (9 mg/dL versus 5.4 mg/dL, P = 0.005). COVID-19 patients with severe disease were also less likely to be treated with ARBs (20% versus 25.4%, P = 0.03) and with ACE inhibitors (16.6% versus 22.6%, P = 0.01). However, in the fully adjusted model, only a lower serum inorganic phosphorus [OR 0.89 (95% CI 0.81–0.99), P = 0.026] evolved as independent risk factor for severe disease.
Performance of risk prediction models for COVID-19 incidence and mortality
Variables entered into the models for prediction of incidence and mortality and their coefficients are shown in Supplementary data, Tables S8 and S9. The model for prediction of COVID-19 incidence revealed an AUC of 0.638 in the original cohort of patients from European countries, which increased to 0.688 in the internal validation cohort, using Boot. The model for prediction of mortality among COVID-19 patients from all countries performed somewhat better, with an AUC of 0.706 in the prediction cohort and 0.788 in the internal validation cohort. Sensitivities and specificities are listed in Table 4.
Table 4.
Performance of predictive models for COVID-19 mortality and incidence in HD patients
| COVID-19 mortality |
COVID-19 incidence in European patients |
|||
|---|---|---|---|---|
| Value | 96%CI | Value | 96%CI | |
| AUC | 0.706 | (0.650–0.763) | 0.638 | (0.619–0.657) |
| AUCBoot | 0.780 | (0.732–0.828) | 0.688 | (0.647–0.728) |
| Sensitivity (%) | 75.7 | (66.8–82.8) | 68.8 | (61.8–75.0) |
| Specificity (%) | 66.2 | (63.0–72.9) | 61.0 | (59.9–62.1) |
| Accuracy (%) | 68.4 | (64.1–72.5) | 61.1 | (60.1–62.2) |
DISCUSSION
In this study, we described COVID-19 epidemiology, identified independent risk factors for symptomatic COVID-19 incidence, mortality and severe disease and developed risk prediction models for incidence and outcomes of COVID-19 in a multinational cohort, comprising >38 000 HD patients from 22 countries. We compared mortality among patients with and without COVID-19, matched for age, sex and diabetes state.
Incidence of COVID-19 was lower in this study than in most previous reports from local or regional dialysis cohorts [2, 4, 16–18], but comparable with reports from national dialysis registries [3, 5] and some regional registries [19, 20]. The relatively low COVID-19 incidence in our report may have been influenced by the COVID-19 contingency/prevention plan, implemented in all participating dialysis centres from February 2020, which included screening of all patients for symptoms of COVID-19, increased body temperature or contact with infected individuals before entering a dialysis facility. As COVID-19 PCR tests were performed in all suspected cases detected during screening, we believe our incidence rates reflect the proportion of symptomatic patients in the cohort quite well. In a recent report, only 9.2% of dialysis patients with SARS-CoV-2 antibodies were estimated to have been diagnosed previously with a COVID-19 PCR test [7], and screening of all patients with SARS-CoV-2 PCR test in a Spanish dialysis centre revealed that 39% of patients with positive test results were asymptomatic [17], suggesting that a considerable number of patients may carry SARS-CoV-2 without developing symptoms. However, some caution is warranted in interpreting these results, as the frequency of positive PCR tests in the former study was estimated from data for the general population and the observational period for the Spanish study was only 11 days.
The strongest independent risk factor for COVID-19 incidence in our cohort of dialysis patients was living in a nursing home, which was in accordance with earlier findings in dialysis patients [18], or general populations [21–23]. This finding may reflect the challenge that infection containment poses in that setting. In spite of a somewhat higher age in infected patients in crude comparison, we found that older age had a protective effect in the fully adjusted model in both, the whole cohort and in European patients. These findings are in accordance with a lower seroprevalence of SARS-CoV-2 antibodies in patients aged 80 or above in a large US dialysis cohort [7] and the absence of age as risk factor for COVID-19 in the French national dialysis registry [3], while a Chinese study found a higher infection risk for patients aged 65 years or above [24]. The seeming contradiction of a protective effect of older age and increased risk for nursing home inhabitants may reflect an increased cautiousness and lesser mobility, resulting in lesser social interaction of elderly people outside nursing homes, earlier acquired unspecific immunity from previous corona—or other infections or vaccinations or more stringent in-centre isolation of patients in this age group. Increasing serum concentration of C-reactive protein and dropping lymphocyte count from the time before pandemics until COVID-19 diagnosis were interpreted as early laboratory signs of COVID-19 infection and confirmed preliminary data in a cohort of 306 dialysis patients [16].
Our finding in European patients gives support to the hypothesis that ARBs may reduce the risk of COVID-19 infection. So far, it has been unclear whether the effect of ARBs is protective or deleterious in COVID-19 infection. A recent meta-analysis found neither positive nor negative associations with COVID-19-related outcomes [25]. The association with incidence of COVID-19 is less well examined. A large population-based study revealed an association of ACE inhibitors, and even more so ARBs, with lower COVID 19 incidence, in line with our findings [26].Experimental studies support both, potentially harmful [27–29] or protective effects [30] via up-regulation of ACE2. Additionally, ARBs are also associated with decreased mortality in patients with pneumonia [31] and anecdotal clinical observations support a potential protective effect of an up-regulation of ACE2 in COVID-19 [32].
In this study, the case fatality rate was 22% among all patients with COVID-19, which is comparable to previous reports [3, 4, 9, 18], whereas reports from Italy revealed higher rates [2, 5]. This study is the largest multi-national report on COVID-19 in dialysis patients, comprising data from a wide variety of countries with considerable variations in reported COVID-19-related mortality in the general population, ranging from around 1% to around 10%, according to data compiled by the Johns Hopkins Coronavirus Resource Centre. In addition, we describe for the first time, dialysis-specific risk factors for COVID-19-associated mortality. A recent report from the European Renal Association COVID-19 database (ERACODA) included 768 dialysis patients with COVID-19 from different European countries and is thus the only other report to date of COVID-19 in a large multi-national cohort [9]. Our findings of a strong association of older age with mortality in COVID-19 patients on dialysis is in accordance with findings in the ERACODA report, which additionally demonstrated an augmentation of age-related mortality by frailty index [9]. In this study, data on frailty was not available. In contrast to the ERACODA report, but in accordance with findings in the general population [33], we identified male sex and ischemic heart disease as independent risk factors for mortality. Possible explanations for this divergence may be the differences in size between this study and the ERACODA (1259 versus 768 patients), in parameters included in the multivariable analyses, and in cohort composition, although we cannot exclude that some patients from this study also were included in the ERACODA report. Infections were overrepresented among causes of death in both, patients with and without COVID-19, which is a discrepancy from other reports of causes of death in dialysis patients [34, 35]. Possible reasons could be an underrepresentation of patients with advanced cardiovascular disease in the current cohort, which consisted mainly of outpatients, the seasonal variation of cardiovascular mortality and a possible over-reporting of infectious causes of death due to increased awareness during the ongoing pandemic.
Reliable prediction of mortality due to COVID-19 has been difficult to accomplish, due to differences in testing practices and definition of COVID-19-related deaths. Thus, estimation of excess mortality during an observational period, compared to previous observational periods, has been suggested as a better measure of the impact of the current COVID-19 pandemic and have resulted in estimates that differ by factor 10 between countries [36]. In contrast, we used propensity score matching and found greatly increased ORs for mortality among COVID-19 patients, which underlines the urgent need for preventive measures in the high-risk HD population. We could not find any other reports on matched comparisons of mortality rates in dialysis patients with and without COVID-19. However, reports from other high-risk populations, such as patients presenting with stroke [37] or myocardial infarction [38], demonstrated increased mortality risks far below those we observed in dialysis patients. In accordance with our findings, a report from the OpenSAFELY health analytics platform identified advanced CKD as one of the strongest predictors of COVID-19 related mortality [33]. Thus, patients with advanced CKD, including dialysis patients, stand out not only as a population with increased susceptibility for COVID-19 infection, but also as a group with one of the highest mortality risks when infected with COVID-19.
The risk prediction models developed in this study comprise exclusively parameters that are readily available in the outpatient settings of HD clinics. Development and validation cohorts included hospital- and community-based clinics from a wide variety of countries, underlining their applicability to different clinical settings. Both, the model for prediction of incidence of COVID-19 and mortality in COVID-19 positive patients, performed acceptably well to excellent, with AUCs between 0.64 and 0.78. No previous descriptions of risk prediction models in dialysis patients exist and reported prediction models for COVID-19-related outcomes were usually developed in small, unselected in-patient cohorts, admitted to single centres for treatment of COVID-19. A large single-centre cohort of unselected outpatients, included at a visit for suspected COVID-19, demonstrated a somewhat better performance of a prediction model for severe disease or mortality, largely based on similar parameters as this study [39]. Moreover, symptoms and chest X-ray findings specific for COVID-19 were also included in the model, which may explain the slightly better performance compared to our results. These parameters were not available in this study. On the other hand, we included dialysis-specific parameters with highly predictive potential, such as type of vascular access and dialysis vintage.
We found univariate associations of severe disease with older age, higher Charlson comorbidity index, diabetes, cardiovascular disease, higher C-reactive protein and lower albumin, although associations did not remain significant in the fully adjusted model. Nevertheless, these factors have previously been related to severity of COVID-19 disease in other settings [2] and are independently associated with increased mortality in the general population and in dialysis patients [40]. Additional factors, highly prevalent in the dialysis population, but not captured by this study, may contribute to the high risk of severe disease in dialysis patients. Indeed, several studies have previously related the compromised immune status of end-stage renal disease patients to a high susceptibility to infectious diseases, poorer response to therapeutic measures and greater propensity for adverse outcomes [41]. Identification of the most vulnerable dialysis patients, implementation of specific preventive measures and timely recognition of the group of patients who would benefit the most from health care interventions could be helpful in the development of strategies to improve outcome related to COVID-19 in dialysis patients.
We defined severe disease as hospitalization or death. These criteria were chosen, as they are objective and readily available in the iRIMS database. However, we cannot exclude the possibility of hospital admissions of patients with mild forms of the disease, according to other definitions of severity, e.g. based on clinical and/or laboratory criteria [1]. Thus, the loss of significances for predictors for severe disease in our fully adjusted model may depend on the uncertain definition of the outcome. Additional studies in this population are needed to clarify, which definition of disease severity is most relevant for management and prognosis of COVID-19 in HD patients.
Strengths of this study were the large size of the cohort, the uniform routines for management of COVID-19 within the dialysis network, and the fact that we had access to detailed demographic and medical data and could relate these to the diagnosis of COVID-19 on an individual patient level. Despite the large number of patients included, we have evaluated simultaneously both European and non-European countries that were in different phases of the pandemic. This may have contributed to a diversity of the sample and, consequently, to the attenuation of potential statistical differences. An extended follow-up of the cohort, covering the peak of the COVID-19 pandemic in all participating countries, may contribute to the clarification of the main factors related to COVID-19 incidence and outcomes, including distinct regional epidemiological factors. Additionally, our work does not include information regarding the clinical characteristics of the COVID-19 episode, limiting the possibility to identify independent risk factors related to severity and prognosis. Future studies, specifically directed to the collection of clinical and interventional data in large dialysis populations may provide additional relevant information regarding potential modifiable factors related to COVID-19 infection. A further limitation of this study is its observational design, which only permits the description of associations. Thus, no causative relationship between COVID-19 and death during the observational period could be established.
In conclusion, we present epidemiologic data for COVID-19 in HD patients from a number of European and non-European countries. We confirm findings from several earlier reports from regional or national cohorts. In addition, we present dialysis-specific risk factors, which have not been presented previously. We also describe for the first time the association of ACE inhibitors and ARBs with incidence and outcome of COVID-19 in HD patients. Our finding of a lower incidence of COVID-19 in patients treated with ARBs in this setting is supported by recent data from the general population. We also present prediction models for incidence and mortality, related to COVID-19 in HD patients, which perform well and which are based on predictors that are readily available in most dialysis clinics. Our data can aid decision-makers and care providers in the development of preventive strategies in preparation for the next wave of the COVID-19 pandemic. Given the high risk for serious complications in HD patients with COVID-19, there is an imminent need for prospective evaluation of preventive and therapeutic strategies.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We want to thank all participating patients and health care personnel, the Diaverum Country Medical Directors and Filiz Akdeniz, RN, Suzanne Pearce, RN, Israel Silva, RN and Marietta Török, MD from the Diaverum Corporate Medical Team for their participation and valuable contributions to this study.
FUNDING
The study was funded by Diaverum AB.
AUTHORS’ CONTRIBUTIONS
Ma.H., C.S., Mi.H. and F.M. developed the research questions, all authors participated in development of study design and analysis plan, C.S. and C.L. supervised data collection, P.V. performed the statistical analyses, Ma.H., C.S., Mi.H., P.V. and F.M. drafted the article and/or revised the final version, Ma.H. wrote the final version of the article, F.M. supervised the study, All authors contributed to the interpretation of data and read and approved the final manuscript. All authors had unrestricted access to all data in the study.
CONFLICT OF INTEREST STATEMENT
Ma.H., C.S., Mi.H., C.L. and F.M. are employees of Diaverum. Ma.H. has received consultation and lecture fees from Resverlogix and Amgen, unrelated to the current research. Mi.H. has received consultation and lecture fees from Siemens Healthcare Diagnostics, Abbott Diagnostics, Roche, Alere, Astute and Baxter, unrelated to the current research. P.M.V. has no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Sheleme T, Bekele F, Ayela T.. Clinical presentation of patients infected with coronavirus disease 19: asystematic review. Infect Dis (Auckl) 2020; 13: 117863372095207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alberici F, Delbarba E, Manenti C. et al. A report from the Brescia renal COVID task force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection.Kidney Int 2020; 98: 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cécile C, Florian B, Carole A.. et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 2020;98: 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corbett RW, Blakey S, Nitsch D. et al. Epidemiology of COVID-19 in an urban dialysis center. J Am SocNephrol 2020;31:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quintaliani G, Reboldi G, Di Napoli A. et al. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society of Nephrology. J Nephrol 2020; 33: 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu X, Sun J, Nie S. et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China.Nat Med 2020; 26: 1193–1195 [DOI] [PubMed] [Google Scholar]
- 7. Anand S, Montez-Rath M, Han J. et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet 2020;396:1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sekine T, Perez-Potti A, Rivera-Ballesteros O. et al. Robust T cell immunity in convalescent Individuals with asymptomatic or mild COVID-19. Cell 2020; 183: 158–168.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hilbrands LB, Duivenvoorden R, Vart P. et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 2020; 35: 1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steyerberg EW, van Veen M.. Imputation is beneficial for handling missing data in predictive models. J Clin Epidemiol 2007; 60: 979. [DOI] [PubMed] [Google Scholar]
- 12. Sterne JA, White IR, Carlin JB. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338: b2393–b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moons KG, Kengne AP, Woodward M. et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012; 98: 683–690 [DOI] [PubMed] [Google Scholar]
- 14. Steyerberg EW, Harrell FE, Borsboom GJ. et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001; 54: 774–781 [DOI] [PubMed] [Google Scholar]
- 15. Moons KG, Altman DG, Reitsma JB. et al. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015; 162: W1–73 [DOI] [PubMed] [Google Scholar]
- 16. Fontana F, Giaroni F, Frisina M. et al. SARS-CoV-2 infection in dialysis patients in northern Italy: a single-centre experience. Clin Kidney J 2020; 13: 334–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rincón A, Moreso F, López-Herradón A. et al. The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin Kidney J 2020; 13: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller N, Chantrel F, Krummel T. et al. Impact of first-wave COronaVIrus disease 2019 infection in patients on haemoDIALysis in Alsace: the observational COVIDIAL study. Nephrol Dial Transplant 2020; 35: 1338–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiong F, Tang H, Liu L. et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysispatients in Wuhan. J Am SocNephrol 2020; 31: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yau K, Muller MP, Lin M. et al. COVID-19 outbreak in an urban hemodialysisunit. Am J Kidney Dis 2020;76: 690–695.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Man P, Paltansing S, Ong DSY. et al. Outbreak of COVID-19 in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin Infect Dis 2020;ciaa1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McBee SM, Thomasson ED, Scott MA. et al. Notes from the field: universal statewidelaboratory testing for SARS-CoV-2 in nursing homes - West Virginia, April 21-May 8, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1177–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kittang BR, Hofacker SV, Solheim SP. et al. Outbreak of COVID-19 at three nursing homes in Bergen. Tidsskr nor Laegeforen 2020; 140 [DOI] [PubMed] [Google Scholar]
- 24. Xu X, Nie S, Sun J. et al. The cumulative rate of SARS-CoV-2 infection in Chinese hemodialysispatients.Kidney Int Rep 2020; 5: 1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo KB, Bhargav R, Salacup G. et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers and outcomes in patients with COVID-19: asystematic review and meta-analysis. Expert Rev CardiovascTher 2020;18:919–930. [DOI] [PubMed] [Google Scholar]
- 26. Hippisley-Cox J, Young D, Coupland C. et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart 2020; 106: 1503–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallagher PE, Ferrario CM, Tallant EA.. Regulation of ACE2 in cardiac myocytes and fibroblasts.Am J Physiol Heart Circ Physiol 2008; 295: H2373–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perico L, Benigni A, Remuzzi G.. Should COVID-19 concern nephrologists? Why and to Whatextent? The emerging impasse of angiotensin blockade.Nephron 2020; 144: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaur U, Acharya K, Mondal R. et al. Should ACE2 be given a chance in COVID-19 therapeutics: A semi-systematic review of strategies enhancing ACE2. Eur J Pharmacol 2020; 887: 173545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imai Y, Kuba K, Rao S. et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436: 112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calò LA, Davis PA, Rigato M. et al. Angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers and risk of COVID 19: information from Bartter's and Gitelman’s syndromes patients. J Hypertens 2020; 38: 1386. [DOI] [PubMed] [Google Scholar]
- 33. Williamson EJ, Walker AJ, Bhaskaran K. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Usvyat LA, Carter M, Thijssen S. et al. Seasonal variations in mortality, clinical, and laboratory parameters in hemodialysis patients: a 5-year cohort study. Clin J Am SocNephrol 2012; 7: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goto S, Hamano T, Ogata S. et al. Seasonal variations in cause-specific mortality and transition to renal replacement therapy among patients with end-stage renal disease. Sci Rep 2020; 10: 2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beaney T, Clarke JM, Jain V. et al. Excess mortality: the gold standard in measuring the impact of COVID-19 worldwide? J R Soc Med 2020; 113: 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin C, Zhou L, Hu Z. et al. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke 2020; 51: 2219–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solano-López J, Zamorano JL, Pardo Sanz A. et al. Risk factors for in-hospital mortality in patients with acute myocardial infarction during the COVID-19 outbreak. Rev EspCardiol (Engl Ed) 2020; 73: 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Banerjee A, Pasea L, Harris S. et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet 2020; 395: 1715–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi SR, Lee YK, Cho AJ. et al. Malnutrition, inflammation, progression of vascular calcification and survival: Inter-relationships in hemodialysis patients. PLoS One 2019; 14: e0216415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease.Nat Rev Nephrol 2013; 9: 255–265 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.