Abstract
The novel coronavirus, called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic in March 2020 by the World Health Organization. Older individuals and patients with comorbid conditions such as hypertension, heart disease, diabetes, lung disease, chronic kidney disease (CKD) and immunologic diseases are at higher risk of contracting this severe infection. In particular, patients with advanced CKD constitute a vulnerable population and a challenge in the prevention and control of the disease. Home-based renal replacement therapies offer an opportunity to manage patients remotely, thus reducing the likelihood of infection due to direct human interaction. Patients are seen less frequently, limiting the close interaction between patients and healthcare workers who may contract and spread the disease. However, while home dialysis is a reasonable choice at this time due to the advantage of isolation of patients, measures must be assured to implement the program. Despite its logistical benefits, outpatient haemodialysis also presents certain challenges during times of crises such as the coronavirus disease 2019 (COVID-19) pandemic and potentially future ones.
Keywords: COVID-19, ESRD, haemodialysis, home dialysis, peritoneal dialysis
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the coronavirus disease 2019 (COVID-19) pandemic, has up to now more than 35 million cases and caused more than 1 million deaths in 235 countries around the world, according to data from the World Health Organization [1]. Its spread has affected the general population with symptoms often similar to those of a common first-airway infection, while in fewer instances it has led to a severe respiratory syndrome with high fever and bilateral interstitial pneumonia, often requiring hospitalization in intensive care wards, and followed by an adverse outcome [2]. The infection fatality rate in general population has been estimated to range from 0.5% to 1% [3], but there is a great variability among countries and clusters of population. Infectivity of the virus is high; it occurs by aerosols through the spread of droplets released by infected people, by coughing, sneezing and even talking in close contact, even if there are no clear symptoms. The disease has a more severe manifestation in some subjects in relation to older age and the presence of comorbid diseases [4] (Table 1). This is especially evident in patients with chronic kidney disease (CKD) and more dramatically in those undergoing haemodialysis (HD) treatment. The spread of the COVID-19 pandemic has highlighted the importance of limiting social activities to decrease the risk of exposure to SARS-CoV-2. Dialysis patients are considered to be at higher risk of developing severe COVID-19 because of their immunocompromised status and the underlying medical condition. Home-based renal replacement therapies have many advantages in this regard.
Table 1.
Risk for hospitalization for COVID-19 infection related to underlying medical conditions
| Underlying medical condition | Increasing risk (aRR) | CI (95%) |
|---|---|---|
| Age 65+ years (uRR) | 2 | 1.8–2.1 |
| Male (uRR) | 1.2 | 1.1–1.4 |
| Asthma | 1.4 | 1.1–1.7 |
| Hypertension | 2.8 | 2.3–3.4 |
| Coronary artery disease | 1.3 | 0.99–1.4 |
| Obesity (BMI ≥30 kg/m2) | 2.9 | 2.3–3.5 |
| Diabetes | 3.2 | 2.5–4.1 |
| CKD | 4.0 | 3.0–5.2 |
| Severe obesity | 4.4 | 3.4–5.7 |
| Two conditions (uRR) | 4.5 | – |
| Three or more conditions (uRR) | 5 | – |
uRR, unadjusted rate ratio; aRR, adjusted rate ratio (modified from Ref. [4]); CI, confidence interval; BMI, body mass index.
GROUNDS FOR INCREASED RISK OF COVID-19 INFECTION IN CKD AND DIALYSIS PATIENTS
CKD patients are particularly vulnerable to respiratory diseases due to a functionally inadequate immune system [5] and to the need for frequent hospital admissions. The dysfunction of the immune system in uraemic patients is related to alterations in both the innate and adaptive immune systems. These two alterations, associated with the presence of uraemic toxins, nutritional deficiencies and immunosuppressive therapies, make infectious diseases the second leading cause of death in CKD patients, after cardiovascular disease. This impairment results in an increased susceptibility to bacterial and viral infections, poor vaccination responses and increased risk of malignancies. These changes in immune system might resemble premature immunological ageing and might reflect an alteration in the lymphoid and myeloid hematopoietic stem cell ratio [6]. In a recent survey [7] conducted by the Italian Society of Nephrology on the risks of infection by SARS-CoV-2 of CKD patients undergoing renal replacement therapy (RRT) during the exponential growth phase of the pandemic in the country, it was found that, during the surveyed period, 1368/60 441 patients (2.26%) had contracted SARS-CoV-2 infection, while the percentage of infection in the general population was estimated to be around 0.4% [8]. A second important risk factor for SARS-CoV-2 infection in dialysis patients can be related to the organization of hospital facilities in providing dialysis treatment.
HAEMODIALYSIS TREATMENT
Replacement dialysis treatment can be performed using three main modalities: HD, peritoneal dialysis (PD) and transplantation. In the previously mentioned survey of the Italian Nephrology Society, out of 60 441 patients on RRT, 51% were on HD, 7% on PD and 42% on transplantation. In the United States Renal Data System (USRDS) registry relating to the year 2017, HD patients accounted for 62.9%, PD patients for 7.1% and transplant patients for 30% [9]. Worldwide, 89% of dialysis patients living in high–medium-income countries are on a HD treatment, and the use of this modality of RRT is growing faster in Latin American countries than in Europe and the USA [10]. Thus, the dialysis treatment in most developed countries is carried out almost exclusively by HD in hospital or limited care Units, at three sessions a week, with an average duration of 4 h each. This dialysis schedule inevitably leads, given the increasing number of patients, to setting up dialysis rooms with more and more patients and that are consequently more crowded. Often the dialysis room is filled with so many dialysis beds that it is not possible to maintain an adequate safety distance between patients. In addition, HD patients are transported to the hospital dialysis Units from their homes in groups, almost never individually, and wait for the start of the HD treatment in a waiting room that, due to spatial problems, often is not able to ensure adequate distance between pateints, and which sometimes does not have proper air circulation. In such a scenario of a pandemic with high diffusivity, it is clear that patients undergoing hospital HD may be more prone to be infected than the general population with comparable comorbidities and age. In light of this, under the pressure of the pandemic, in order to reduce the risk of infection by SARS-CoV-2 in dialysis patients it seems crucial to explore new management modalities. There may be options other than intra-hospital dialysis performed with a 3-weekly treatment schedule of 4 h, although so far they have not had extensive application. Home HD (HHD), incremental HD, nocturnal HD and PD are treatment modalities that are well known, well-structured and have already been used for a long time, with varying success [11]. Their level of use differs widely: with regard to extra-hospital techniques, it ranges from a minimum of 3% in Japan to 28% in New Zealand, reaching 46% in Hong Kong (Figure 1).
FIGURE 1:
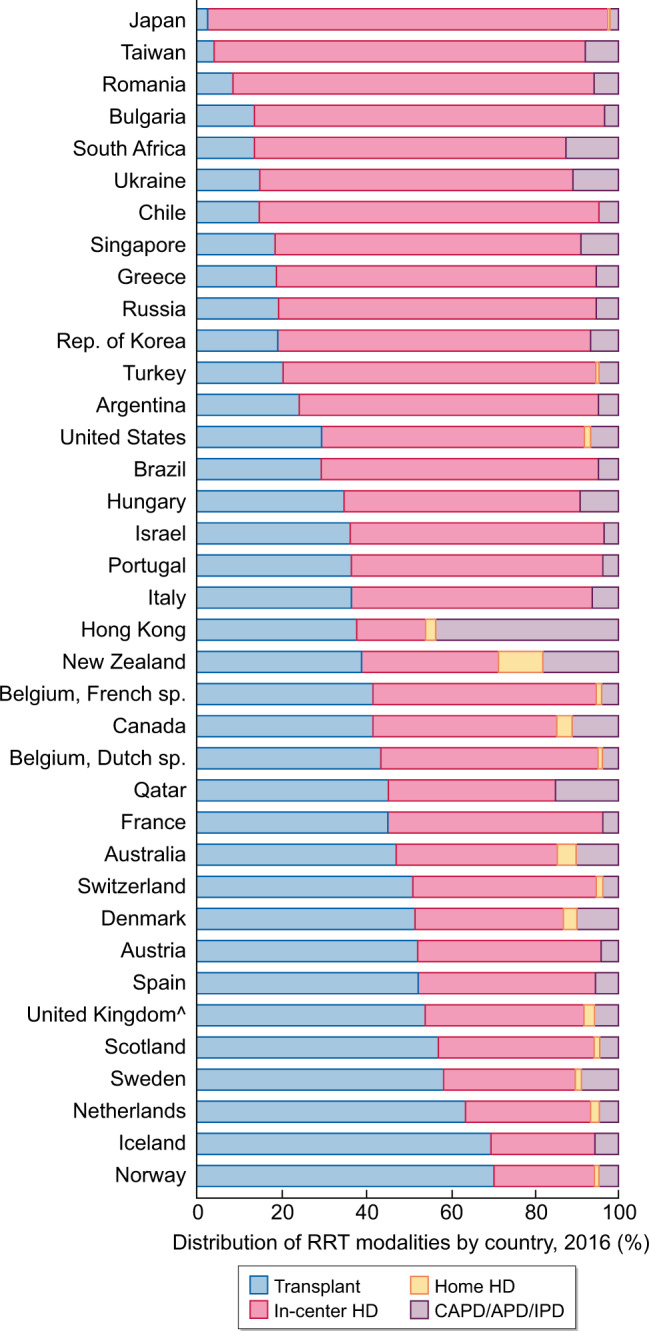
Per cent distribution of RRT modalities, by country, 2016 (modified from Ref. [12]). CAPD, continuous ambulatory peritoneal dialysis; IPD, intermittent peritoneal dialysis.
DEVELOPMENT AND SHRINKAGE OF HHD
The rise of HHD began in the 1960s and 1970s in the USA and the UK due to the pressing need to provide dialysis treatment to an increasing number of patients in the face of a shortage of intra-hospital dialysis workstations [13]. In the early 1970s, in fact, 59% of patients in the UK and 32% in the USA were performing HD at home, especially overnight [14]. In the last 50 years, there has been a huge growth in the number of patients with end-stage renal disease (ESRD) worldwide, with a corresponding exponential growth in the number of dialysis patients. Despite this exponential growth of the total number of dialysis patients, the percentage of those in HD at home since the 1970s has decreased and remains surprisingly low [12], while the number of patients dialysed in hospital and satellite Units has increased significantly. It is quite complicated to get accurate information about the number of dialysis patients that are home-treated in the world. Not all dialysis registries collect this data, and in addition, there is a significant difference between the number of patients starting an HHD program and those who continue one for a substantial period of time. According to a historical paper (2006), the prevalence of HD at home showed a wide variability between the various countries, ranging from a high use in Australia and New Zealand (39 and 58.4%, respectively) to a much lower one in the USA (4.6%) and in other European countries (Scotland and Holland, 8.6 and 6.2%, respectively) [15]. More recent data show a further reduction in the percentage of HHD in Europe with a percentage <2%, and only slightly higher (4%) in the UK [16]. Currently, data referring to Italy show a prevalence of HHD of 1.08% of a total of 46 000 patients in hospital or limited care HD [17]. This underutilization of HHD seems in contrast to the several benefits that, even in non-COVID times, are widely described in the literature [12]. HHD is in fact able to provide maximum flexibility in treatment schedules to customize the dialysis treatment by modifying blood flow and dialysate, treatment duration and frequency. This flexibility can also be adapted to the patient's needs to leave much of the day available for the patient's activities, by varying the timetable (night/day and so on). The clinical benefits of HHD are manifested in both improved survival and reduced morbidity compared with 3 weekly in dialysis Units [18, 19]. HHD allows patients a more physiological treatment scheme as it can be performed daily or at least avoiding the long inter-dialytic gap, reducing cardiovascular stress by using a lower ultrafiltration rate. It is well known, in fact, that high ultrafiltration rates during treatment are an important cardiac risk factor [20, 21]. In addition to the advantages related to a reduction of left ventricular hypertrophy, an appropriate HHD schedule allows better management of blood pressure and phosphate control with a reduction of drugs in both instances [22, 23]. Also from an economic point of view, both nocturnal and daily HHD have proven to be cost-effective compared with intra-hospital dialysis [24, 25].
OVERCOMING BARRIERS TO HHD
In the clinical practice mainly adopted so far in Western countries, the choice to encourage patients to adhere to an HHD treatment program has encountered several obstacles, partly patient dependent, partly nephrologist dependent. On the patient’s side, there may be no willingness to adhere for fear of making mistakes, fear of not having an adequate treatment equal to that provided in hospital dialysis, fear of being left alone to manage their life-saving treatment, fear of needle puncture, etc. [26]. In addition, nephrologists themselves are not convinced enough of the usefulness of the procedure so that it is not even proposed to the patient or implemented among the dialysis modalities. The possibility to perform HHD should be discussed with all CKD Stages 4–5 patients, to try to promote independence and autonomy, to improve quality of life and to obtain clinical benefits. Clearly, the counselling process prior to dialysis initiation appears to be in favour of the choice of HHD over conventional in-centre dialysis, and pre-ESRD educational intervention has been proven to be the best contributor to orientation toward HHD [27, 28]. Furthermore, often a high percentage of patients are placed under nephrologist care too late, just a few weeks before they have to start dialysis [29]. So starting HD in hospital as a late referral, these patients are not aware of the possibility of HHD treatment. It is advisable that all patients with ESRD are properly educated on the choice of method when their glomerular filtration rate is such that there is still time before the need to begin replacement therapy, although a well-structured educational program can also begin after an urgent-start dialysis, with good results of a transition to home modality [30]. Appropriate identification of suitable patients should be a priority for dialysis Units so they can refer as many patients as possible to a home treatment. Some years ago the International Society of Haemodialysis published, with the contribution of nephrologists and nurses experienced in the management of home dialysis programs, a comprehensive manual [31] that should provide adequate support and knowledge to expand the home program, useful for those nephrologists not trained in home dialysis. The recent initiative called ‘Advancing American Kidney Health’ designed to reduce risk of kidney failure has three important goals, one of which is to send 80% of new patients with ESRD to home dialysis or transplant by 2025, with the aim, if successful, to transform the lives of CKD patients in the USA and change most of the clinical practice of nephrology [32]. To date, in the presence of the COVID-19 pandemic, specific data on the epidemiology and clinical picture of the infection in patients on HHD are still not well defined. However, it is reasonable to assume that, since there are no substantial differences in comorbidities, immunocompromised state and metabolic alterations, the theoretical susceptibility to COVID-19 does not differ between patients in HD Units and those at home. The theoretical advantage of home dialysis for patients is that they can perform dialysis treatment at home without the need to move to the hospital setting, thus reducing the possibility of exposure to the risk of infection. While benefiting from this undoubted advantage during a period of pandemic, patients on HHD may suffer from forced isolation, from problems in the supply of dialysis products, from difficulty in carrying out blood tests and from reduced ability to have direct contact with healthcare staff [33]. The possible expansion of the use of HHD in the COVID-19 era will have an impact on the organization of dialysis Units.
Our experience, gained during the COVID-19 pandemic, offers the opportunity to modify the current dialysis care system through a new approach oriented to the clinical and social priorities of the patient. HHD could be a tool to improve patient outcomes, developing new procedures under the umbrella of local and national rules, in which macroeconomic and regulatory innovations should be made by central decision-makers [34].
The manufacturing companies should receive input for the development and research of materials and devices that are more biocompatible and efficient, easy to manage at the patient's home, and with bidirectional communication capabilities with the dialysis units. Educational programs should be activated early in the advanced stages of the disease. These should accomplish a knowledge and familiarization with all the available treatment modalities, presenting the intrinsic pros and cons, providing patients with the theoretical and practical tools to overcome possible difficulties. This educational activity can only be managed by trained and committed medical and nursing staff who are very confident in these proposed choices. One factor that may explain the under use of educational benefits is that nephrologists do not receive extensive training in HHD. In fact, it has been shown that only ∼15% of nephrologists feel confident in their approach to HHD patients, and those who feel confident in HHD tend to prescribe it to a greater extent [35]. The use of bidirectional communication technologies operating in real-time would achieve many goals: (i) limiting to the lowest possible extent the patient’s fear of treatment responsibility (the self-puncturing problem for instance); (ii) limiting the coming of patients to the hospital; (iii) monitoring the patient’s clinical condition; and (iv) providing at the same time an accurate control of patient compliance. Telehealth and telemedicine systems must be integrated with equipment software, which must have a simple and intuitive patient interface. During the COVID-19 pandemic in the New York City, it was possible for a care provider to offer the possibility to perform monthly visits to 150 patients on PD and 60 on HHD remotely, through systems integrated with the software of the PD and HD machines [36]. The experiences brought about by necessity due to the COVID-19 pandemic should stimulate new perspectives to implement the best treatment for each patient with ESRD. Telehealth and telemonitoring procedures are necessary to ensure treatment safety by expanding the range of treatments to as many patients as possible.
Furthermore, dialysis costs are high and likely are continuing to rise due to increased life expectancy and morbidities in ESRD. RRT accounts for 5–7% of the total healthcare budgets, despite affected patients only representing 0.1–0.2% of the general population [37]. HHD treatments (HD and PD) have economic advantages over in-hospital dialysis, but despite this, except in countries such as Hong Kong and Thailand where the choice of treatment is not free, their use is marginal, likely due to the presence of barriers and conflicting opinions among practitioners and policymakers [38]. It is therefore not possible to produce a universal paradigm, given that from an economic perspective, conditions may vary greatly between different countries. In this regard, data from the Australian dialysis registry show a 38% difference in treatment costs between in-hospital HD and HHD, with the cost of PD very similar to the latter [39].
For better HHD outcomes and patient acceptance, remote counselling should be organized with dedicated staff able to ensure the widest possible temporal coverage, enhancing the patient’s life choices. The use of the latest generation of dialysis machines can allow the monitoring of dialysis adequacy and minimize the need for blood tests, which could be performed at the patient’s home [11, 33]. Precise pathways for the treatment of urgent situations that cannot be solved by remote assistance should be organized, along with psychological support from experienced dialysis practitioners. The prescription of flexible treatment schedules in terms of duration and frequency to improve quality of life along with the preservation of residual kidney function are needed. Last but not least, the rearrangement of the patients’ training procedures, the choice of the home treatment to be proposed according to their attitudinal features, and the ability to overcome possible staff shortages due to quarantine or infection need to be provided to ensure that the home programs do not have to stop but possibly expand to reduce the risk of infection to the patients.
Although considerable progress has been made in recent years in the treatment of uraemic patients, survival data cannot yet be considered optimal. The periodicity of volume and metabolic control are likely to be improved. Home dialysis treatment allows flexibility to achieve optimal purification without increasing costs. With regard to the current COVID-19 pandemic scenario, home treatment adds an additional benefit by reducing the risk of virus exposure and infection. The increase in home treatments also allows a reduction in hospital dialysis crowding and also increases the ability to not exclude any patient from treatment. HHD treatment reduces the need for 3-weekly trips from home to the hospital dialysis Unit and back, which may constitute another risk of infection, thus also saving money and reducing polluting emissions. However, to achieve an increase in the use of home care dialysis techniques, several conditions are also needed, such as the availability of user-friendly dialysis equipment, telematics monitoring and counselling assistance by telehealth systems, reorganization of dialysis Units and retraining of dedicated staff providing a systematic approach to the problem, with the supply of adequate incentives for doctors and patients to choose a home care treatment [40]. The shift of dialysis treatment modalities towards home dialysis is certainly not quick to perform, and needs first of all a change of thinking in the nephrologists, in the administrators and lastly in the patients themselves. The COVID-19 pandemic has dramatically highlighted the inadequacy of the current healthcare organization in the control of infectious risk in highly sensitive populations due to the need for frequent attendance for treatment in hospital wards.
PD TO REDUCE THE RISK OF INFECTION
PD in ESRD during the pandemic
The ‘stay-at-home’ messages given during the pandemic could increase the choice to provide RRT through PD. In fact, PD offers the opportunity to manage patients remotely, thus reducing contagion during an epidemic [41]. The clear goal is to break the chain of transmission through the dialysis unit. Compared with in dialysis Units, PD can be easily performed by patients themselves at home. Physicians can conduct telemedicine consultations and PD prescriptions. In addition to the need for high volumes of clean dialysate, machines, circuits and space, dialysis Units require a large number of healthcare workers such as physicians, nurses, technicians and janitors. However, the number of such healthcare workers may be reduced due to illness or quarantine. In the era of the COVID-19 pandemic, maintaining an adequate dialysis workforce is challenging. It is worth reducing a patient's chances of coming into contact with other people or going to healthcare facilities, thereby minimizing the risk of SARS-CoV-2 infection.
Previously, during the 2009 H1N1 influenza pandemic, it was demonstrated that the risk of infection in dialysis patients was lower among PD patients. This finding suggests the possibility of differential influenza susceptibility by dialysis modality, suggesting a previously unidentified risk factor for influenza incidence [42].
On 28 March 2020, the International Society of Peritoneal Dialysis published their strategies regarding COVID-19 prevention in PD patients. This statement endorses the strategy of keeping people on PD at home and suggests that hospital visits should be minimized for only urgent indications (such as suspected peritonitis or fluid overload), and that consultations should otherwise be conducted by telehealth [43]. In the UK, by 29 April 2020, 2.9% of patients on PD were reported to have contracted COVID-19, compared with 9% of patients on HD [44].
The survey of the Italian Society of Nephrology regarding the exposure to novel coronavirus in patients on RRT published on June 2020 reported a lower incidence of COVID-19 in PD patients compared with HD (1.38% versus 3.55%, respectively), but an higher fatality rate compared with HD (45% versus 33.76%, respectively) [7].
A study on 818 patients on maintenance PD in the Wuhan population showed that only 8 patients were diagnosed with COVID-19 during the pandemic period, and the incidence of symptomatic SARS-CoV-2 infection was 2.44 per 1000 person-months in general population. At presentation, PD patients with COVID-19 infection present classical symptoms, radiological features and laboratory findings similar to the general population. According to their findings, the incidence of symptomatic COVID-19 in patients on PD was close to that of the general population, indicating that the PD population was not a high-risk population for COVID-19 [45].
Similarly, Ronco et al. described the incidence of SARS-CoV-2 pneumonia in dialysis population of the Veneto region and Vicenza Area in Italy during the pandemic period: they demonstrated that, compared with HD, PD patients had a significantly lower rate of COVID-19 (ratio 1:3). Moreover, they found a significantly lower rate of all-cause hospitalization (2:5 patients/month) in PD patients compared with HD patients. None of the PD patients was admitted to hospital for COVID-19 symptoms. Compared with the same period of the previous year, the number of hospital visits was reduced and the incidence of peritonitis episodes was low (maintained below 0.2/patient/year). The rate of hospitalization for causes independent from PD treatment was 2 patients/month, a reduction of 76% compared with the same months of the previous year. They also reported a significant hospital access restriction for cases that could be deferred (elective surgery, diagnostic procedures). Nonetheless, patients reported a sense of attention and care being given to their problems, and there were no complaints about lack of care or lack of attention to specific problems [46].
Alfano et al. presented a different PD perspective during the COVID-19 pandemic, endorsing PD as the preferred RRT modality for ESRD patients. Among the advantages, they mention the minimized risk of viral transmission through interpersonal contacts, as well as the use of telemedicine to deliver renal care without exposure of the patient to the risks of contacts [47].
PD in COVID-19-associated AKI
We need to consider the high incidence of acute kidney injury (AKI) during SARS-CoV-2 pneumonia and the possibility of PD as RRT in AKI. PD in patients with AKI provides an acceptable form of treatment, in fact, recent studies have suggested that outcomes with PD are as good as with extracorporeal RRTs. Certainly, PD could provide some advantages for PD to manage patients with AKI [48].
A previous study compared PD with continuous RRT (CRRT) in critically ill patients with AKI and found that patients in the PD group had a lower mortality rate at 28 days, faster recovery of renal function and fewer complications of infections [49]. Furthermore, SARS-CoV-2 is a highly contagious airborne disease. The patients should be kept in an isolation room, should not be transported and should be minimally exposed to healthcare providers. When applying this concept to dialysis for COVID-19 patients, automated PD (APD) is preferred because the cycler can be moved and installed anywhere. Moreover, the PD fluid exchanges occur automatically and only one nurse can manage the cycler. Such minimum contact with the patient reduces not only the risk of contagion, but also the need for personal protective equipment (PPE) use [50]. Lastly, the pandemic could determine a different resource allocation in healthcare because of scarce resource settings (clinics/hospitals). PD is simple and efficient, it provides continuous steady fluid removal and requires less equipment when compared with extracorporeal dialysis. Moreover, it requires lower work intensity, with a single nurse being able to manage different PD cyclers simultaneously [51].
Such an efficient resource management method helps address the shortage of medical equipment and reduce exposure of the healthcare staff, which are critical issues during the pandemic. Because of the above-mentioned benefits, PD should be considered as one of the options to treat COVID-19-related AKI, and APD is the preferred form to minimize the risk of exposure.
The first experience of PD in AKI during SARS-CoV-2 pneumonia was made by Hugh Cairns in the intensive care units (ICUs) at Kings College Hospital, London. The main background was the shortage of continuous veno-venous HD machines. Thirty-two patients were considered eligible for PD: there were five failures because of unsuccessful catheter insertion, and 27 patients were treated through APD in the ICU. The reported patient outcomes were as follows: 7 recovered renal function, 3 died because of COVID-19 and 17 remained on chronic PD [52]. The second experience was that of Dr Mihran Naljayan from the Louisiana State University, who treated 18 patients with PD and reported the following outcomes: 12 on AKI-PD, 2 discharged to an outpatient PD unit, 1 recovered renal function and 3 died [53]. Clinical experiences and studies support the use of PD in AKI situations; the outcomes are not different from the ones reported with other dialytic techniques such as CRRT, sustained low-efficiency dialysis and intermittent HD. A shortage of CRRT and HD machines and supplies, as well as of staff and PPE, has brought PD to the forefront. The preliminary experience reported here provides evidence that PD can contribute to reduce the contribution of kidney disease to COVID-19 evolution.
Furthermore, we have to consider the haemodynamic advantage of PD. Cardiac involvement was also observed in patients with COVID-19. Cardiac injury may result from myocarditis, profound systemic inflammation or microvascular dysfunction [54]. Thus, RRT should be performed with caution. The gentle and prolonged removal of body fluids and toxins during PD reduces the risk of haemodynamic instability, which makes PD a treatment of choice for critically ill patients both with AKI and with ESRD [49]. As shown, PD has comparable, or even better, outcomes. Frequent, routine trips for dialysis treatments at healthcare facilities, where individuals with advanced age, a large comorbidity burden and high rates of hospitalization are cohorted together, fosters a high-risk situation for SARS-CoV-2 transmission. The advantages of PD for reducing infection rate are clear, and reflect not only the reduced social contact between patients and health-workers but also clinical aspects. Reports we described confirm that home-based PD limits the risk of SARS-CoV-2 infection. In Table 2, we summarize the main experiences with PD as RRT during the pandemic both for ESRD and AKI.
Table 2.
Studies/experiences describing use of PD during the pandemic
| Study | Participants | Design | Result |
|---|---|---|---|
| Italian Society of Nephrology COVID Survey 2020 | 4139 chronic PD patients | Observational | Incidence of COVID-19 in PD patient was lower than HD patient (1.38 versus 3.55%) |
| Jiang et al., China 2020 | 818 chronic PD patients | Observational | Incidence of symptomatic COVID-19 in PD patient was close to that of the general population |
| Ronco et al., Veneto 2020 | 130 chronic PD patients | Observational | PD allow to have a significant lower rate of COVID-19 infections |
| Alfano et al., Modena 2020 | 2 chronic PD patients | Observational | PD is the preferred RRT in ESRD |
| Cairns et al., Kings College Hospital, London 2020 | 32 AKI patients | Observational |
27 AKI PD treated successfully No complications were recorded |
| Naljayan et al., Louisiana State University School of Medicine 2020 | 18 AKI patients | Observational |
12 on AKI-PD 2 discharged to an outpatient PD unit 1 recovered renal function 3 died |
What we learned from the pandemic
In Table 3, we summarize the main messages of our review: current barriers to home dialysis therapies, best practices on the basis of the lessons learnt during the pandemic and the long-term changes needed to improve use of home dialysis therapies.
Table 3.
Lessons from pandemic and considerations regarding home dialysis treatments
| Best practices | Barriers | Suggestions | |
|---|---|---|---|
| Home dialysis treatment | Limit exposure to the hospital setting |
Isolation Acute intradialytic problems |
Reinforcement of telemedicine |
| Remote counselling | Avoid isolation | Fragile and elderly patients | Follow an ‘urgent pathway’ |
| Flexibility—empowerment | Better survival and quality of life | Dialysis prescriptions | Reinforcement of remote counselling |
| Home biochemical controls | Practical, reducing the need for going to a laboratory or hospital | Standard pre- and post-dialysis controls may be difficult to organize | Frequency of controls would not be reduced |
| Family involvement | Important psychological support | The burden may be heavy and create tension | Psychological aid could be needed |
| Residual kidney function | Better preserved in tailored dialysis | Slow loss of kidney function could go unnoticed | Considering not only remote monitoring |
| Assisted home dialysis | Limited exposure to the hospital setting and no travel time | May fail to guarantee privacy | Attention on potential carriers |
| Reduction of travel time and carbon footprint | ‘Green’ advantages | Waste management needs to be organized in advance | Limiting need to travel is an advantage in a ‘lockdown’ |
The COVID-19 pandemic has highlighted the advantage of receiving home dialysis, and in particular PD, thus we can consider the pandemic as an opportunity to re-evaluate and refine existing models of healthcare delivery at home and overcome barriers to increasing the use of home-based dialysis. Beside the high risk of communicable infectious disease, transmission of viral hepatitis and colonization with multidrug-resistant microbes, factors such as poverty, housing instability, care-givers limitations and lack of storage space present real challenges to home dialysis use, and there may also be issues around patients’ capacity for learning PD. Some of these factors may be overcome by proactively providing assistance. Initiating PD is far more time intensive than HD. It requires more extensive discussions with patients and their caregivers, identifying and collaborating with other specialists and primary care providers, and empowering patients and their families to take a leadership role in their own care. Previous studies have shown the association between appropriate education and the proportion of patients who choose PD [55]. With this in mind, great effort should be made to increase the proportion of patients who choose PD. For example in the UK and Canada, assistance is available for older people to have PD in their home or nursing homes. Previous studies have demonstrated a high level of satisfaction in patients with assisted PD [56].
These efforts include providing patients with adequate education about PD, exploring models of providing assistance and developing robust telemedicine strategies that minimize in-person interactions. During the pandemic, the key role of telehealth has emerged. In fact, telemedicine allows maintenance of the social isolation of many patients, encouraging at-risk group professionals to work from home, preserving the health of many who are at the forefront by decreasing the flow of people in the clinic environment.
Another major factor to be aware of is that in general among most countries, PD is more cost-effective than HD. The USRDS 2012 Annual Report reports an annual cost of HD per patient around US $87 500 per year, while that of PD is around US $66 750 [57]. So increasing the use of PD in the US would produce an important saving to the healthcare system over the years. These advantages go beyond the emergency state imposed by the COVID-19 pandemic to offer an opportunity that can be considered over time to increase the number of patients receiving PD.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This article is part of a supplement supported by Fresenius Medical Care without any influence on its content.
REFERENCES
- 1. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (6 October 2020, date last accessed)
- 2. Kliger AS, Silberzweig J.. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol 2020; 15: 707–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19
- 4. Ko JY, Danielson ML, Town M. et al. ; COVID-NET Surveillance Team. Risk factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis 2020; ciaa1419: doi: 10.1093/cid/ciaa1419 (online ahead of print) [Google Scholar]
- 5. Kato S, Chmielewski M, Honda H. et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008; 3: 1526–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michiel GHB. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol 2013; 9: 255–265 [DOI] [PubMed] [Google Scholar]
- 7. Quintaliani G, Reboldi G, Di Napoli A. et al. ; on behalf of the Italian Society of Nephrology COVID-19 Research Group. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society. J Nephrol 2020; 33: 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data
- 9. Saran R, Robinson B, Abbott KC. et al. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2020; 75 (Suppl 1): Svi–Svii [DOI] [PubMed] [Google Scholar]
- 10. Himmelfarb J, Vanholder R,. Mehrotra R et al. The current and future landscape of dialysis. Nat Rev Nephrol 2020; 16: 574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cozzolino M, PiccoliGB, , Alp Ikizler T. et al. The COVID-19 infection in dialysis: are home-based renal replacement therapies a way to improve patient management. J Nephrol 2020; 33: 629–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 13. Walke RC, Howard RK, Morton RL.. Home hemodialysis: a comprehensive review of patient-centered and economic considerations. Clinicoecon Outcomes Res 2017; 9: 149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blagg CR. Home haemodialysis. BMJ 2008; 336: 3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacGregor MS, Agar JWM, Christopher RB.. Home haemodialysis—international trends and variation. Nephrol Dial Transplant 2006; 21: 1934–1945 [DOI] [PubMed] [Google Scholar]
- 16. Cherukuri S, Bajo M, Colussi G. et al. Home hemodialysis treatment and outcomes: retrospective analysis of the Knowledge to Improve Home Dialysis Network in Europe (KIHDNEy) cohort. BMC Nephrol 2018; 19: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. https://www.edtnaerca.org/resource/edtna/files/Grand-Rounds-Brunori%20(1).pdf
- 18. Jun M, Jardine MJ, Gray N. et al. Outcomes of extended-hours hemodialysis performed predominantly at home. Am J Kidney Dis 2013; 61: 247–253 [DOI] [PubMed] [Google Scholar]
- 19. Rivara MB, Adams SV, Kuttykrishnan S. et al. Extended-hours hemodialysis is associated with lower mortality risk in patients with end-stage renal disease. Kidney Int 2016; 90: 1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foley RN, Gilbertson DT, Murray T. et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107 [DOI] [PubMed] [Google Scholar]
- 21. Jefferies HJ, Virk B, Schiller B. et al. Frequent hemodialysis schedules are associated with reduced levels of dialysis induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol 2011; 6: 1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rocco MV, Lockridge RS Jr, Beck GJ. et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 2011; 80: 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tennankore K, Nadeau-Fredette AC, Chan CT.. Intensified home hemodialysis: clinical benefits, risks and target populations. Nephrol Dial Transplant 2014; 29: 1342–1349 [DOI] [PubMed] [Google Scholar]
- 24. Agar JW, Knight RJ, Simmonds RE. et al. Nocturnal haemodialysis: an Australian cost comparison with conventional satellite haemodialysis. Nephrology 2005; 10: 557–570 [DOI] [PubMed] [Google Scholar]
- 25. Kroeker A, Clark WF, Heidenheim AP. et al. An operating cost comparison between conventional and home quotidian hemodialysis. Am J Kidney Dis 2003; 42: 49–55 [DOI] [PubMed] [Google Scholar]
- 26. Trinh E, Christopher TC.. The rise, fall, and resurgence of home hemodialysis. Semin Dial 2017; 30: 174–180 [DOI] [PubMed] [Google Scholar]
- 27. Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol 2002; 13: 1279–1287 [DOI] [PubMed] [Google Scholar]
- 28. Little J, Irwin A, Marshall T. et al. Predicting a patient’s choice of dialysis modality: experience in a United Kingdom renal department. Am J Kidney Dis 2001; 37: 981–986 [DOI] [PubMed] [Google Scholar]
- 29. Mehrotra R, Marsh D, Vonesh E. et al. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 2005; 68: 378–390 [DOI] [PubMed] [Google Scholar]
- 30. Rioux JP, Cheema H, Bargman JM. et al. Effect of an in-hospital chronic kidney disease education program among patients with unplanned urgent-start dialysis. Clin J Am Soc Nephrol 2011; 6: 799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Implementing Hemodialysis in the Home: A Practical Manual First Edition Copyright © 2016. International Society for Hemodialysis. http://www.ishd.org/home-hd-toolkit/
- 32. Mehrotra R. Advancing American kidney health: an introduction. Clin J Am Soc Nephrol 2019; 14: 1788–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yerram P, Misra M.. Home dialysis in the coronavirus disease 2019 era. Adv Chronic Kidney Dis 2020; 27: 442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Himmelfarb J, Vanholder R, Mehrotra R et al.. The current and future landscape of dialysis. Nat Rev Nephrol 2020; 16: 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Merighi JR, Schatell DR, Bragg-Gresham JL. et al. Insights into nephrologist training, clinical practice, and dialysis choice. Hemodial Int 2012; 16: 242–251 [DOI] [PubMed] [Google Scholar]
- 36. Srivatana V, Liu F, Levine DM. et al. Early use of telehealth in home dialysis during the COVID-19 pandemic in New York City. Kidney360 2020; 1: 524–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vanholder R, Annemans L, Brown E. et al. ; on behalf of the European Kidney Health Alliance. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 38. Hager D, Ferguson TW, Komenda P.. Cost controversies of a “home dialysis first” policy. Can J Kidney Health Dis 2019; 6: 205435811987154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Financial Support for Home Dialysis Patients in Australia – Kidney Health Australia – The Home Network: https://kidney.org.au/uploads/resources/home-dialysis-financial-assistance-electricity-water-and-subsidies.pdf (19 December 2020, date last accessed)
- 40. Lockridge R Jr, Weinhandl E, Kraus M. et al. A systematic approach to promoting home hemodialysis during end stage kidney disease. Kidney360 2020; 1: 993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tranæus A, Yao Q.. Immune dysfunction in dialysis patients–prevention and treatment strategies. Perit Dial Int 2008; 28: 161–166 [PubMed] [Google Scholar]
- 42. Cho J-H, Do J-Y, Kim S-H. et al. Impact of dialysis modality on the incidence of 2009 pandemic H1N1 influenza in end-stage renal disease patients. Perit Dial Int 2011; 31: 347–358 [DOI] [PubMed] [Google Scholar]
- 43. Brown E, De Arteaga J, Chow J. et al. ISPD: Strategies regarding COVID-19 in PD patients Adapted from Peking University First Hospital, 2020
- 44.UK Renal Registry: Weekly COVID-19 Surveillance. Report for Renal Centres in the UK. Bristol: UK Renal Registry, 2020
- 45. Jiang H-J, Tang H, Xiong F. et al. COVID-19 in peritoneal dialysis patients. Clin J Am Soc Nephrol 2021; 16: 121–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ronco C, Manani SM, Giuliani A. et al. Remote patient management of peritoneal dialysis during COVID-19 pandemic. Perit Dial Int 2020; 40: 363–367 [DOI] [PubMed] [Google Scholar]
- 47. Alfano G, Fontana F, Ferrari A. et al. Covid-19 Working Group (MoCo19). Peritoneal dialysis in the time of coronavirus disease 2019. Clin Kidney J 2020; 16: 265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chionh CY, Soni SS, Finkelstein FO. et al. Use of peritoneal dialysis in AKI: a systematic review. Clin J Am Soc Nephrol 2013; 8: 1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Almeida CP, Ponce D, de Marchi AC. et al. Effect of peritoneal dialysis on respiratory mechanics in acute kidney injury patients. Perit Dial Int 2014; 34: 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ponce Andre D, Balbi L, Durand JB. et al. Acute peritoneal dialysis in the treatment of COVID-19-related acute kidney injury. Clin Kidney J 2020; 13: 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ikizler TA, Kliger AS.. Minimizing the risk of COVID-19 among patients on dialysis. Nat Rev Nephrol 2020; 16: 311–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cairns H, Bowes E, Naljayan M. Use of PD for COVID-19-Associated AKI: Clinical Experience and Updated 2020 ISPD Guidelines. https://academy.theisn.org/isn/2020/covid-/293275/edwina.brown.brett.cullis.hugh.cairns.elaine.bowes.mihran.naljayan.simon.html?f¼menu%3D13browseby%3D8sortby%3D2label%3D19791 (23 April 2020, date last accessed)
- 53. Inciardi RM, Lupi L, Zaccone G. et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng YY, Ma YT, Zhang JY. et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020; 17: 259–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Devoe DJ, Wong B, James MT. et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis 2016; 68: 422–433 [DOI] [PubMed] [Google Scholar]
- 56. Brown EA, Johansson L, Huson L. et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol 2016; 11: 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karopadi AN, Mason G, Rettore E. et al. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 2013; 28: 2553–2569 [DOI] [PubMed] [Google Scholar]


