Abstract
Background
Many drugs approved for other indications can control the growth of tumor cells and limit adverse events (AE).
Data sources
Literature searches with keywords ‘repurposing and cancer’ books, websites: https://clinicaltrials.gov/, for drug structures: https://pubchem.ncbi.nlm.nih.gov/
Areas of agreement
Introducing approved drugs, such as those developed to treat diabetes (Metformin) or inflammation (Thalidomide), identified to have cytostatic activity, can enhance chemotherapy or even replace more cytotoxic drugs. Also, anti-inflammatory compounds, cytokines and inhibitors of proteolysis can be used to control the side effects of chemo- and immuno-therapies or as second-line treatments for tumors resistant to kinase inhibitors (KI). Drugs specifically developed for cancer therapy, such as interferons (IFN), the tyrosine KI abivertinib TKI (tyrosine kinase inhibitor) and interleukin-6 (IL-6) receptor inhibitors, may help control symptoms of Covid-19.
Areas of controversy
Better knowledge of mechanisms of drug activities is essential for repurposing. Chemotherapies induce ER stress and enhance mutation rates and chromosome alterations, leading to resistance that cannot always be related to mutations in the target gene. Metformin, thalidomide and cytokines (IFN, tumor necrosis factor (TNF), interleukin-2 (IL-2) and others) have pleiomorphic activities, some of which can enhance tumorigenesis. The small and fragile patient pools available for clinical trials can cloud the data on the usefulness of cotreatments.
Growing points
Better understanding of drug metabolism and mechanisms should aid in repurposing drugs for primary, adjuvant and adjunct treatments.
Areas timely for developing research
Optimizing drug combinations, reducing cytotoxicity of chemotherapeutics and controlling associated inflammation.
Keywords: thalidomide, metformin, cytokine storm, unfolded protein response (UPR), proteosome inhibitors, interferons (IFN), tumor necrosis factor (TNF), interleukin-2 (IL-2), Covid-19 adjuvant treatments
Introduction
Using compounds approved for one clinical use in another disease or syndrome is referred to as ‘repurposing’. Most of the drive for repurposing is the high cost of developing a drug, and the very long time it can take to determine the safety and specificity of a completely new drug. The timeline for any new cancer drug to go through enough clinical trials to obtain approval can be years, or even decades. Even for chronic myeloid leukemia (CML), which may affect about 0.2% of the population during their lifetime, over a decade was required for one of the first therapeutic KIs, Gleevec, to become standard of care. For smaller patient pools or drugs with less clear results in treatment, the delay can be even longer. Approval of omacetaxine, a plant alkaloid that inhibits protein translation, for treating TKI-resistant CML took more than 30 years.1 A phase 1 trial (NCT02081378) for asciminib (an allosteric inhibitor of the ABL kinase) to treat TKI-resistant CML and Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL), began in 2014, is ongoing in August of 2020 with an estimated completion date of 2024.
Repurposing, per se, is an old concept in oncology. Indeed, the first chemotherapy drugs might be regarded as repurposed chemical weapons, arising as they did from research on the ‘mustard gas’ (which has no relation to mustard and is liquid at room temperature) that caused so many deaths and chronic illness in the wars of the 20th century. While treating survivors of these attacks, doctors realized that the toxins, in addition to their vesicant (i.e. blister-inducing) activity, might have antitumor potential. In the medical equivalent of beating guns into plowshares, in this case converting a toxic compound to a therapeutic one, chemists and doctors worked together in a decade-long search for compounds with lower toxicity and enhanced cytostatic activity, with the hope of finding treatments to prolong the lives of their cancer patients.2 After many explorations of conjugates with different biological molecules, two alkylating agents, chlorambucil (Leukeran) and busulfan (Myleran) (Fig. 1), were developed to treat chronic lymphocytic and myeloid leukemias (CLL and CML). Despite the advent of many other chemotherapies, these simple drugs continue to be used to this day.
Fig. 1.

Alkylating agents used for treating leukemias, chlorambucil (left) and bufulfone (myleran, right) were patterned after toxic nitrogen mustard gas (center) during many design iterations. 3D chemical structures are from Pubchem; atom colors are: carbon, gray; hydrogens, small white; nitrogen, blue; sulfur, yellow; chloride, green; oxygen, red.
This review will discuss two basic meanings of repurposing in cancer therapy. The first is adapting drugs used in other areas, for example anti-infectives or treatments for chronic diseases, for their observed cytostatic activity.3 A second meaning is using drugs that were designed primarily to treat other illnesses to enhance the effects of chemotherapy or manage side effects. Examples of these two areas are shown below. Section ‘Repurposing cancer therapies as antivirals and specifically anti-Covid-19 treatments’ introduces the recent testing of cancer medications for treating Covid-19 infections and how this may have future benefit for repurposing in oncology.
Adapting common drugs with cytostatic potential for cancer therapy
The great dream of the many waves of drug design in cancer is to achieve a drug that will only kill cancer cells, leaving most normal cells untouched. No chemotherapy has ever achieved this lofty goal. Unlike the careful design of, for example, drugs targeting phosphorylation cascades4,5 and orally available TKIs,6 there is no clear cellular target for most early therapies. Chemotherapy infusions cannot be typically done at home, as there is risk of anaphylaxis and many are so toxic that accidental extravasation7 can lead to difficult to treat blisters. Directing treatments to specific organs often leads to the escape of a few wayward bandits, abnormal cells that will eventually find their way into another tissue and reinitiate tumorigenesis. Oncologists are thus always on the lookout for drugs with fewer side effects that can be used to treat cancer as a chronic disease or even prevent it.8,9 Drugs designed to treat many different indications have been introduced into cancer therapy10 and hundreds more have been reported to inhibit the growth of tumor cells in culture (see https://depmap.org/repurposing for the growth inhibitory activity of approved drugs against 578 human cancer cell lines.11).
One area for repurposing is to replace current therapies with others that are cytostatic, rather than cytotoxic. Two repurposed drugs that have recently shown the most success are metformin, used since 1995 in the USA for diabetes, and thalidomide and its derivatives, which were developed to treat diseases such as psoriasis and inflammation related to infections.
Metformin as a cytostatic agent
Metformin (Fig. 2) traces its roots to a plant extract whose primary ingredient was guanidine, used throughout the Middle ages to treat diabetes symptoms.12 Metformin was first synthesized in 1922, but due to the advent of insulin, only advanced as ‘Glucophage’ 30 years later when it was approved in France. It has become the first-line treatment for type 2 diabetes. Pertinent to this review, metformin is playing an increasing role as a cytostatic cancer treatment, thanks to its low toxicity (its primary adverse event (AE) is the rarely occurring lactic acidosis.) The first cancer trials arose from reports that diabetics taking metformin daily had lower rates of breast13 and other cancers, augmented by studies showing cells from metformin-treated diabetics do not grow well in culture.14
Fig. 2.
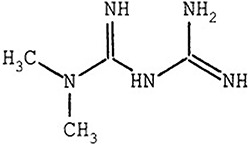
Metformin (dimethylbiguanide) used for diabetes treatment.
The cytostatic effects reported led to introducing metformin as an adjunct therapy for different types of cancers, some of which have reported remarkable success. In a recent phase II trial, 139 lung adenocarcinoma patients, whose tumors contained driver epidermal growth factor receptor (EGFR) mutations, were treated with TKIs (erlotinib hydrochloride, afatinib dimaleate or Gefitinib at standard dosage) plus or minus 500 mg/day of metformin (i.e. well within the normal dosage for treating diabetes). Adding metformin increased the progression-free survival (PFS) by about a third; it nearly doubled overall survival (OS).15
Metformin also improved PFS and OS in advanced, previously untreated non-small cell lung cancer (NSCLC) when used in combination with platinum-based chemotherapy with or without the anti-VEGF inhibiting antibody, bevacizumab (Avastin) in two phase II studies. These included 33 non-diabetic patients of whom 70% had some history of smoking, with KRAS, EGFR and LKB1 mutation prevalence of 48, 26 and 8.3%, respectively. The PFS and OS for metformin-treated patients were especially improved in those with KRAS mutations. This suggests that determining molecular subgroups should be used to guide therapy in the future, especially in light of the paucity of direct KRAS inhibitors.16
As exciting as these results are, metformin does not always improve survival.17 The effects seen in the various clinical trials, many of which are ongoing, are patient specific. Several metabolic pathways altered by metformin treatment may account for the heterogeneity in response. It might be logical to assume that the overall effect of metformin, by lower circulating glucose concentration, would specifically starve tumor cells (which have an enhanced metabolic need for glucose), leading to decreased proliferation and metastasis. Various other explanations have been given for its observed effects in diabetes and in preventing cell growth,18 whereby the inability of metformin to enter many cells has not always been accounted for in studies of its effects on metabolism.19 One is that metformin can metabolically reprogram cancer cells by activating 5′ AMP-activated kinase (AMPK), by increasing the ratio of AMP to ATP to some extent in cells (whereby the ratio was much higher after Rosiglitazone treatment). Other studies attributed the lower rates of breast cancer to metformin’s role in controlling fatty acid oxidation.20
Identifying the patients most likely to be helped relies on accurately identifying the cells most affected by metformin and determining if those types predominate in the patient tumors. A study of normal murine mammary cells indicated that metformin had the highest effect on hormone receptor positive luminal cells, where it decreased the total cell number, progenitor capacity and DNA damage. The authors suggest that identifying this type of cells in humans would indicate those most likely to benefit from metformin treatment.21 To shed further light on this question, whole transcriptome RNA sequencing22 of 40 breast cancer patients before and after 13–21 days of dose escalating metformin (from 500 mg/day to 1500 by day 6, which is still within the dose range for diabetes treatment) revealed that patients’ profiles correlated with an optimal antiproliferative response. One commonality was that metformin treatment increased glucose flux in the tumor (as measured by 18-fluorodeoxy glucose uptake via PET-CT) as well as in other tissues.
Thalidomide and derivatives in cancer therapy
Thalidomide derivatives have a variety of uses in modern cancer therapy. Indeed, someone who awoke suddenly from a 60-year sleep would be amazed that this notorious drug would be in such widespread use today. The clinical tragedy associated with its first introduction remains a cautionary tale for all involved in drug research.23 Thalidomide was first developed to treat morning sickness and sold over the counter to pregnant women in Germany in the 1950s, with recommended doses in the range of aspirin treatments (300–500 mg). The drug's side effects, including peripheral neuropathy, stopped its approval in the USA. However, thalidomide was only withdrawn worldwide in 1962 after it was linked to severe birth defects. As discussed elsewhere,23 even after this withdrawal, thalidomide remained in clinical use for treating Hansen disease (leprosy). The major use of thalidomide and its derivatives for many years was immunomodulatory. There are now a variety of thalidomide-related compounds to choose from that have been designed to specifically control different pathways in immune cells. The relatively low cost of treatment for this family of drugs means it can play a role in many cancer therapies, both for its tumor growth inhibition and its anti-inflammatory activities.
The earliest introduction of thalidomide derivatives to cancer therapy was to control inflammation. Eventually, their ability to prevent the growth of certain cancer cells was recognized. Thalidomide and its more potent structural relatives, lenalidomide and pomalidomide,23 are used to treat multiple myeloma,24 mantle cell lymphoma, and myelodysplastic syndromes associated with the deletion 5q abnormality. On the other hand, apremilast (Otezla) was specifically designed to inhibit PDE425,26 and is now used to control psoriasis, lupus erythematosus and rheumatoid arthritis.27 PDE4 is a phosphatase that degrades cAMP, a small molecule that can modulate inflammatory responses. Targeted PDE4 inhibitors are in preclinical trials for cancer.28,29
At this point, the mechanistic basis for using the thalidomide drug family in cancer becomes confusing, as they have pleiomorphic effects. For example, their anti-inflammatory activity has been linked to their ability to inhibit secretion of tumor necrosis factor (TNF)-α and other cytokines.30,31 Anti-TNF antibodies such as Humira have revolutionized the treatment of psoriasis and rheumatoid arthritis; however, inhibiting TNF may enhance inflammatory central nervous system syndromes such as multiple sclerosis.32
A recent discovery suggests another reason for thalidomide-related compounds’ action in cancer cells: their ability to bind cereblon,33 a protein involved in limb outgrowth. The devastating teratogenic effects of thalidomide when taken in early pregnancy have also been linked to this binding. Even more intriguing are current attempts to manipulate thalidomide’s binding to cereblon to induce specific protein degradation in cancer cells.34 Early results indicated that thalidomide induced specific degradation of repressors in T-cells that can lead to activation and increased IL-2 secretion, thus providing another way to stimulate the immune system to fight cancer cells.35
Cytokine-based therapies
There are repeated trials of cytokines as co-therapies. The earliest of the cytokines to enter cancer trials were recombinant interferons (IFN), introduced in the 1980s.36–39 The IFNs were identified for their antiviral activity; their first use in cancer was for the control of leukemias. They have also been tested for a variety of blood and solid cancers. IFN-α was used for many years to treat hairy cell leukemia,40 CML and myelofibrosis41; it may still be resorted to alone or as adjunct therapy for CML patients resistant to multiple TKIs.1 A recent paper reported deep molecular remission in four of nine CML patients treated with Imatinib plus Ropeginterferon-α2b, with few AE.42 IFNs have also been suggested to enhance treatment with temozolomide by inhibiting the MGMT repair enzyme.43 However, chromosome instability in AML has been shown to directly upregulate IFN-stimulated genes,44 suggesting that IFN itself will not be helpful in treatment. The cost, need for injection or infusion, and side effects of IFNs suggest they could be replaced with small molecule, intracellular inducers, such as STING activators,45,46 which may also require injection, or with compounds that may activate select steps in the IFN-induced pathways.
Other cytokines39,47 have been tried repeatedly as co-treatments. Two of these proteins, TNF48 and IL-2, were highly anticipated as potential anticancer therapies. Early tests showed some successes, but their toxic effects thwarted widespread clinical use. As with IFNs, there are continuing attempts to repurpose IL-2 in cancer therapy,49–52 as an additive to immunotherapy, or for treating patients who have failed to respond to immunotherapy.53 More study on how to control the AEs in IL-2 treatments54,55 may lead to safer ways to use this molecule in cancer.
TNF is also problematic as a cancer treatment. Direct clinical use of TNF has been limited by side effects, such as cachexia and fever. TNF has been implicated in the origin of many different types of tumors, possibly precluding it use as a treatment.56 Furthermore, TNF levels in the body rise with age,57,58 along with increased incidence of cancer. TNF levels should certainly be checked in those who fail to respond to immune therapies.
However, things may turn around for this protein; a recent paper using mouse models suggests that the TLR-5 antagonist entolimod may control the toxic effects of TNF without affecting its antitumor activity.59 If this proves true in human trials, this may unveil a new future use of TNF in cancer therapy.
Other drugs in testing
Other agents designed to treat a variety of different diseases are in testing against cancers. Statins have been tested as anticancer agents, as they can inhibit the activity of many GTPase oncogenes. While statins have not performed well as cancer drugs,60 disulfiram (Antabuse), developed to treat alcoholism, is in testing with copper to treat metastatic breast cancer (NCT03323346). Nelfinivir, an AKT inhibitor developed to treat HIV, is in phase I trials for treating solid tumors,61 (NCT01445106).
Gamma-secretase inhibitors, developed to treat Alzheimer disease (AD), are in multiple tests as anti-cancer drugs (alone [NCT01981551, NCT03785964, NCT03691207] or in combination with Car-T therapy [NCT 03502577]) as they also inhibit Notch 1 and signal peptidases.62 And in turn, the cancer drug saracatinib (AZD-0530), designed to inhibit the SRC and BCR-ABL kinases, is now being tested for its effects on AD,63 based on its inhibition of the Fyn Kinase, which may contribute to synaptotoxicity.
Repurposing drugs to control the effects of or enhance chemotherapy
Oncologists are also combining chemotherapeutics with compounds to control their effects on normal cells or improve their overall activity. Many FDA-approved chemotherapy drugs have severe side effects, ranging from blistering at the site of infusion to hair and teeth loss. While cooling the scalp or chewing ice during the infusion can partially control these side effects,64 additional anti-inflammatory compounds are being sought. The orally available inhibitors of poly (ADP-ribose) polymerase (PARP) and kinases are easy to administer, but they may induce side effects such as nausea,65,66 which may require co-treatment with antiemetics. However, use of proton pump inhibitors, used to control GERD symptoms in as many as 30% of cancer patients, has been shown to limit the effect of chemotherapy and worsen OS.67
Especially patients with cardiac problems may benefit from co-treatment during chemotherapy with drugs such as β-blockers and aspirin.68,69 Low-dose aspirin has long been recommended both to control inflammation and inhibit coagulation and was previously recommended to control stroke and heart attack incidence. However, ASPREE, a 4.7-year placebo controlled trial of >19 000 individuals older than 65–70 determined there was no advantage of taking aspirin. The risk of being diagnosed with stage 3 or 4 cancers and increased mortality was higher in the aspirin-treated patients than in the placebo group.70
The usefulness of β-blockers to control tumor growth especially has been explored as propranolol decreased proliferation, migration and invasion of triple negative breast cancer cells in vitro.71 Topical treatment with the β-blockers propranolol and timolol is a validated treatment for complicated infantile hemangiomas.72,73 There are also many studies indicating that β-blockers can control the growth of vascular sarcomas and other endothelial cell tumors.74 Their use in other cancers has shown less benefit. Multiple retrospective studies of patients treated with combination therapies including β-blockers have shown little indication of overall efficacy in ovarian cancer,75 lung cancer76 or in preventing cancer recurrence.77
However, β-blocker co-treatment with anthracyclines can significantly reduce chemotherapy cardiotoxicity and preserve left ventricle function.78,79 Furthermore, topical treatments with propranolol and timolol, such as those developed for infantile hemangiomas, can also shorten the recovery time when applied to painful swelling around the nails that can develop after treatment with EGFR inhibitors.80
Another repurposed drug, dexrazoxane, may be superior for this use. Originally developed as an antimitotic, dexrazoxane, the (+)-enantiomorph of razoxane, has now been approved by the FDA for repurposing as a cotreatment to prevent anthracycline-induced extravasation injuries81 and cardiomyopathy. Dexrazone’s effect may be due to its ability to inhibit the formation of a toxic iron-anthracycline complex.82 A recent multicenter study83 of over 1000 AML patients treated with daunorubicin or mitoxantrone showed that co-treatment with dexrazone significantly lowered cardiac problems and also reduced treatment-related mortality. Dexrazone cotreatment is also used for immunosuppressive purposes.
The blood pressure medication, Mibefradil, which slows the excretion of many common drugs, can be used short term to enhance the activity of several different cancer drugs.84 However, this may be counterproductive as increases in a drug’s plasma concentration can induce cytokine-release syndrome (also called ‘cytokine storm’). Drugs that can prevent the release of several inflammatory cytokines are hence useful. Abivertinib85 is a novel small molecule TKI targeting mutant forms of both EGFR and Bruton’s tyrosine kinase (BTK). In addition to slowing cell growth, abivertinib binds irreversibly and prevents phosphorylation of the BTK receptor, thus inhibiting the release of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α.
Leucovorin (folinic acid), developed to treat pernicious and megaloblastic anemias,86 can control the side effects of methotrexate and chemotherapy drugs. Combinations of leucovorin with 5-fluorouracil and either oxaliplatin or irinotecan (the FOLFOX or FOLFIRI regimens) are standard treatments for colorectal cancers. However, alternatives to oxaliplatin should be sought, as there are severe and long-term neurotoxic AEs associated with this combination.87 Artemisinin (malaria treatment) derivatives may be an alternative to oxaliplatin, as they are cytotoxic against colon cancer cells lines at low concentrations when used in combination with leucovorin and 5-fluorouracil (FOLNSC combination).88
Another problem arising in cancer is enhanced coagulation, leading to stroke and heart attacks, due to chemotherapy or disease progression. While aspirin can reduce coagulation, other compounds specifically designed to control clotting, such as apixaban (Eliquis), a factor Xa inhibitor developed to treat atrial fibrillation,89 may be preferable as cancer progresses. Apixaban does not have the same effect on platelet interaction as warfarin and heparin and may thus be a safer alternative.
One problem with combining inhibitors is that treatment with a wide variety of cytotoxic agents enhances mutations and treatment resistance by inducing ER stress and the unfolded protein response (UPR). UPR-induced autophagy supports tumorigenesis and the development of resistance to treatment.90,91 One way to handle the unfolded protein response to chemotherapy in general is to control the proteasome, which regulates protein expression by removing ubiquitylated proteins. Proteosome inhibitors such as ixazomib (Ninlaro®) can be combined with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy. Bortezomib (Velcade) is used in multiple myeloma and mantle cell lymphoma. Bortezomib caused a rapid and dramatic change in the levels of intracellular peptides that are produced by the proteasome,92 due to the inhibitor’s direct interaction with subunits of the proteasome. Bortezomib has been suggested as an alternative to vincristine (and to treat neuropathy associated with vincristine treatment) for pediatric ALL.93
Of course, no drug is without AEs. A recent report 94 suggests that administration of the antihistamine, ketotifen, can control the ocular effects of bortezomib.
Repurposing cancer therapies as antivirals and specifically anti-Covid-19 treatments
There is a clear overlap between the needs of cancer and severe Covid-19 patients for drugs to control inflammation and coagulation. Thus, there has been a recent spate of papers on repurposing drugs, including chemotherapy agents, to treat Covid-19 (and before the current pandemic, Ebola virus). The reader will not be surprised that there are trials planned of metformin and thalidomide as possible adjuvant treatments (e.g. NCT04510194, NCT04273529). A recent review summarizes the plethora of ongoing clinical trials of anti-cancer drugs being tested.95 Among these are many phase 1–3 trials of IFNs and Janus associated kinase (JAK) inhibitors, alone or in combination with antiviral drugs. The JAK inhibitor, baricitinib, has also been reported to be efficacious in treating Covid-19.96 There are also intriguing reports that those with mutations in IFN-related genes97 or auto-antibodies against IFNs have a more severe disease course.98 Covid-19, along with other β-coronaviruses, is known to interfere with the early immune response that is based on IFN and the genes it stimulates.
However, while early treatment with IFN (types I and III) may have benefit, later in the disease course, it can cause damage to the lung epithelia that can lead to superinfections.99 This is because IFNs can also play a role in cytokine release syndrome or ‘cytokine storm’, which may be responsible for mortality associated with Covid-19. Other tests are ongoing to determine whether inhibition of specific inflammatory cytokines is beneficial. A recent report found high levels of TNF in T-cells from Covid-19 patients with a fatal outcome and suggested the cytokine may have inhibited the immune response to the virus.100 As this increase may inhibit normal humoral responses,100 it is possible that TNF inhibitors, such as those developed for psoriasis, might be beneficial in treatment.
There are also many ongoing clinical trials for tocilizumab and other inhibitors of interleukin-6, a cytokine associated with inflammation. These IL-6 inhibitors were previously approved to treat multiple myeloma, lymphoproliferative disorders and Castleman’s syndrome. Abivertinib, a cancer therapy TKI, is in phase 2 clinical trials for repurposing to prevent cytokine storm in Covid-19 patients (NCT04440007).
While these trials are dedicated to finding better treatments for Covid-19 in this hour of emergency, it is clear that the results can have impact on the future course of cancer therapy. There are particular ramifications for how to control the inflammatory AEs of immune therapy, which often limit its usefulness in treating fragile patients who have endured many types of chemotherapy. Companies should be conscious of the advantages of combining repurposed compounds with novel therapies, during their clinical trials. Any additional costs will be more than paid for if the therapy is then more acceptable to patients.
A few words in parting
While the examples here show the positive side of repurposing, it should be noted that not all chemotherapy agents can be re-used for every cancer. Combining therapies does not always bring better results. For example, recent results of a breast cancer trial101 indicate that adding the anthracycline Epirubicin (+5-fluoruracil and cyclophosphamide) to neoadjuvant chemotherapy (including trastuzumab and pertuzumab) had little effect on event-free survival but increased AE, including two women who developed acute leukemia. As chemotherapy may primarily act in breast cancer by inducing terminal menopause, adding it to treatments designed to reduce estrogen levels may be superfluous. A 1999 study of metastatic (stage IV) melanoma showed there was no survival advantage to combining dacarbazine with cisplatin, carmustine and tamoxifen compared to high-dose dacarbazine alone. Only 25% of the patients on either regimen survived more than a year.102 Fortunately, new immunotherapy drugs, such as a combination of CTLA-4 and PD-1 inhibitors (ipilimumab and nivolumab), have revolutionized the outlook for patients with metastatic melanoma. A recent report showed 1-year survival exceeded 80%, with 4-year rates >50%.103
A second caution is that while many FDA-approved compounds have been found to have antitumor cell activity in vitro in ‘high-throughput’ screening, moving them into human treatments is difficult. Not every new combination of drugs can be tested in a random control trial; the costs, not to mention the lack of suitable patients, would be prohibitive. Improving selection from ‘shot in the dark’ assays of whole cells requires better understanding of why a given drug is cytostatic, and skepticism is called for when effects in culture require unrealistically high concentrations. For example, emetine, better known as an active ingredient of Ipecac syrup used to induce vomiting, binds ribosomes104 and has been reported to selectively kill AML cells.105 Whether its side effects can be overcome sufficiently to justify testing as a cancer therapeutic is not currently clear.
Investigating the metabolic basis for a ‘hit’ can be time consuming and costly. Funding is often lacking for such trials,106 especially for off-patent drugs. In this respect, there should be more public and pharmaceutical coalition funding available for off-label testing. Another major difficulty in repurposing compounds is the need to assemble a proper patient pool for a blinded control study. One must take into account that cancer patients, especially those who are older, have additional disease, or have survived many different treatments and accompanying AE, are fragile. For example, a paper reporting a complete response to Ipilumab therapy following treatment with BRAF/MEK inhibitors ended by reporting the patient’s death from side effects of the therapy.107 Many trials, even of new drugs, are abandoned because they do not meet their target patient pool within a reasonable time frame, or the company funding it changes direction. Dosages that may be well tolerated in trials conducted using healthy subjects or when used for treating the diseases, the drug was originally intended for may not be achievable in cancer patients. Combination therapies only complicate these problems. However, individual treatments with older drugs can lead to remarkable cures.108 Still, the best approach for a patient who has no clear alternative therapy may be to recommend an ongoing trial. Another alternative, for patients with genetic markers that correlate with the anticipated activity of a test intervention, is the N-of-1 trial.109 Here, the patient serves as his/her own control. The success of the therapy, or basis for continuation, can be based for example on blood levels of selected metabolites and proteins that should lead to reduced disease or tumor growth.
Conclusions
The examples included in this review and related references show that the pantheon of approved drugs is a rich source of solutions for many problems in oncology. Replacing cytotoxic with cytostatic drugs targeting specific cellular pathways promises to further enhance treatment while limiting AEs. Combinations with repurposed inhibitors designed to control inflammation can control the toxicity of chemotherapeutics or cytokines to normal cells and provide new treatments for resistant tumors.
The pace of research on repurposing compounds from all clinical areas is breathtaking and has contributed to increasing survival and easing the effects of chemotherapy on cancer patients. In the proper setting, established drugs can be smart therapies that can replace untargeted toxins relied upon in the past.
Data Availability Statement
No new data were generated or analyzed in support of this research.
Funding
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases, USA, R21 AI105985-01 (to CHS) and R01 AI137332-01.
Conflict of interest statement
The authors have no potential conflicts of interest.
References
- 1. Winer ES, DeAngelo DJ. A review of omacetaxine: a chronic myeloid leukemia treatment resurrected. Oncol Ther 2018;6:9–20. doi: 10.1007/s40487-018-0058-6 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haddow A. On the biological alkylating agents. Perspect Biol Med 1973;16:503–24. doi: 10.1353/pbm.1973.0029 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 3. Berns A, Ringborg U, Celis JE, et al. Towards a cancer mission in horizon Europe: recommendations. Mol Oncol 2020;14:1589–615. doi: 10.1002/1878-0261.12763 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sugiyama Y, Kameshita I. Multi-PK antibodies: powerful analytical tools to explore the protein kinase world. Biochem Biophys Rep 2017;11:40–5. doi: 10.1016/j.bbrep.2017.06.005 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braun W, Schein CH. Membrane interaction and functional plasticity of inositol polyphosphate 5-phosphatases. Structure 2014;22:664–6. doi: 10.1016/j.str.2014.04.008 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 6. Duenas-Gonzalez A, Garcia-Lopez P, Herrera LA, et al. The prince and the pauper. A tale of anticancer targeted agents. Mol Cancer 2008;7:82. doi: 10.1186/1476-4598-7-82 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang R, Murray N. Management of anthracycline extravasation into the pleural space. Oxf Med Case Rep 2016;2016:omw079. doi: 10.1093/omcr/omw079 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turanli B, Grotli M, Boren J, et al. Drug repositioning for effective prostate cancer treatment. Front Physiol 2018;9:500. doi: 10.3389/fphys.2018.00500 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeter JM, Bowles TL, Curiel-Lewandrowski C, et al. Chemoprevention agents for melanoma: a path forward into phase 3 clinical trials. Cancer 2019;125:18–44. doi: 10.1002/cncr.31719 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta SC, Sung B, Prasad S, et al. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci 2013;34:508–17. doi: 10.1016/j.tips.2013.06.005 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 11. Corsello SM, Nagari RT, Spangler RD, et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nature Cancer 2020;1:235–48. doi: 10.1038/s43018-019-0018-6 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas I, Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatr Diabetes 2017;18:10–6. doi: 10.1111/pedi.12473 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 13. Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 14. Palazzolo G, Mollica H, Lusi V, et al. Modulating the distant spreading of patient-derived colorectal cancer cells via aspirin and metformin. Transl Oncol 2020;13:100760. doi: 10.1016/j.tranon.2020.100760 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arrieta O, Barron F, Padilla MS, et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol 2019;e192553. doi: 10.1001/jamaoncol.2019.2553 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parikh AB, Marrone KA, Becker DJ, et al. A pooled analysis of two phase II trials evaluating metformin plus platinum-based chemotherapy in advanced non-small cell lung cancer. Cancer Treat Res Commun 2019;20:100150. doi: 10.1016/j.ctarc.2019.100150 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 17. De A, Kuppusamy G. Metformin in breast cancer: preclinical and clinical evidence. Curr Probl Cancer 2020;44:100488. doi: 10.1016/j.currproblcancer.2019.06.003 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 18. Pacal L, Kankova K. Metformin in oncology—how far is its repurposing as an anticancer drug? Klin Onkol 2020;33:107–13. doi: 10.14735/amko2020107 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 19. Spiering MJ. The mystery of metformin. J Biol Chem 2019;294:6689–91. doi: 10.1074/jbc.CL119.008628 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lord SR, Collins JM, Cheng WC, et al. Transcriptomic analysis of human primary breast cancer identifies fatty acid oxidation as a target for metformin. Br J Cancer 2020;122:258–65. doi: 10.1038/s41416-019-0665-5 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shehata M, Kim H, Vellanki R, et al. Identifying the murine mammary cell target of metformin exposure. Commun Biol 2019;2:192. doi: 10.1038/s42003-019-0439-x [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lord SR, Cheng WC, Liu D, et al. Integrated pharmacodynamic analysis identifies two metabolic adaption pathways to metformin in breast cancer. Cell Metab 2018;28:679, e4–88. doi: 10.1016/j.cmet.2018.08.021 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schein CH. Repurposing approved drugs on the pathway to novel therapies. Med Res Rev 2020;40:586–605. doi: 10.1002/med.21627 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma 2013;54:683–7. doi: 10.3109/10428194.2012.728597 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corral LG, Kaplan G. Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis 1999;58:I107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 1999;163:380–6. [PubMed] [Google Scholar]
- 27. De Souza A, Strober BE, Merola JF, et al. Apremilast for discoid lupus erythematosus: results of a phase 2, open-label, single-arm, pilot study. J Drugs Dermatol 2012;11:1224–6. [PubMed] [Google Scholar]
- 28. Massimi M, Ragusa F, Cardarelli S, et al. Targeting cyclic AMP signalling in hepatocellular carcinoma. Cell 2019;8. doi: 10.3390/cells8121511 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yougbare I, Belemnaba L, Morin C, et al. NCS 613, a potent PDE4 inhibitor, displays anti-inflammatory and anti-proliferative properties on A549 lung epithelial cells and human lung adenocarcinoma explants. Front Pharmacol 2020;11:1266. doi: 10.3389/fphar.2020.01266 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martiniuk F, Giovinazzo J, Tan AU, et al. Lessons of leprosy: the emergence of TH17 cytokines during type II reactions (ENL) is teaching us about T-cell plasticity. Journal of drugs in dermatology: JDD 2012;11:626–30. [PMC free article] [PubMed] [Google Scholar]
- 31. Petzold G, Fischer ES, Thoma NH. Structural basis of lenalidomide-induced CK1alpha degradation by the CRL4(CRBN) ubiquitin ligase. Nature 2016;532:127–30. doi: 10.1038/nature16979 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 32. Kunchok A, Aksamit AJ Jr, Davis JM 3rd, et al. Association between tumor necrosis factor inhibitor exposure and inflammatory central nervous system events. JAMA Neurol 2020;77:937–46. doi: 10.1001/jamaneurol.2020.1162 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mori T, Ito T, Liu S, et al. Structural basis of thalidomide enantiomer binding to cereblon. Sci Rep 2018;8:1294. doi: 10.1038/s41598-018-19202-7 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chamberlain PP, Cathers BE. Cereblon modulators: low molecular weight inducers of protein degradation. Drug discovery today. Dent Tech 2019;31:29–34. doi: 10.1016/j.ddtec.2019.02.004 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 35. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN). Br J Haematol 2014;164:811–21. doi: 10.1111/bjh.12708 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schellekens H, de Reus A, Bolhuis R, et al. Comparative antiviral efficiency of leukocyte and bacterially produced human alpha-interferon in rhesus monkeys. Nature 1981;292:775–6. [DOI] [PubMed] [Google Scholar]
- 37. Weissmann C, Nagata S, Boll W, et al. Structure and expression of human alpha-interferon genes. In: Merrigan TC (ed.). Interferons. Academic Press, 1982;295–396 [PubMed] [Google Scholar]
- 38. Palva I, Lehtovaara P, Kaariainen L, et al. Secretion of interferon by Bacillus subtilis. Gene 1983;22:229–35. [DOI] [PubMed] [Google Scholar]
- 39. Schein CH. The shape of the messenger: using protein structure information to design novel cytokine-based therapeutics. Curr Pharm Des 2002;8:2113–29. [DOI] [PubMed] [Google Scholar]
- 40. Silva WFDJ, Teixeira LLC, Rocha V, et al. Current role of interferon in hairy cell leukemia therapy: a timely decision. Hematol Transfus Cell Ther 2019;41:88–90. doi: 10.1016/j.htct.2018.04.004 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bewersdorf JP, Giri S, Wang R, et al. Interferon therapy in myelofibrosis: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk 2020;20:e712–23. doi: 10.1016/j.clml.2020.05.018 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heibl S, Buxhofer-Ausch V, Schmidt S, et al. A phase 1 study to evaluate the feasibility and efficacy of the addition of ropeginterferon alpha 2b to imatinib treatment in patients with chronic phase chronic myeloid leukemia not achieving a deep molecular response (MR 4.5)—AGMT_CML 1. Hematol Oncol 2020. doi: 10.1002/hon.2786 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 43. Vazquez-Blomquist D, Leenstra S, van der Kaaij M, et al. A co-formulation of interferons type I and II enhances temozolomide response in glioblastoma with unmethylated MGMT promoter status. Mol Biol Rep 2020;47:5263–71. doi: 10.1007/s11033-020-05604-2 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 44. Jin N, Lera RF, Yan RE, et al. Chromosomal instability upregulates interferon in acute myeloid leukemia. Genes Chromosomes Cancer 2020;59:627–38. doi: 10.1002/gcc.22880 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harabuchi S, Kosaka A, Yajima Y, et al. Intratumoral STING activations overcome negative impact of cisplatin on antitumor immunity by inflaming tumor microenvironment in squamous cell carcinoma. Biochem Biophys Res Commun 2020;522:408–14. doi: 10.1016/j.bbrc.2019.11.107 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 46. Ritter JL, Zhu Z, Thai TC, et al. Phosphorylation of RAB7 by TBK1/IKKepsilon regulates innate immune Signaling in triple-negative breast cancer. Cancer Res 2020;80:44–56. doi: 10.1158/0008-5472.CAN-19-1310 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schein CH. From interleukin families to glycans: relating cytokine structure to function. Curr Pharm Des 2004;10:3853–5. doi: 10.2174/1381612043382512 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 48. Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012;119:651–65. doi: 10.1182/blood-2011-04-325225 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li G, Sachdev U, Peters K, et al. The VE-PTP inhibitor AKB-9778 improves antitumor activity and diminishes the toxicity of interleukin 2 (IL-2) administration. J Immunother 2019;42:237–43. doi: 10.1097/CJI.0000000000000290 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pachella LA, Madsen LT, Dains JE. The toxicity and benefit of various dosing strategies for interleukin-2 in metastatic melanoma and renal cell carcinoma. J Adv Pract Oncol 2015;6:212–21. [PMC free article] [PubMed] [Google Scholar]
- 51. Payne R, Glenn L, Hoen H, et al. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a community hospital biotherapy program. J Immunother Cancer 2014;2. doi: 10.1186/2051-1426-2-13 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alwan LM, Grossmann K, Sageser D, et al. Comparison of acute toxicity and mortality after two different dosing regimens of high-dose interleukin-2 for patients with metastatic melanoma. Target Oncol 2014;9:63–71. doi: 10.1007/s11523-013-0276-7 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 53. Buchbinder EI, Dutcher JP, Daniels GA, et al. Therapy with high-dose interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer 2019;7:49. doi: 10.1186/s40425-019-0522-3 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carey PD, Wakefield CH, Guillou PJ. Neutrophil activation, vascular leak toxicity, and cytolysis during interleukin-2 infusion in human cancer. Surgery 1997;122:918–26. doi: 10.1016/s0039-6060(97)90333-0 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 55. Maybauer DM, Maybauer MO, Szabo C, et al. Lung-protective effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 in interleukin-2 induced toxicity. Biochem Biophys Res Commun 2008;377:786–91. doi: 10.1016/j.bbrc.2008.10.066 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Donia M, Kjeldsen JW, Svane IM. The controversial role of TNF in melanoma. Onco Targets Ther 2016;5:e1107699. doi: 10.1080/2162402X.2015.1107699 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Puchta A, Naidoo A, Verschoor CP, et al. TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog 2016;12:e1005368. doi: 10.1371/journal.ppat.1005368 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Verschoor CP, Loukov D, Naidoo A, et al. Circulating TNF and mitochondrial DNA are major determinants of neutrophil phenotype in the advanced-age, frail elderly. Mol Immunol 2015;65:148–56. doi: 10.1016/j.molimm.2015.01.015 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 59. Haderski GJ, Kandar BM, Brackett CM, et al. TLR5 agonist entolimod reduces the adverse toxicity of TNF while preserving its antitumor effects. PLoS One 2020;15:e0227940. doi: 10.1371/journal.pone.0227940 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abdullah MI, de Wolf E, Jawad MJ, et al. The poor design of clinical trials of statins in oncology may explain their failure—lessons for drug repurposing. Cancer Treat Rev 2018;69:84–9. doi: 10.1016/j.ctrv.2018.06.010 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 61. Blumenthal GM, Gills JJ, Ballas MS, et al. A phase I trial of the HIV protease inhibitor nelfinavir in adults with solid tumors. Oncotarget 2014;5:8161–72. doi: 10.18632/oncotarget.2415 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ran Y, Hossain F, Pannuti A, et al. Gamma-secretase inhibitors in cancer clinical trials are pharmacologically and functionally distinct. EMBO Mol Med 2017;9:950–66. doi: 10.15252/emmm.201607265 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nygaard HB, Wagner AF, Bowen GS, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer's disease. Alzheimers Res Ther 2015;7:35. doi: 10.1186/s13195-015-0119-0 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vasconcelos I, Wiesske A, Schoenegg W. Scalp cooling successfully prevents alopecia in breast cancer patients undergoing anthracycline/taxane-based chemotherapy. Breast 2018;40:1–3. doi: 10.1016/j.breast.2018.04.012 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 65. Veneris JT, Matulonis UA, Liu JF, et al. Choosing wisely: selecting PARP inhibitor combinations to promote anti-tumor immune responses beyond BRCA mutations. Gynecol Oncol 2020;156:488–97. doi: 10.1016/j.ygyno.2019.09.021 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 66. Deangelo DJ. Managing chronic myeloid leukemia patients intolerant to tyrosine kinase inhibitor therapy. Blood Cancer J 2012;2:e95. doi: 10.1038/bcj.2012.30 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 2020;31:525–31. doi: 10.1016/j.annonc.2020.01.006 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 68. Moran TB, Plana JC. Management of Patients with acute coronary syndrome and cancer. Curr Cardiol Rep 2020;22:159. doi: 10.1007/s11886-020-01409-8 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 69. Regulska K, Regulski M, Karolak B, et al. Beyond the boundaries of cardiology: still untapped anticancer properties of the cardiovascular system-related drugs. Pharmacol Res 2019;147:104326. doi: 10.1016/j.phrs.2019.104326 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 70. McNeil JJ, Gibbs P, Orchard SG, et al. Effect of aspirin on cancer incidence and mortality in older adults. J Natl Cancer Inst 2020. doi: 10.1093/jnci/djaa114 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Spini A, Roberto G, Gini R, et al. Evidence of beta-blockers drug repurposing for the treatment of triple negative breast cancer: a systematic review. Neoplasma 2019;66:963–70. doi: 10.4149/neo_2019_190110N34 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 72. Hagen R, Ghareeb E, Jalali O, et al. Infantile hemangiomas: what have we learned from propranolol? Curr Opin Pediatr 2018;30:499–504. doi: 10.1097/MOP.0000000000000650 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 73. Price A, Rai S, McLeod RWJ, et al. Topical propranolol for infantile haemangiomas: a systematic review. J Eur Acad Dermatol Venereol 2018;32:2083–9. doi: 10.1111/jdv.14963 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 74. Wagner MJ, Cranmer LD, Loggers ET, et al. Propranolol for the treatment of vascular sarcomas. J Exp Pharmacol 2018;10:51–8. doi: 10.2147/JEP.S146211 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cho MA, Jeong SY, Sohn I, et al. Impact of angiotensin receptor blockers, beta blockers, calcium channel blockers and thiazide diuretics on survival of ovarian cancer patients. Cancer Res Treat 2020;52:645–54. doi: 10.4143/crt.2019.509 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Coelho M, Squizzato A, Cassina N, et al. Effect of beta-blockers on survival of lung cancer patients: a systematic review and meta-analysis. Eur J Cancer Prev 2020;29:306–14. doi: 10.1097/CEJ.0000000000000544 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 77. Yap A, Lopez-Olivo MA, Dubowitz J, et al. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth 2018;121:45–57. doi: 10.1016/j.bja.2018.03.024 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 78. Kheiri B, Abdalla A, Osman M, et al. Meta-analysis of carvedilol for the prevention of anthracycline-induced cardiotoxicity. Am J Cardiol 2018;122:1959–64. doi: 10.1016/j.amjcard.2018.08.039 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 79. Shah P, Garris R, Abboud R, et al. Meta-analysis comparing usefulness of beta blockers to preserve left ventricular function during anthracycline therapy. Am J Cardiol 2019;124:789–94. doi: 10.1016/j.amjcard.2019.05.046 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 80. Sibaud V, Casassa E, D'Andrea M. Are topical beta-blockers really effective "in real life" for targeted therapy-induced paronychia. Supportive Care Cancer 2019;27:2341–3. doi: 10.1007/s00520-019-04690-8 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 81. Jordan K, Behlendorf T, Mueller F, et al. Anthracycline extravasation injuries: management with dexrazoxane. Ther Clin Risk Manag 2009;5:361–6. doi: 10.2147/tcrm.s3694 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Langer SW. Dexrazoxane for the treatment of chemotherapy-related side effects. Cancer Manag Res 2014;6:357–63. doi: 10.2147/CMAR.S47238 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Getz KD, Sung L, Alonzo TA, et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: a report from the Children's Oncology Group. J Clin Oncol 2020;38:2398–406. doi: 10.1200/JCO.19.02856 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Krouse AJ, Gray L, Macdonald T, et al. Repurposing and rescuing of Mibefradil, an antihypertensive, for cancer: a case study. Assay Drug Dev Technol 2015;13:650–3. doi: 10.1089/adt.2015.29014.ajkdrrr [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 85. Wang H, Pan R, Zhang X, et al. Abivertinib in patients with T790M-positive advanced NSCLC and its subsequent treatment with osimertinib. Thoracic cancer 2020;11:594–602. doi: 10.1111/1759-7714.13302 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Girdwood RH. The relationships between vitamin B12, folic acid and folinic acid. Br J Nutr 1952;6:315–24. doi: 10.1079/bjn19520033 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 87. Selvy M, Pereira B, Kerckhove N, et al. Long-term prevalence of sensory chemotherapy-induced peripheral neuropathy for 5 years after adjuvant FOLFOX chemotherapy to treat colorectal cancer: a Multicenter cross-sectional study. J Clin Med 2020;9. doi: 10.3390/jcm9082400 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Elhassanny AEM, Soliman E, Marie M, et al. Heme-dependent ER stress apoptosis: a mechanism for the selective toxicity of the dihydroartemisinin, NSC735847, in colorectal cancer cells. Front Oncol 2020;10:965. doi: 10.3389/fonc.2020.00965 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fazeel ZA. Apixaban: an oral anticoagulant having unique mechanism of action with better safety and efficacy profile. MAMC J Med Sci 2016;2:63–8. doi: 10.4103/2394-7438.182723 [published Online First: Epub Date]. [DOI] [Google Scholar]
- 90. Bhardwaj M, Leli NM, Koumenis C, et al. Regulation of autophagy by canonical and non-canonical ER stress responses. Semin Cancer Biol 2019. doi: 10.1016/j.semcancer.2019.11.007 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Martelli AM, Paganelli F, Chiarini F, et al. The unfolded protein response: a novel therapeutic target in acute leukemias. Cancer 2020;12. doi: 10.3390/cancers12020333 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gelman JS, Sironi J, Berezniuk I, et al. Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib. PLoS One 2013;8:e53263. doi: 10.1371/journal.pone.0053263 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Joshi J, Tanner L, Gilchrist L, et al. Switching to bortezomib may improve recovery from severe vincristine neuropathy in pediatric acute lymphoblastic Leukemia. J Pediatr Hematol Oncol 2019;41:457–62. doi: 10.1097/MPH.0000000000001529 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 94. Dennis M, Maoz A, Hughes D, et al. Bortezomib ocular toxicities: outcomes with ketotifen. Am J Hematol 2019;94:E80–2. doi: 10.1002/ajh.25382 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 95. Borcherding N, Jethava Y, Vikas P. Repurposing anti-cancer drugs for COVID-19 treatment. Drug Des Devel Ther 2020;14:5045–58. doi: 10.2147/DDDT.S282252 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stebbing J, Sanchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv 2020. doi: 10.1126/sciadv.abe4724 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020;370. doi: 10.1126/science.abd4570 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370. doi: 10.1126/science.abd4585 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 2020;369:712–7. doi: 10.1126/science.abc2061 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kaneko N, Kuo H-H, Boucau J, et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 2020;183:143–57 e13. doi: 10.1016/j.cell.2020.08.025 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Van der Voort A, Van Ramshorst MS, Van Werkhoven ED, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2-blockade for HER2-positive breast cancer (TRAIN-2): a randomized phase III trial. J Clin Oncol 2020;38:501–1. doi: 10.1200/JCO.2020.38.15_suppl.501 [published Online First: Epub Date]. [DOI] [Google Scholar]
- 102. Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745–51. doi: 10.1200/JCO.1999.17.9.2745 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 103. Smylie MG. Use of immuno-oncology in melanoma. Curr Oncol 2020;27:S51–8. doi: 10.3747/co.27.5135 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wong W, Bai XC, Brown A, et al. Cryo-EM structure of the plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. Elife 2014;3. doi: 10.7554/eLife.03080 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cornet-Masana JM, Moreno-Martinez D, Lara-Castillo MC, et al. Emetine induces chemosensitivity and reduces clonogenicity of acute myeloid leukemia cells. Oncotarget 2016;7:23239–50. doi: 10.18632/oncotarget.8096 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pantziarka P, Verbaanderd C, Huys I, et al. Repurposing drugs in oncology: from candidate selection to clinical adoption. Semin Cancer Biol 2020. doi: 10.1016/j.semcancer.2020.01.008 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 107. Gonzalez-Cao M, Boada A, Teixido C, et al. Fatal gastrointestinal toxicity with ipilimumab after BRAF/MEK inhibitor combination in a melanoma patient achieving pathological complete response. Oncotarget 2016;7:56619–27. doi: 10.18632/oncotarget.10651 [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Magge T, Shaikh H, Chaudhary R. Complete response to temozolomide in metastatic melanoma after failure of 5 lines of treatment. Am J Ther 2020. doi: 10.1097/MJT.0000000000001186 [published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 109. Kronish IM, Cheung YK, Shimbo D, et al. Increasing the precision of hypertension treatment through personalized trials: a pilot study. J Gen Intern Med 2019;34:839–45. doi: 10.1007/s11606-019-04831-z [published Online First: Epub Date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.


