Background:
Numerous approaches have been employed to treat chronic lymphocele and cutaneous lymphatic fistulas (LFs) with little success. Given a high incidence and substantial consequences for patients, there is an ongoing demand for effective therapeutic and preventive strategies. The aim of this study was to evaluate the results after microscopic lymphatic ligation (MLL) and lymphovenous anastomosis (LVA) as a therapeutic and preventive approach in this context.
Methods:
Demographic data, surgical characteristics, complications, and the overall outcome of all patients undergoing surgery for postoperative LF from 2014 to 2019 were collected retrospectively. Patients were categorized in accordance with predefined inclusion/exclusion criteria and with their treatment. Statistical analysis was conducted using descriptive, summary statistics to identify a central tendency.
Results:
Thirty-four patients underwent indocyanine-green-lymphangiography guided revision surgery for LF. Two patients were lost to follow-up at 6 months. LF was successfully treated in all patients (n = 32) with a multimodal approach. Only MLL was performed in 22 patients and MLL/LVA in 10 patients. LF resolved in 78% of all patients with MLL only or MLL/LVA. In the remaining 22%, LF resolved after additional sclerotherapy within 3 months.
Conclusions:
Treatment of LF should follow a standardized staged surgical approach to optimize outcome. LF was treated successfully in all our patients. We therefore propose a multimodal interdisciplinary approach to this common clinical problem that includes adjunctive sclerotherapy.
INTRODUCTION
The exact pathophysiology of postoperative chronic fluid accumulation has been vague for decades. The term seroma was historically given to describe this frequent phenomenon and a multifactorial causality was acknowledged. Studies have shown that the fluid is in fact an accumulation of lymphatic fluid deriving from severed afferent lymphatics at the site where tissue was removed accumulating initially in a nonepithelialized cavity.1,2 Postoperative lymphatic fistulas (LFs) can result whenever lymphatic vessels are injured during surgical dissection. They present either as cutaneous fistula with lymphorrhea or as a collection of lymph accumulating initially in a nonepithelialized cavity referred to as lymphocele. A closed lymphocele can evolve into a cutaneous LF, if wound breakdown occurs. Some LFs resolve spontaneously, whereas others become chronic. Anatomical studies reveal that divergent and alternative lymphatic drainage pathways exist.3,4 Differences in vessel depth or vessel diameter may be contributing factors favoring transport through 1 vessel over another and thus leading to a prolonged leakage in some cases.5
Although after sentinel-lymph node biopsy (SLNB) the formation of a symptomatic lymphocele appears to be less than 7%, incidences following lymphadenectomy (LAD), in particular of the groin, are higher and are reported between 40% and 50%.6–8 Following excision of a soft-tissue tumor, incidences range between 10% and 36% and reach almost 100% after extended soft-tissue resections in the proximal medial thigh.9 Predictive factors include the amount of excised tissue as well as adjuvant chemotherapy and/or radiotherapy of the operating site.10
In addition, LF is associated with further complications such as wound breakdown, infection of the operating site including underlying vascular grafts or other prostheses, prolonged treatment duration, recurrent hospitalization, lymphangitis, fever, and sepsis.10,11 Muscle flaps are efficient in dead space reduction and thus assist in the prevention of chronic postoperative fluid accumulation but do not address the fistula itself.7,11 Various other approaches have been introduced to prevent and treat LF without scientific evidence such as drainage systems, compression dressings, quilting sutures, fibrin glue and repeated percutaneous aspirations, embolization, or injection of sclerosants. However, in high-risk cases, for example, patients under immunosuppressive medication or reduced wound-healing ability due to radiotherapy, conservative treatment is known to be less effective and can even increase morbidity.
In the era of lymphatic supermicrosurgery, modern therapeutic approaches include investigation of the wound bed with Indocyanine-Green (ICG) lymphography to detect severed lymphatic vessels.12 Selective ligation of lymph vessels for the treatment of recurrent inguinal LF using titanium clips or nonabsorbable sutures appears to be an appropriate treatment modality in small-scale reports.13,14 The successful use of reconstructive lymphatic microsurgery has been described as a more physiologic option in a limited number of patients with LF so far and may provide more reliable results.15–17 However, not all patients are suitable for lymphatic reconstruction since lymphatics may not be suitable for anastomosis due their size or quality and venules may not always be available.
Therefore, the purpose of this study is to evaluate the outcomes of microscopic lymphatic ligation (MLL) and lymphovenous anastomosis (LVA) for the treatment of LF. We propose a comprehensive treatment algorithm with a multimodal interdisciplinary approach.
MATERIALS AND METHODS
The approval for this study was granted by the Cantonal Ethics Committee of Zurich, Switzerland (BASEC-Nr. 2019-00841).
Surgical Technique and Algorithm
In our unit, a standardized interdisciplinary approach in cooperation with the interventional radiologists was developed since 2014 to provide a clear treatment algorithm for patients with postoperative LF depending on wound status (open/closed) and the presence of immunosuppression (Fig. 1). This algorithm has been used increasingly in our unit over the past 5 years and our sclerotherapy protocol has been published recently.18
Fig. 1.
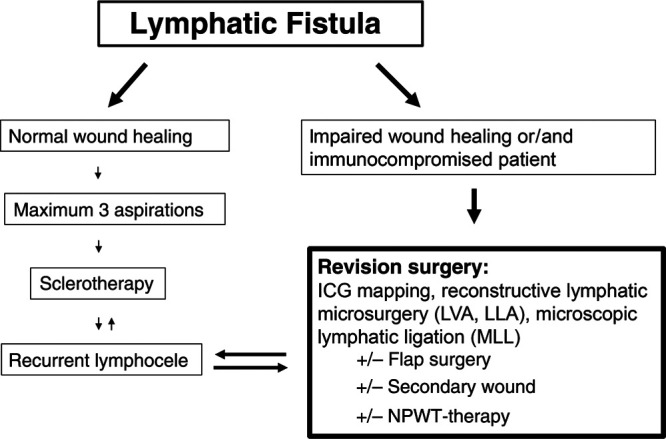
Flowchart on the standardized staged surgical approach depending on wound status (open/closed) and the presence of immunosuppression in patients with postoperative LF.
The diagnosis for postoperative LF was made by clinical examination only and treatment was offered whenever it became symptomatic by size and patient reported discomfort. If the surgical wound remains closed, sclerotherapy is preferred as a first approach after 3 unsuccessful attempts of percutaneous fluid aspirations. We proceed to revision surgery directly in the case of wound breakdown or if the patient is immunosuppressed, for example, after heart transplantation and resect epithelialized cavities if present. In the setting of cutaneous LF or infection, antibiotics are given and staged debridement with negative pressure wound therapy is applied. During the final revision surgery, the wound bed is examined meticulously under the microscope guided by ICG to investigate the existence of leaking lymphatic vessels (Figs. 2 and 3). (See Video 1 [online], which displays an intraoperative video of chronic LF with visible lymphorrhea.)
Fig. 2.
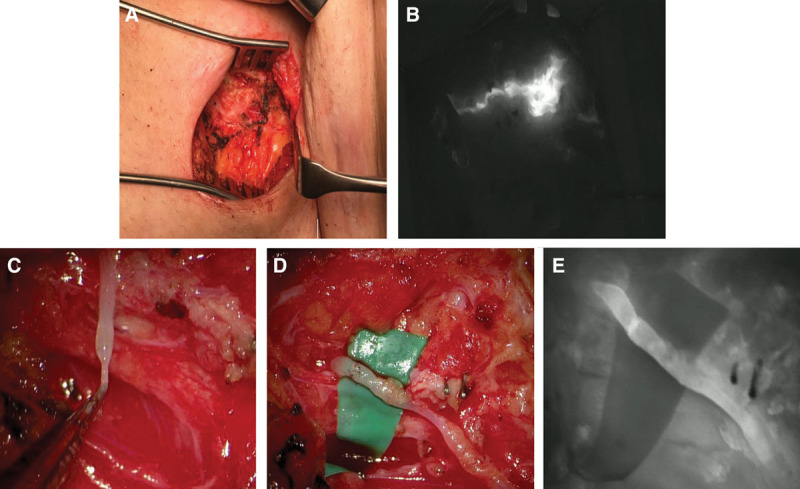
Surgical treatment of chronic lymphocele. A, Intraoperative situs of a patient with a high-flow fistula (300–500 ml/d) after lymph node biopsy in the right groin. B, Intraoperative ICG-lymphangiography of the situs showing lymphatic leakage. C, Intraoperative view of an injured lymphatic vessel with high flow under the microscope. D, Lymphovenous anastomosis of the severed lymphatic vessel to an adjacent vein. E, Intraoperative ICG-lymphangiography confirmed patency of the lymphovenous anastomosis.
Fig. 3.
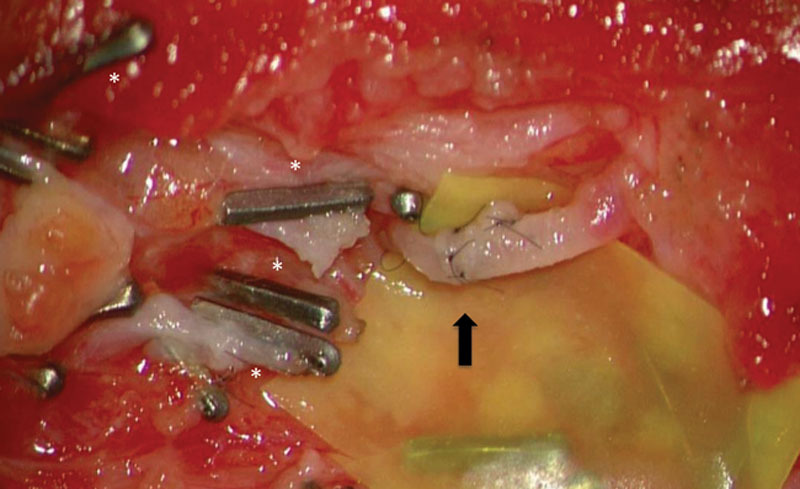
Intraoperative situs with MLLs and lymphovenous anastomosis (black arrow). The 0.2-mm titanium microclips and 10-0 nylon sutures were used.
Video 1. Video 1 from “Lympho-Venous Anastomoses and Microscopic Lymphatic Ligations for the Treatment of Persistent Lymphocele and Cutaneous Lymphatic Fistula”.
We prefer to implement end-to-end LVA whenever possible and combine it with MLL using microtitanium clips. During surgery, 0.2–0.3 ml of ICG is injected intradermally in the dorsal interdigital spaces I–IV of hand or foot and, in addition, above the elbow/knee or around the wound depending on the localization. Severed lymphatic vessels and subsequent lymphatic leakage are detected by ICG-angiography using a high-resolution fluorescent microscope (OPMI Pentero, Zeiss, Germany) (Fig. 2). (See Video 2 [online], which displays intraoperative video (fluorescence mode) of ICG-leakage of the same lymphatic fistula, which will make it easier to detect.) If a suitable vein is available, LVA is performed using an end-to end technique with Nylon 11-0 or 12-0 interrupted sutures. Unfortunately, wounds are often scarred after numerous operations and attempts of sclerotherapy and a vein may not be available. In these cases, we perform selective microscopic ligations with microtitanium clips. A flap usually was always added if impaired wound healing can be expected due to the size of the skin defect or if the soft-tissue coverage is insufficient. A Blake drain is placed and kept for at least 10–14 days to allow for sufficient wound healing. We ideally remove the drain when discharge is less than 20 ml/d. Bed rest is required for 3 days postoperatively. Patients are discharged after 5 days the earliest and depending on wound and general condition either with or without drain. Amoxicillin 1 g twice daily is given as long as the drain is in place and antibiotics are constantly adapted according to microbiologic specimen results. Patients are advised physical activity restriction for a total of 14–21 days and are usually followed up to 12 month postoperatively in an ambulatory setting. If minor LF persists after revision surgery, additional sclerotherapy is performed. We usually wait 3–4 weeks after MLL/ LVA before referring patients to sclerotherapy.18
Video 2. Video 2 from “Lympho-Venous Anastomoses and Microscopic Lymphatic Ligations for the Treatment of Persistent Lymphocele and Cutaneous Lymphatic Fistula”.
Study Design and Patients
This retrospective analysis included a review of 150 operation reports and clinical follow-up data of all male and female patients (>18 years) who underwent revision surgery for LF between January 2014 and April 2020. Patients who did not receive ICG-lymphangiography were excluded from this study. LFs were refractory to conservative or interventional management for at least 4 weeks. Patients were excluded if they did not consent for retrospective data analysis or if the institutional informed consent was missing. Other exclusion criteria were preexisting severe skin diseases such as atopic dermatitis or acne inversa, concomitant oncologic chemotherapy or concomitant radiotherapy, ongoing intravenous drug abuse, and revision surgery without special consideration given to lymphatics. Patients were further categorized according to their treatment in 2 cohorts: MLL and MLL combined with lymphatic reconstruction. All patients were operated by the senior author (N.L.). Demographic data, surgical characteristics and complications, as well as the overall outcome of treatment were abstracted from patients’ computerized charts and electronic records and are presented in a descriptive manner. Lymphedema was monitored clinically. Diagnosis was based on signs and symptoms, and the International Society of Lymphology Staging System is our method of choice to further classify lymphedema along with imaging modalities. We document all signs that may be subjective observations of our ambulatory patients such as a feeling of heaviness in the affected extremity.
Statistical Analysis
Statistical analysis was conducted in a descriptive manner. Data were analyzed using Microsoft Excel Version 14.3.6. (Microsoft Corp., Redmond, Wash.). This study was conducted according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
RESULTS
Thirty-four patients undergoing ICG-lymphangio-graphy–guided revision surgery between January 2014 and April 2020 were included, and 2 patients were lost to follow-up. A flowchart of patients reporting on the numbers of individuals at each stage of study is provided in Figure 4.
Fig. 4.
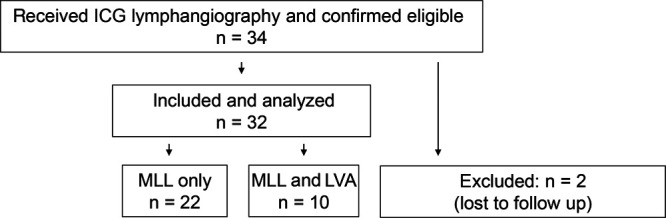
Flowchart of patients reporting on selection and numbers of individuals.
Main Causes for LF Development
The primary causes for LF were vascular cannulation for extracorporeal membrane oxygenation (n = 11), other vascular access procedures/vein harvest (n = 8), lymph node biopsy/lymph node dissection (n = 7), dermolipectomy (n = 3), soft-tissue tumor resection (n = 3), flap donor site (n = 1), and knee prosthesis procedure (n = 1). The LF was located at the groin and medial thigh in 31 patients, the neck in 2 patients, and abdominal (subcutaneous) in 1 patient.
Demographics and Results in Summary
Patients were pretreated in accordance with our previously described standardized staged surgical approach depending on wound status (open/closed) and presence of immunosuppression. Two patients were lost to follow up after revisional surgery for LF and were excluded from further analysis: One patient in the MLL cohort had a persistent low-grade infection of his femoral vascular graft and had to be operated on by vascular surgeons before and after our revision surgery. After a 3-month consultation with an ongoing LF, the patient unfortunately never presented again at our department. Another severely immunosuppressed patient with persisting LF after LVA and MLL died within the study period. Eventually, 32 patients with persistent LF were included into further analysis.
Mean age was 61 ± 16 years. Mean body mass index was 29 ± 6 kg/m2. None of the patients had preoperative signs and symptoms of secondary lymphedema before treatment. Twenty-eight percent (9/32) patients were immunosuppressed. All patients received perioperative antibiotic therapy.
The mean follow-up period was 11 months. Twenty-seven of 32 patients were followed up at 12 months and 4 patients were followed up at 6 months postoperatively. One patient was followed up at 3 months postoperatively and is expected to return for further follow-ups.
In summary, LF was successfully treated in all patients (n = 32) (Fig. 1; Table 1). LF resolved in 78% (25/32) of all patients with MLL only or MLL with LVA. In the remaining 22% (7/32), LF resolved after additional sclerotherapy after 1.5 months and (range 1–3) in 2.5 sclerotherapy sessions on average (range 1–4). MLL alone was conducted in 22 and MLL was combined with LVA in 10 patients.
Table 1.
Characteristics of Patients and Procedures—Revision Surgery with ICG-lymphangiography and MLL
| Nb | Age | Sex (M/F) | IS | Malignant Disease | Primary Intervention | Localization of LF | Previous Therapy | Additional Intervention | Complications | Secondary Sclerotherapy | Outcome (12 mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | F | — | — | ECMO | IR | NPWT | Pedicled muscle flap | — | OK-432 | Resolved |
| 2 | 56 | M | — | — | Endoscopic vein harvest | MLTL | NPWT | — | — | — | Resolved |
| 3 | 62 | M | — | — | Implantation of knee prosthesis | MLTR | NPWT, ST | Pedicled muscle flap | — | — | Resolved |
| 4 | 84 | M | — | Pleomorph, high-grade liposarcoma | nRT, tumor resection, vascular graft placement | MUTL | ST | Pedicled muscle flap | — | OK-432 | NA |
| 5 | 72 | M | MTX | — | Endoscopic vein harvest | MUTL | NPWT | — | — | Ethanol | Resolved |
| 6 | 79 | M | — | Adeno-CA of colon [pT3pN1a (1/26) pM1 (HEP)] | Vascular bypass surgery | IR | NPWT | — | — | OK-432 | Resolved |
| 7 | 83 | F | — | — | Femoral artery catheterization | IL | NPWT | — | — | — | Resolved |
| 8 | 72 | M | — | Melanoma of unknown origin (IIIB, pT0N1bM0) | Lymphadenectomy | IL | ST | — | SWD | — | Resolved |
| 9 | 74 | M | — | Pleomorph, spindelcell type of sarcoma (G4; 14 × 8 × 8 cm, M1) | nRT, tumor resection, lymphadenectomy | MUTL | ST | Fasciocutaneous flap | — | — | Resolved |
| 10 | 48 | F | KTPL | Posttransplant lymphoproliferative disorder | Lymph node biopsy | IL | PA | — | — | — | Resolved |
| 11 | 68 | M | — | — | Vascular graft placement | IL | NPWT | — | — | — | Resolved |
| 12 | 64 | M | HTPL | — | ECMO | IR | NPWT | Pedicled ALT flap | — | — | Resolved |
| 13 | 53 | M | — | — | ECMO | IL | NPWT | Pedicled ALT flap | SWD | OK-432 | Resolved |
| 14 | 56 | M | — | — | ECMO | IR | NPWT | Pedicled muscle flap | — | — | Resolved |
| 15 | 31 | M | LTPL | — | ECMO | IR | none | — | — | — | Resolved |
| 16 | 68 | F | — | Vulvar melanoma [T4aN3 (3/12) M1a] | Lymphadenectomy | IL | NPWT | Pedicled muscle flap | — | — | Resolved |
| 17 | 58 | M | HTPL | — | ECMO | IR | — | Capsula excision | — | — | Resolved |
| 18 | 64 | F | — | — | Dermolipectomy | MUTR | PA | — | — | — | Resolved |
| 19 | 46 | F | — | — | Dermolipectomy | MUTL | PA | — | — | — | Resolved |
| 20 | 44 | F | — | — | Dermolipectomy | MUTL | PA | — | — | — | Resolved |
| 21 | 53 | F | — | Breast cancer [ypT1a (1mm) pN0 (sn 0/4) M0R0, G3, ER 10%, PR−, HER2+] | SIEA harvest | Abdomen | PA | Capsula excision | — | — | Resolved* |
| 22 | 83 | M | — | — | Lymphadenectomy | IL | PA, ST | — | — | — | Resolved* |
| 23 | 64 | M | HTPL | — | ECMO | IL | NPWT | Pedicled muscle flap, STSG | SWD | — | Resolved† |
ALT, anterolateral thigh; F, female; ECMO, extracorporeal membrane oxygenation; HTPL, heart transplant immunosuppressive therapy; IL, inguinal left; IR, inguinal right; IS, immunosuppressive therapy; KTPL, kidney transplant immunosuppressive therapy; LF, Lymphatic fistula; LTPL, lung transplant immunosuppressive therapy; M, male; MLTL, medial lower thigh left; mo, month; MTX, methotrexate; MUTL, medial upper thigh left; MUTR, medial upper thigh right; NA, lost to follow-up; Nb, patient number; NPWT, negative pressure wound therapy; nRT, neoadjuvant radiotherapy; OK-432, Picibanil; PA, percutaneous aspiration; SIEA, superficial inferior epigastric artery perforator flap; ST, sclerotherapy; STSG, split thickness skin graft; SWD, superficial wound dehiscence.
*Six-month follow-up.
†Three-month follow-up.
MLL Only
Twenty-two patients with postoperative LF were treated with MLL without perioperative complications. Mean follow-up period was 11 months. Six out of 22 patients (27%) were immunosuppressed. In 6 out of the 22 cases, additional muscle flaps were performed. In 2 out of 22 cases, a major tissue defect was reconstructed with a pedicled anterolateral thigh fasciocutaneous flap. Although 82% (18/22) of all patients were successfully treated with MLL, 18% (4/22) underwent further secondary sclerotherapy (Fig. 5). None of them had to be reoperated on due to surgical site infection or major wound breakdown (Table 1). We did not see any secondary lymphedema in this cohort after treatment.
Fig. 5.
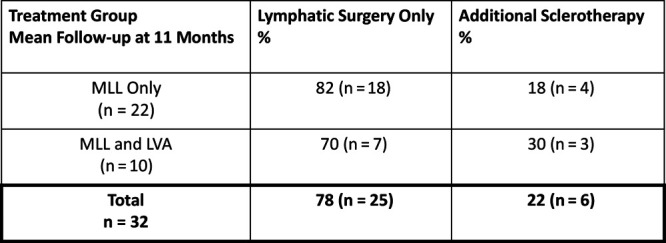
Subanalysis regarding need for additional secondary sclerotherapy in patients receiving MLL only and MLL and LVA.
MLL and LVA
In 10 patients, LVAs were performed in combination with MLL without perioperative complications (Table 2). In total, 14 LVAs were performed (Table 3). Mean follow-up period was 11 months. Three out of 10 patients (30%) were immunosuppressed. One patient received a pedicled muscle flap and 1 patient received a chimeric pedicled flap. Although 70% (7/10) of all patients were successfully treated with LVA in combination with MLL, 30% (3/10) underwent further secondary sclerotherapy (Fig. 5). One patient developed a postoperative hematoma and had to be operated emergently. This patient developed transient lymphedema at 6 month that was not apparent after 12 months postoperatively.
Table 2.
Characteristics of Patients and Procedures—Revision Surgery with ICG-lymphangiography, MLL, and LVA
| Nb | Age (years) | Sex (M/F) | IS | Malignant Disease | Primary Intervention | Localization of LF | Previous Therapy | Additional Intervention | Complications | Secondary Sclerotherapy | Outcome (12 mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | HTPL | — | ECMO | IR | NPWT | Chimeric pedicled flap (ALT & M. vastus lateralis) | — | Ethanol | NA |
| 2 | 27 | M | HTPL | — | ECMO | IR | PA, ST, NPWT | Chimeric pedicled flap (ALT & M. vastus lateralis) | — | Ethanol | Resolved |
| 3 | 82 | F | — | Rectal cancer (T1N0MX) | Vein harvest | MUTL | PA | — | H | — | Resolved |
| 4 | 50 | F | — | Synovial cardiac cancer* | Carotid artery thrombectomy | NR | NPWT | Pedicled muscle flap | — | — | Resolved |
| 5 | 52 | M | — | Hypopharyngeal cancer (cT2cN3bM0) | nRT, CT, modified radical neck dissection level II–IV | NL (severed thoracic duct) | NPWT | — | — | — | Resolved |
| 6 | 43 | M | C | — | Lymph node biopsy | IL | PA, ST | — | — | — | Resolved |
| 7 | 74 | M | — | Melanoma (pT2aN1M0) | Lymph node biopsy | IR | NPWT | — | — | OK-432 | resolved |
| 8 | 62 | F | — | Vaginal cancer (pT4pN1(1/26)V0Pn0R0 G2-3) | Lymphadenectomy | IR | NPWT | — | — | — | Resolved† |
| 9 | 81 | M | — | — | Iliac EVAR | IR | NPWT, ST | — | — | Ethanol | Resolved† |
| 10 | 21 | F | HTPL | — | ECMO | IR | NPWT | — | — | — | Resolved |
| 11 | 74 | M | HTPL | — | ECMO | IL | NPWT | — | — | — | Resolved |
ALT, anterolateral thigh; C, cortisone; CT, chemotherapy; EVAR, endovascular aneurysm repair; F, female; ECMO, extracorporeal membrane oxygenation; H, hematoma; HTPL, heart transplant immunosuppressive therapy; IL, inguinal left; IR, inguinal right; IS, immunosuppressive therapy; KTPL, kidney transplant immunosuppressive therapy; LF, Lymphatic fistula; LTPL, lung transplant immunosuppressive therapy; M, male; MLTL, medial lower thigh left; mo, month; MTX, methotrexate; MUTL, medial upper thigh left; MUTR, medial upper thigh right; NA, lost to follow-up; Nb, patient number; NL, neck left; NPWT, negative pressure wound therapy; NR, neck right; nRT, neoadjuvant radiotherapy; OK-432, Picibanil; PA, percutaneous aspiration; SIEA, superficial inferior epigastric artery perforator flap; ST, sclerotherapy; STSG, split thickness skin graft; SWD, superficial wound dehiscence.
*Tumor stage not available.
†Follow-Up 6 month.
Table 3.
Number of LVA in Each Patient
| Patient Number | Nb of LVA |
|---|---|
| 1 | 1 |
| 2 | 3 |
| 3 | 1 |
| 4 | 1 |
| 5 | 1 |
| 6 | 1 |
| 7 | 1 |
| 8 | 2 |
| 9 | 1 |
| 10 | 2 |
| 11 | 1 |
Nb, patient number.
DISCUSSION
Different concepts about the anatomical characteristics of the lymphatic system exist in an attempt to explain why LF becomes chronic in some patients. In the lower limbs, there are 2 major routes of lymphatic flow. Their distribution is similar to veins of the lower limb and can be divided into 2 major groups: superficial vessels and deep vessels. Furthermore, cadaver studies demonstrate that the lymphatic vessels of the thigh form 3 layers: the first layer, immediately below the surface of the subcutaneous fat; the second layer, between the first and third layers; and the third layer, on the deep fascia.19,20 The medial aspect of the thigh in particular contains rich vertical lymphatic vessels.20 According to the concept of lymphosomes, superficial lymphatic vessels diverge and merge on their way to the lymph node and do not cross each other in humans, demarking and dividing the skin into certain lymphatic territories, which are referred to as lymphosomes.21 Preferential lymphatic drainage patterns seem to exist such that for a given tissue space, fluid drainage is the primary responsibility of one single vessel, whereas any additional vessels in the area serve as overflow or reserve transport routes for large fluid loads.22 If these connections are not present and/or high-flow afferent lymphatic vessels are injured, LF might be promoted. Multiple studies show that supermicrosurgical lymphatic reconstruction is effective for creating new lymphatic pathways enabling the smooth flow of lymph fluid from the affected area to the trunk.15–17,23 The saphenous vein is often resected during LAD and soft-tissue tumor resections of the upper medial thigh. The anatomy of the venous system of the thigh still allows the implementation of intralesional LVA as superficial veins perforate the deeper layer and connect with the deep venous systems.
Macroscopic ligation of lymphatic vessels bears the risk of subsequent clinical lymphedema because the normal lymphatic drainage function may be impaired and is therefore not recommended.24 Additionally, macroscopic ligation often does not actually close the microscopic severed lymphatic vessel. Selective MLL under the fluorescence microscope using microtitanium clips as well as lymphatic mapping and ligation of severed lymph vessels with nonabsorbable sutures have been described to be useful for the treatment of refractory LF.14,25 In the case of a high lymphatic upload through a main pathway producing high pressure, simple ligation might come off or loosen. Therefore, LVA might be superior to MLL alone, if possible, because these will reduce the pressure within the lymphatic system and allow a physiological drainage of the severed lymphatic vessel into the venous system. Other studies have described LVA for LF15–17,23,26–28 demonstrating successful treatment by LVA in patients with pelvic LF after gynecologic cancer treatment combined with lymphocele capsule resection and in patients with LF after SLNB. One patient with chronic LF after inguinal hernia repair was treated successfully by surrounding LVA for chronic groin lymphocele treatment.28 In our experience, the main advantage of performing LVA over MML is that the fistula usually is treated more reliably as the technique addresses the physiology of the condition directly. One danger of clipping only may be that the clips fall off during wound healing and the fistula returns. Reconstruction of alternative drainage pathways for severed lymphatics will be the better option than just ligating those and should be the primary goal. Results may be more stable and the risk of lymphedema might be lower. However, not all patients are suitable for LVA, especially in the case of chronic LF, in which the patients was usually been operated many times and the tissue is extremely scarred. Lymphatics may be too small in caliber or fibrotic, and there may be no appropriate venules in close proximity. We therefore propose to use both techniques to treat LF.
Some authors claim that extralesional LVA/LLA have similar effects compared to intralesional LVA/LLA.15,28 So far, there is no evidence that all patients benefit from this technique. Moreover, there is a considerable risk of developing secondary lymphedema following these procedures. We did not perform extralesional LVA/LLA since we believe that these should be preserved for patients with a preexisting lymphedema. We also did not treat patients with concomitant lymphedema, whereas the whole patient collective described by Giacalone et al15 had lymphedema before treatment. None of our patients had lymphedema before the treatment and none developed manifest lymphedema at the end of follow-up. Our mean follow-up time was 11 months, and most patients were followed up at 12 months postoperatively. Only 1 patient developed mild symptoms of lymphedema after LVA and MLL, which was transient and symptoms disappeared at 6 months. Our results show that MLL and LVA can safely be performed in patients with LF. Giacalone et al15 further have presented a highly homogenous patient population in contrast to our cohort. Furthermore, all patients were immunocompetent. On the contrary, the patient collective in clinical practice is very inhomogeneous and a patient-specific approach is advised (eg, immunosuppression, tissue defect, infection, localization, previous irradiation, etc.).
Twenty-eight percent patients were immunosuppressed [MLL only cohort 27% (6/22) and LVA/MLL cohort 30% (3/10)]. Most of the patients included into this study did not receive an additional flap (72%, ie, 23/32). A flap was usually added if impaired wound healing was suspected due to the size of the skin defect or if the soft-tissue coverage was insufficient. Especially in immunocompromised patients, wound healing can remain a problem even after LF is treated. In these cases, tissue reconstruction is necessary. Eventually, the fact that wound closure is achieved at all is a big success in some patients. Moreover, coverage with a flap alone does not stop the lymphocele. It will at best turn the open wound with LF into a closed would with a LF underneath. Studies have shown that muscle flaps do not reduce morbidity after lymphadenectomies.7,29
In summary, we have demonstrated how diverse the collective of LF patients is in daily clinical practice, and our numbers and demographics are mirroring the reality at our maximum care university hospital with a busy reconstructive surgery unit. We do not aim to confirm a definitive best way of treating LF as the present study design will not allow for such a conclusion. Based on our experiences and the results of this study, we recommend a multimodal and patient-centered strategy that may include adjunct sclerotherapy to optimize outcomes in each individual case. MLL and LVA may complement each other. LF can be very difficult to manage and patients usually spent months in the hospitals before they finally are referred to us. Primary sclerotherapy alone often was not successful due to a high-flow lymphatic fistula in these patients. The proposed algorithm intends to act as a guide and assist in controlling an LF in a comprehensive way for a seemingly minor, but eventually severe problem, since no real treatment options exist, if sclerotherapy alone is not successful. However, sclerotherapy may very well be useful as an adjunct therapy after surgical repair for a much smaller remaining fistula.
Another interesting aspect of our results is that although symptomatic axillary LF occurs in 14% of patients undergoing breast-conserving surgery with SLNB, we did not see any patients with axillary LF.30 Management infrequently requires more than simple aspiration.30 An explanation might be that the groin is permanently exposed to shear stress whenever the patient ambulates, whereas the axilla can easily rest. In addition, a significant higher lymphatic flow can be expected from a leg when compared to an arm.
Some limitations apply to this study. First, the study has a retrospective, descriptive nature and further long-term follow-up data should be collected. Especially, because it is not possible to determine whether chronic lymphedema is present during a 1-year follow-up period alone. Due to lack in documentation, aspirated volumes, drainage output volumes and lymphocele sizes could not be described. The effectiveness of each treatment modality and their combination as well as complication rates and side effects have to be tested separately and prospectively. Also, postoperative imaging evaluation should be used to assess for lymphedema and verify the significance of cases in which an LVA could be performed. In a next scientific step, we are planning a long-term follow-up study with a larger cohort to identify confounders and to compare different treatments and their efficacies.
In the present study, we have demonstrated that our standardized staged surgical approach depending on wound status (open/closed) and the presence of immunosuppression in patients with postoperative LF is feasible and highly successful at our institution. We are not aware of a study that discusses a staged surgical approach to LF with multiple techniques complementing each other as presented here. To our knowledge, we report the largest cohort treated so far with this approach. In addition, we adopted this technique to lesions of the thoracic duct. LVA was successfully performed to reconstruct a thoracic duct transection by lymphovenous anastomosis of the thoracic duct to the external jugular vein after neck dissection for hypopharyngeal cancer.31
CONCLUSIONS
In conclusion, the treatment of LF is very challenging and should follow a standardized staged approach to optimize each patient’s outcome. LF was treated successfully in all our patients. We therefore strongly advise a multimodal interdisciplinary approach to this common clinical problem that includes adjunctive sclerotherapy. Reconstructive lymphatic supermicrosurgery should be considered as a preferred causal therapy. MLL remains important in combination and whenever reconstructive microsurgery is not feasible.
Footnotes
Published online 18 February 2021.
Presented at the 30th EURAPS (European Association of Plastic Surgery) meeting in Helsinki, Finland (2019), and the DAM (German-speaking Society for Microsurgery of Peripheral Nerves and Vessels) meeting in Lugano, Switzerland (2018).
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Montalto E, Mangraviti S, Costa G, et al. Seroma fluid subsequent to axillary lymph node dissection for breast cancer derives from an accumulation of afferent lymph. Immunol Lett. 2010;131:67–72. [DOI] [PubMed] [Google Scholar]
- 2.Bonnema J, Ligtenstein DA, Wiggers T, et al. The composition of serous fluid after axillary dissection. Eur J Surg. 1999;165:9–13. [DOI] [PubMed] [Google Scholar]
- 3.Pan WR, Zeng FQ, Wang DG, et al. Perforating and deep lymphatic vessels in the knee region: an anatomical study and clinical implications. ANZ J Surg. 2017;87:404–410. [DOI] [PubMed] [Google Scholar]
- 4.Pan WR, Levy SM, Wang DG. Divergent lymphatic drainage routes from the heel to the inguinal region: anatomic study and clinical implications. Lymphat Res Biol. 2014;12:169–174. [DOI] [PubMed] [Google Scholar]
- 5.Gashev AA, Nagai T, Bridenbaugh EA. Indocyanine green and lymphatic imaging: current problems. Lymphat Res Biol. 2010;8:127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cigna E, Gradilone A, Ribuffo D, et al. Morbidity of selective lymph node biopsy for melanoma: meta-analysis of complications. Tumori. 2012;98:94–98. [DOI] [PubMed] [Google Scholar]
- 7.Greuter L, Klein JH, Rezaeian F, et al. Evaluation of factors in seroma formation and complications in sentinel and radical lymph node dissections in skin cancer patients. Eur J Plast Surg. 2017;40:39–46. [Google Scholar]
- 8.Contreras N, Jakub JW. The achilles heel of minimally invasive inguinal lymph node dissection: seroma formation. Am J Surg. 2020;219:696–700. [DOI] [PubMed] [Google Scholar]
- 9.Poon-Chue A, Menendez L, Gerstner MM, et al. MRI evaluation of post-operative seromas in extremity soft tissue sarcomas. Skeletal Radiol. 1999;28:279–282. [DOI] [PubMed] [Google Scholar]
- 10.Sanniec KJ, Swanson S, Casey WJ, III, et al. Predictive factors of wound complications after sarcoma resection requiring plastic surgeon involvement. Ann Plast Surg. 2013;71:283–285. [DOI] [PubMed] [Google Scholar]
- 11.Fischer JP, Nelson JA, Mirzabeigi MN, et al. Prophylactic muscle flaps in vascular surgery. J Vasc Surg. 2012;55:1081–1086. [DOI] [PubMed] [Google Scholar]
- 12.Rebecca AM, Mahabir RC, Pflibsen L, et al. Indocyanine green lymphangiography as an adjunct for the optimal identification and management of lymphatic leaks in the groin. J Reconstr Microsurg. 2019;35:83–89. [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Aliano K, Stavrides S, et al. Lymphatic mapping in the treatment of chronic seroma: a case series. Eplasty. 2015;15:e7. [PMC free article] [PubMed] [Google Scholar]
- 14.Toyserkani NM, Nielsen HT, Bakholdt V, et al. Ligation of lymph vessels for the treatment of recurrent inguinal lymphoceles following lymphadenectomy. World J Surg Oncol. 2016;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacalone G, Yamamoto T, Hayashi A, et al. Lymphatic supermicrosurgery for the treatment of recurrent lymphocele and severe lymphorrhea. Microsurgery. 2019;39:326–331. [DOI] [PubMed] [Google Scholar]
- 16.Gentileschi S, Servillo M, Salgarello M. Supramicrosurgical lymphatic-venous anastomosis for postsurgical subcutaneous lymphocele treatment. Microsurgery. 2015;35:565–568. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Yoshimatsu H, Koshima I. Navigation lymphatic supermicrosurgery for iatrogenic lymphorrhea: supermicrosurgical lymphaticolymphatic anastomosis and lymphaticovenular anastomosis under indocyanine green lymphography navigation. J Plast Reconstr Aesthet Surg. 2014;67:1573–1579. [DOI] [PubMed] [Google Scholar]
- 18.Uyulmaz S, Puippe G, Büyükakyüz N, et al. Sclerotherapy with OK-432 for the treatment of symptomatic lymphocele after lymph node dissection: a retrospective comparative cohort study. Ann Plast Surg. 2020;85:407–412. [DOI] [PubMed] [Google Scholar]
- 19.Kubik S, Manestar M. Topographic relationship of the ventromedial lymphatic bundle and the superficial inguinal nodes to the subcutaneous veins. Clin Anat. 1995;8:25–28. [DOI] [PubMed] [Google Scholar]
- 20.Pan WR, Wang DG, Levy SM, et al. Superficial lymphatic drainage of the lower extremity: anatomical study and clinical implications. Plast Reconstr Surg. 2013;132:696–707. [DOI] [PubMed] [Google Scholar]
- 21.Suami H, Scaglioni MF. Lymphatic territories (lymphosomes) in the rat: an anatomical study for future lymphatic research. Plast Reconstr Surg. 2017;140:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiler M, Dixon JB. Differential transport function of lymphatic vessels in the rat-tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imaging. Front Physiol. 2013;4:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morihisa Y, Inoue Y, Kiyokawa K, et al. Objective assessment of the efficacy of supermicrosurgical lymphaticovenous anastomosis and microsurgical lymphaticovenous implantation in a case of axillary lymphorrhea. J Reconstr Microsurg. 2008;24:29–32. [DOI] [PubMed] [Google Scholar]
- 24.Van den Brande P, von Kemp K, Aerden D, et al. Treatment of lymphocutaneous fistulas after vascular procedures of the lower limb: accurate wound reclosure and 3 weeks of consistent and continuing drainage. Ann Vasc Surg. 2012;26:833–838. [DOI] [PubMed] [Google Scholar]
- 25.Lavie O, Karmeli R, Mansano R, et al. Treatment of recurrent inguinal lymphocele by lymphatic leakage mapping and subsequent ligation of lymphatic vessel endings: a case report. Gynecol Oncol. 2002;84:155–156. [DOI] [PubMed] [Google Scholar]
- 26.Todokoro T, Furniss D, Oda K, et al. Effective treatment of pelvic lymphocele by lymphaticovenular anastomosis. Gynecol Oncol. 2013;128:209–214. [DOI] [PubMed] [Google Scholar]
- 27.Boccardo F, Dessalvi S, Campisi C, et al. Microsurgery for groin lymphocele and lymphedema after oncologic surgery. Microsurgery. 2014;34:10–13. [DOI] [PubMed] [Google Scholar]
- 28.Ayestaray B, Esnault M, Godard M, et al. Treatment of refractory groin lymphocele by surrounding supermicrosurgical lymphaticovenous anastomosis. Arch Plast Surg. 2018;45:290–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Judson PL, Jonson AL, Paley PJ, et al. A prospective, randomized study analyzing sartorius transposition following inguinal-femoral lymphadenectomy. Gynecol Oncol. 2004;95:226–230. [DOI] [PubMed] [Google Scholar]
- 30.Gunn J, Gibson T, Li Z, et al. Symptomatic axillary seroma after sentinel lymph node biopsy: incidence and treatment. Ann Surg Oncol. 2016;23:3347–3353. [DOI] [PubMed] [Google Scholar]
- 31.Lindenblatt N, Puippe G, Broglie MA, et al. Lymphovenous anastomosis for the treatment of thoracic duct lesion: a case report and systematic review of literature. Ann Plast Surg. 2020;84:402–408. [DOI] [PubMed] [Google Scholar]


