Dear Editor
Between 1 February and 19 May 2020, 16 200 adult patients had surgery at Greater Paris University Hospitals. SARS-CoV-2 reverse transcriptase (RT)–PCR results were available for 6740 patients (41.6 per cent), with a mean(s.d.) age of 58.2(19.7) years (51 per cent men). The percentage of operated patients with SARS-CoV-2 RT–PCR results available increased over time, from 846 of 3108 (27.2 per cent) between 1 and 15 February, to 1951 of 2510 (77.7 per cent) between 16 and 30 April, showing progressive awareness of COVID-19 infection in the surgical community.
Patients undergoing surgery were categorized into five groups: group 1, positive RT–PCR test within 5 days before surgery; group 2, positive RT–PCR test within 5 days after surgery; group 3, positive RT–PCR test between 5 and 30 days after surgery; group 4, positive RT–PCR at least 5 days before surgery; and group 5, negative RT–PCR test(s) or positive RT–PCR test more than 30 days after surgery. Procedures were graded according to a complexity index ranging from 1 to 4 independent of outcomes1. Three time intervals were identified: prepandemic phase, 1 February to 16 March 2020; peak and lockdown phase, 16 March to 15 April 2020; and postpeak phase, 15 April to 19 May 2020.
Among the 6740 operated patients with SARS-CoV-2 RT–PCR tests available, there were 164 (2.4 per cent) in group 1, 88 (1.3 per cent) in group 2, 147 (2.2 per cent) in group 3, 129 (1.9 per cent) in group 4, and 6212 (92.2 per cent) in group 5.
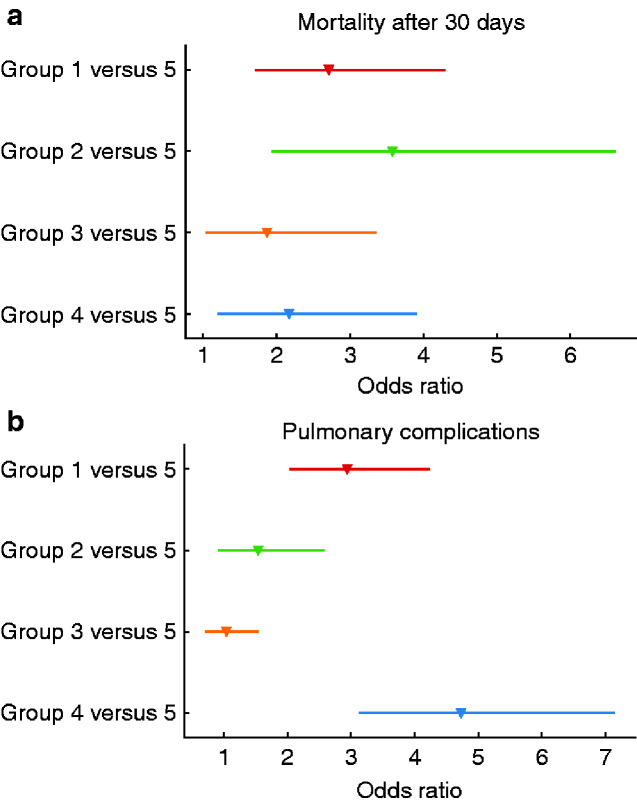
The overall death rate within 30 days after surgery was 4.9 per cent, with the highest rate in group 1 (17.7 per cent) and the lowest in group 5 (4.1 per cent). Mortality was also higher during the pandemic peak phase (125 of 1463, 8.5 per cent) than during the prepandemic (69 of 1811, 3.8 per cent) or postpeak (136 of 3466, 3.9 per cent) phases. Compared with group 5, the adjusted odds ratios (ORs) for 30-day mortality were 2.71 (95 per cent c.i. 1.71 to 4.30), 3.58 (1.93 to 6.62), and 2.17 (1.20 to 3.91) for groups 1, 2, and 4 respectively (Fig. 1a).
Fig. 1.
Adjusted odds ratios for mortality and pulmonary complications within 30 days after surgery
a Mortality and b pulmonary complications. Group 1, positive reverse transcriptase (RT)–PCR test within 5 days before surgery; group 2, positive RT–PCR test within 5 days after surgery; group 3, positive RT–PCR test between 5 and 30 days after surgery; group 4, positive RT–PCR at least 5 days before surgery; and group 5, negative RT–PCR test(s) or positive RT–PCR test more than 30 days after surgery.
The overall pulmonary complication rate was 18.5 per cent, with 51.9 and 55.5 per cent in groups 1 and 4 respectively, and 16.5 per cent in group 5. Pulmonary complications were more frequent during the prepandemic (440 of 1791, 24.6 per cent) and pandemic peak (362 of 1445, 25.1 per cent) phases than during the postpeak (418 of 3446, 12.5 per cent) phase. Compared with group 5, the ORs for 30-day pulmonary complications were 2.94 (2.04 to 4.24), 1.54 (0.91 to 2.58), and 4.73 (3.13 to 7.14) for groups 1, 2, and 4, respectively (Fig. 1b).
Similar results for death and pulmonary complications were obtained after adjustment for the complexity index of the first operation rather than for the highest complexity index of all procedures for each patient; adjustments for pandemic phases led to similar conclusions. Further background information and full results are provided in Tables S1–S8 and Figs S1–S3.
In brief, patients operated during the incubation period for COVID-191 (groups 1 and 2) and those with a history of COVID-192 (group 4) had increased risks of death and pulmonary complications. Potential nosocomial SARS-CoV-2 infection was associated with higher death and complication rates (groups 2 and 3). High mortality and complication rates in group 5 were probably due to false-negative SARS-CoV-2 RT–PCR results3. Mortality rates were different during peak pandemic (8.5 per cent), prepandemic (3.8 per cent), and postpeak (3.9 per cent) phases.
The SARS-CoV-2 pandemic led to large-scale cancellations of elective surgical procedures and to the establishment of priorities for vital procedures4. Nevertheless, better evaluation of risk is still needed to develop guidelines for surgery in patients with COVID-195. Based on a large controlled consecutive cohort study, the present data confirm previous results2,4 and suggest that: during active phases of the pandemic, surgical patients should be isolated for at least 5 days before operation to avoid any contamination risk; and patients with a previous history of SARS-COV-2 infection should be considered at risk for surgery.
Supplementary material
Supplementary material is available at BJS online.
Supplementary Material
Acknowledgements
Data used in preparation of this article were obtained from the AP-HP COVID Clinical Data Warehouse (CDW). As such, members of the AP-HP COVID CDW Initiative (ACCI) contributed to the design and implementation of the database, but did not participate in the analysis or writing of this report. A complete list of ACCI members can be found at https://eds.aphp.fr/COVID-19.
Disclosure. The authors declare no conflict of interest.
References
- 1. Lei S, Jiang F, Su W, Chen C, Chen J, Mei W. et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EclinicalMedicine 2020;21:100331 (CrossRef)(10.1016/j.eclinm.2020.100331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glasbey JC, Nepogodiev D, Omar O.. Delaying surgery for patients with a previous SARS-CoV-2 infection. Br J Surg 2020;107:e601–e602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woloshin S, Patel N, Kesselheim AS.. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med 2020;383:e38 (CrossRef)(10.1056/NEJMp2015897) [DOI] [PubMed] [Google Scholar]
- 4. Nepogodiev D, Glasbey JC, Li E.. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020;396:27–38 (CrossRef)(10.1016/S0140-6736(20)31182-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nepogodiev D, Omar OM, Glasbey JC.. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg 2020;107:1440–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.