Abstract
A high incidence of pancreatitis in COVID-19 has been reported. Although a high proportion of critically unwell patients with COVID-19 have raised serum amylase levels, this does not necessarily reflect acute pancreatitis or a clinically important pancreatic injury.
Dear Editor
COVID-19 is a global health emergency that principally causes respiratory illness. However, a myriad of extrapulmonary organ systems may be affected, and it has been proposed that pancreatic injury occurs as a result of SARS-CoV-2 infection. A recent study1 reported pancreatic injury in 17 per cent of 52 patients admitted with severe COVID-19, although this was defined by an increase in serum amylase level alone. In a further study2 of 64 patients with severe COVID-19, 18 per cent had an increased serum amylase concentration, which was also attributed to pancreatic injury. Further observations, case reports, and a case series of patients with COVID-19 suffering from pancreatitis have been published, strengthening the notion of COVID-19-related pancreatic injury3–5. However, it should be acknowledged that the majority of these articles reported isolated observations and, as reported in BJS, there is a deficiency in ‘serum amylase measurements exist[ing] in the international literature, requiring urgent rectification’6. Altogether, ‘actual evidence of clinical pancreatitis secondary to SARS-CoV-2 is dubious’5.
The authors therefore undertook a retrospective analysis of all patients with COVID-19 admitted to the ICUs at three London teaching hospitals between 14 March and 22 May 2020 in order to investigate the prevalence of either biochemical and/or radiological evidence of pancreatic injury. Peak and mean serum amylase concentrations were studied in relation to ICU mortality. An abnormally raised peak amylase concentration refers to a peak concentration greater than the upper limit of normal (ULN) (over 100 units/l).
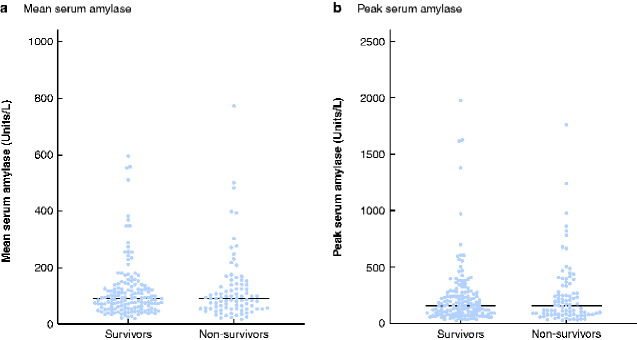
Among 234 patients included in the study, the ICU mortality rate was 37.2 per cent (87 of 234) (Table 1). In total, 158 of 234 patients (67.5 per cent ) had an abnormally raised peak amylase level during ICU admission, and in 52 (22.2 per cent) the peak value was more than three times greater than the ULN. Furthermore, 97 patients in this cohort (41.5 per cent) had a mean amylase concentration greater than the ULN. Sixty-one patients (26.1 per cent) met local clinical criteria for cross-sectional imaging of the abdomen, after which four (1.7 per cent) met the revised Atlanta criteria (rAC) for acute pancreatitis. Of these four patients, two survived ICU admission and two did not. There was no statistically significant difference in mean and peak amylase concentrations between ICU survivors and non-survivors (P = 0.890 and P = 0.925 respectively) (Fig. 1).
Table 1.
Patient demographics, duration of organ support, and mean and peak amylase concentrations in survivors and non-survivors
|
All patients
(n = 234) |
Survivors
(n = 147) |
Non-survivors
(n = 87) |
|
|---|---|---|---|
| Age (years)* | 59 (51.0–64.3) | 56.0 (47.0–63.0) | 61.0 (57.0–66.0) |
| Men | 167 (71.4) | 101 (60.5) | 66 (39.5) |
| BMI (kg/m2)* | 27.7 (24.5–31.4) | 27.6 (24.8–32.7) | 27.6 (23.9–30.3) |
| Duration of ICU stay (days)* | 14.7 (6.0–31.1) | 16.0 (5.6–35.6) | 12.1 (6.0–20.0) |
| Duration of advanced respiratory support days (days)*† | 13.0 (4.0–25.3) | 13.0 (3.0–29.0) | 13.0 (6.0–2–.0) |
| Mean amylase (units/l)* | 90.3 (56.2–136.5) | 90.0 (56.3–132.9) | 90.5 (55.1–139.1) |
| Mean amylase > ULN (100 units/l) | 97 (41.5) | 63 (65) | 34 (35) |
| Peak amylase (units/l)* | 158 (82.8–274.5) | 157 (90.0–273.0) | 160 (79.0–330.0) |
| Peak amylase > 3 × ULN (300 units /l) | 52 (22.2) | 30 (58) | 22 (42) |
| Peak amylase > 2 × ULN (200 units/l) | 94 (40.2) | 58 (62) | 36 (38) |
| Peak amylase > ULN (100 units/l) | 158 (67.5) | 103 (65.2) | 55 (34.8) |
| Peak amylase ≤ ULN (100 units/l) | 76 (32.5) | 44 (57.9) | 32 (42.1) |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.).
Mechanical ventilatory support via endotracheal tube. ULN, upper limit of normal.
Fig. 1.
Mean and peak serum amylase concentrations among survivors and non-survivors
a Mean and b peak serum amylase. Horizontal line indicates median value. Upper limit of normal 100 units/l.
These results demonstrate that, although a significant proportion of critically unwell patients with COVID-19 disease had a raised serum amylase concentration, only a minority (1.7 per cent) had a confirmed diagnosis of acute pancreatitis as defined by the rAC. Furthermore, there was no statistical difference in amylase concentrations between patients who survived and those who died. Previous studies1–4 have suggested that patients with COVID-19 are at increased risk of pancreatic injury, defined as an increase in serum amylase concentration beyond the normal laboratory range. Yet, the reported increases in pancreatic biomarkers were only mild to moderately abnormal, and the data do not corroborate this theory of pancreatic injury. A raised serum amylase level may be multifactorial given that multiple organs can produce and/or secrete amylase, for example in generalized gut inflammation or impaired renal excretion. Therefore, in practice, acute pancreatitis is usually diagnosed by multimodal criteria and the rAC recommends correlating biochemical data (amylase level) with radiological imaging and clinical findings (such as typical abdominal pain).
The present results suggest that CT of the abdomen is not necessarily indicated in the context of a raised serum amylase level alone. It is proposed that a raised serum amylase value in patients with severe COVID-19 is most frequently not immediately attributable to acute pancreatitis or a clinically important pancreatic injury, but is more likely to be a non-specific manifestation of shock/critical illness. The authors hope this gives clinical teams reassurance when managing critically ill patients with COVID-19 who have an abnormally raised amylase level, and suggest that cross-sectional imaging should not be instigated upon this finding, independent of other clinical suspicions of pancreatic injury.
Acknowledgements
The authors thank the clinical teams at Imperial College NHS Trust for their unrelenting care of these patients, and the research nurses for assistance with data collection.
Disclosure. The authors declare no conflict of interest.
References
- 1. Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q.. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology 2020;159:367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu F, Long X, Zou W, Fang M, Wu W, Li W. et al. Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection. medrxiv 2020; DOI: 10.1101/2020.02.28.20029181 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spinella A, Pellino G.. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg 2020:107:785–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szatmary P, Arora A, Raraty MGT, Dunne DFJ, Baron RD, Halloran CM.. Emerging phenotype of SARS-CoV2 associated pancreatitis. Gastroenterology 2020;159:1551–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samanta J, Gupta R, Singh MP, Patnaik I, Kumar A, Kochhar R.. Coronavirus disease 2019 and the pancreas. Pancreatology 2020:20:1567–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mukherjee R, Smith A, Sutton R.. COVID-19-related pancreatic injury. Br J Surg 2020;107:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]