Abstract
Profound T-cell lymphopenia is the hallmark of severe coronavirus disease 2019 (COVID-19). T-cell proliferation is telomere length (TL) dependent and telomeres shorten with age. Older COVID-19 patients, we hypothesize, are, therefore, at a higher risk of having TL-dependent lymphopenia. We measured TL by the novel Telomere Shortest Length Assay (TeSLA), and by Southern blotting (SB) of the terminal restriction fragments in peripheral blood mononuclear cells of 17 COVID-19 and 21 non-COVID-19 patients, aged 87 ± 8 (mean ± SD) and 87 ± 9 years, respectively. TeSLA tallies and measures single telomeres, including short telomeres undetected by SB. Such telomeres are relevant to TL-mediated biological processes, including cell viability and senescence. TeSLA yields 2 key metrics: the proportions of telomeres with different lengths (expressed in %) and their mean (TeSLA mTL), (expressed in kb). Lymphocyte count (109/L) was 0.91 ± 0.42 in COVID-19 patients and 1.50 ± 0.50 in non-COVID-19 patients (p < .001). In COVID-19 patients, but not in non-COVID-19 patients, lymphocyte count was inversely correlated with the proportion of telomeres shorter than 2 kb (p = .005) and positively correlated with TeSLA mTL (p = .03). Lymphocyte count was not significantly correlated with SB mTL in either COVID-19 or non-COVID-19 patients. We propose that compromised TL-dependent T-cell proliferative response, driven by short telomere in the TL distribution, contributes to COVID-19 lymphopenia among old adults. We infer that infection with SARS-CoV-2 uncovers the limits of the TL reserves of older persons.
Clinical Trials Registration Number: NCT04325646.
Keywords: COVID-19, Lymphocytes, Telomeres
A drop in T-cell count is the principal cause of coronavirus disease 2019 (COVID-19) lymphopenia (1–3), whose magnitude is an indicator of COVID-19 severity (2–4). The drop in T cells reflects a failure of T-cell proliferation to keep up with the T-cell loss from the circulation during the course of the illness. Accelerated T-cell proliferation is essential, therefore, to offset the development of and steer the recovery from COVID-19 lymphopenia.
As T-cell proliferative capacity is telomere length (TL) dependent (5,6), we have reasoned that short telomeres might compromise the ability to offset T-cell loss in the face of COVID-19 (7). The ensuing deficit between T-cell loss and production would result in lymphopenia, diminish the ability to clear severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and increase the risk of severe COVID-19. This process might partially explain the increased severity and fatality of COVID-19 in seniors (7), because TL shortens with age (8).
Epidemiologic studies have used leukocyte TL (LTL) as a proxy for an individual’s TL in different leukocyte lineages and somatic cells. This approach is justified, since the interindividual variation in TL far exceeds TL differences among leukocyte lineages and somatic cells within an individual (9–11). These studies as well as more fundamental telomere research are based on the premise that telomeres experience attrition with somatic cell replication, a process ultimately generating short telomeres that signal the cellular machinery to stop replication. Such a signal is triggered, however, by the shortest telomeres—not the mean of the different lengths of telomeres in the nucleus (12,13). Almost all previous telomere studies have employed techniques that generate data based on mean TL (mTL) rather than the shortest telomeres in DNA samples. In the present study, we employed a novel method coined the Telomeres Shortest Length Assay (TeSLA) that also measures and tallies short telomeres in the TL distribution (14). This approach allowed us to test the relation between lymphocyte counts and short TL in patients with COVID-19, using a more direct and biologically meaningful parameter of short TL. We also quantified mTL by Southern blotting (SB) of the terminal restriction fragments (15), to facilitate comparison with a standard TL measurement method. Notably, we focused on older adults because of their comparatively shorter LTL (8) and susceptibility to severe COVID-19.
Method
Subjects
We studied 38 older participants (aged 65–104 years) enrolled in the CORSER 2e branch of a SARS-CoV-2 study in France (N° ID-RCB: 2020-A00406-33; ClinicalTrials.gov identifier: NCT04325646). They were admitted to the Geriatric Department of the University of Nancy. Of these, 17 patients were hospitalized for COVID-19, confirmed by RT-PCR for SARS-CoV-2 in nasopharyngeal samples and clinical manifestations of COVID-19. The other 21 non-COVID-19 patients were hospitalized for different reasons; they had negative RT-PCR for SARS-Cov-2 and no clinical or radiological findings were consistent with COVID-19. We recorded lymphocyte count from the first blood sample collected after the RT-PCR confirmation of COVID-19 or admission blood sample in non-COVID-19 patients. Lymphocyte count was obtained after a complete blood count test using an automated hematology analyzer SYSMEX XN-9100 on whole blood sample. Eighteen participants signed an informed consent approved by the COMITE DE PROTECTION DES PERSONNES (CPP) ILE DE France III. CPP File N°: Am8448-6-3765. Next of kin signed the informed consent for the other 20 patients who were unable to sign the document. None of the participants, including COVID-19 patients, died within 15 days after being enrolled. The COVID-19 participants do not represent all older COVID-19 patients admitted to the Geriatric Department of the University of Nancy, because some patients died before attending physicians were able to secure their informed consent.
Measurements of TL Parameters
TeSLA and SB measurements are detailed in previous studies (14,15). Although these 2 methodologies of TL measurements are correlated (see below and under Supplementary Material), they do not produce identical information. The SB method is the “gold standard” of TL measurements against which the validity of all other techniques is judged, but it was designed to measure the mean length of the 92 telomeres of the q and p arms of the 23 human chromosome pairs (SB mTL). SB typically generates a weak signal for TLs below 3 kb and is therefore usually applied to a range of 3–20 kb (Supplementary Figure S1). TeSLA, in contrast, generates signals for single telomeres in the TL distribution and covers a TL range from 18 kb to below 0.8 kb (Supplementary Figure S1). TeSLA measurements yield 2 key metrics: (i) the proportion of short telomeres (expressed in %) with different TL thresholds for which there is as yet no criterion based on ultimate end points, and (ii) TeSLA mTL (in kb), which is the mTL of the telomeres captured by TeSLA measurements.
Statistical Analyses
Proportions of short telomeres, comorbidities, and sex were compared using a chi-squared test, and correlations were tested using linear regression implemented in R (16). We included age and sex as fixed effects in the regression models, because age and sex could confound the relationship of TL to lymphocyte count (8,17). However, excluding age and sex from the models did not change the conclusions. The primary hypothesis we tested focused on the correlation of the lymphocyte count (dependent variable) with the proportion of telomeres <2 kb among COVID-19 patients. We also used 2 alternative thresholds, that is, <3 and <1.6 kb, to ascertain that results did not reflect an arbitrary choice of threshold (this yielded the same conclusions, results not shown). We next tested the correlation between lymphocyte count and TeSLA mTL and SB mTL. Although SB mTL and TeSLA mTL are correlated (Supplementary Figure S2A), the proportions of short telomeres, for example, <2 kb, correlate better with TeSLA mTL (Supplementary Figure S2B) than with SB mTL (Supplementary Figure S2C). Finally, we performed these analyses in non-COVID-19 patients, and compared correlations with those among COVID-19 patients.
Ethics Approval
This study was approved by the COMITE DE PROTECTION DES PERSONNES, ILE DE France III. CPP File N °: Am8448-6-3765, and by the Rutgers University, New Jersey Medical School IRB, Pro2020000669.
Results
General characteristics of COVID-19 and non-COVID-19 subjects are displayed in Table 1. TL parameters by TeSLA and SB for the 2 patient groups are displayed in Supplementary Table S1.
Table 1.
General Characteristics of the 2 Groups of Patients
| COVID-19 | COVID-19 | p | |
|---|---|---|---|
| No (N = 21) | Yes (N = 17) | ||
| Characteristics | |||
| Women, N (%) | 12 (57) | 9 (53) | .79 |
| Age (years) | 87 ± 9 | 87 ± 8 | .96 |
| BMI (kg/m2) | 24.7 ± 5.6 | 26.2 ± 6.0 | .62 |
| Comorbidities, N (%) | |||
| Cardiovascular disease | 11 (52) | 11 (65) | .44 |
| Pulmonary disease | 3 (14) | 3 (18) | .78 |
| Cancer | 4 (19) | 3 (18) | .91 |
| Neurological disease | 8 (38) | 9 (53) | .36 |
| Diabetes mellitus | 7 (33) | 2 (12) | .12 |
| Hypertension | 15 (71) | 13 (76) | .73 |
| Medications | |||
| Number of chronic medications | 6.6 ± 3.2 | 6.4 ± 3.7 | .58 |
| Blood and kidney parameters | |||
| Lymphocytes (109/L) | 1.50 ± 0.50 | 0.91 ± 0.42 | <.001 |
| Leucocytes (109/L) | 6.49 ± 2.06 | 5.72 ± 2.21 | .27 |
| Hemoglobin (g/dL) | 12.0 ± 1.8 | 11.5 ± 1.8 | .41 |
| CRP (mg/L) | 17.9 ± 13.5 | 63.2 ± 53.2 | <.001 |
| Creatinine clearance (mL/min/1.73m2)a | 79.1 ± 21.1 | 74.1 ± 25.8 | .30 |
Notes: BMI = body mass index; CRP = C-reactive protein. Data are presented as mean ± SD.
aModification of diet in renal disease method.
Lymphocyte count (109/L) was significantly lower in COVID-19 patients compared to non-COVID-19 patients (difference: −0.59 ± 0.15; p < .001), and this difference remained virtually unchanged (difference: −0.57 ± 0.098; p < .001) when controlling for sex and age.
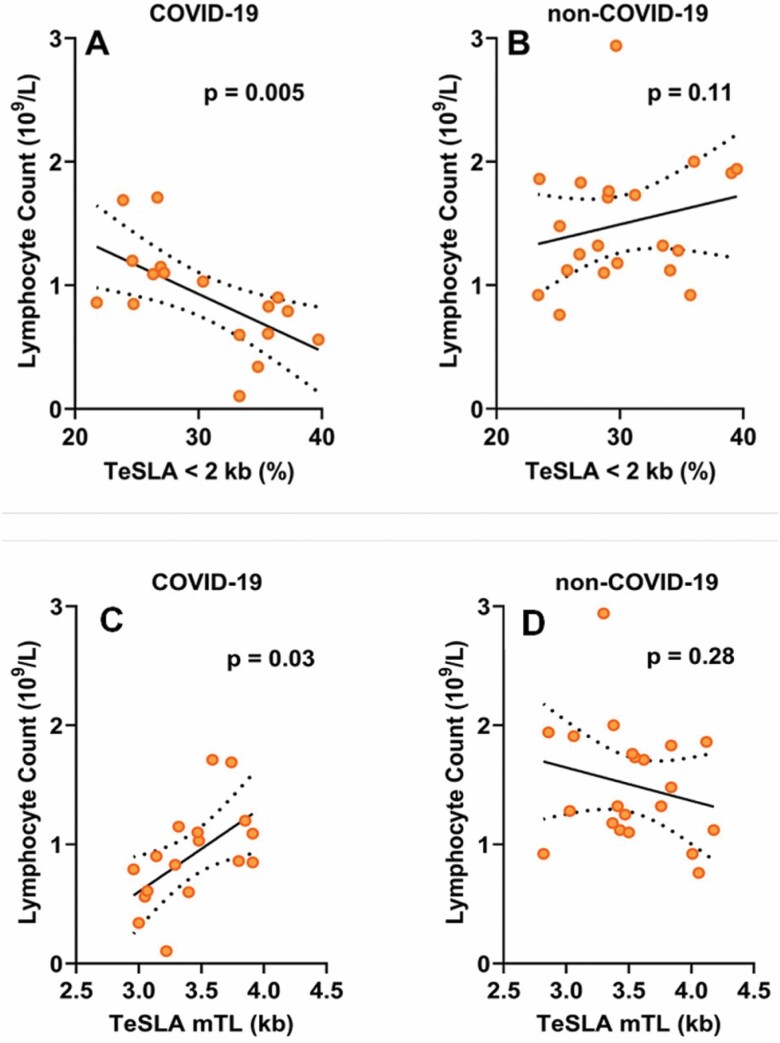
Among COVID-19 patients, lymphocyte count showed significant inverse correlations with the proportion of short telomeres (p = .005; Figure 1A) and this relationship also remained unchanged after adjusting for age and sex (p = .005; Supplementary Table S2). Lymphocyte count in non-COVID-19 patients showed no significant correlation with the proportion of short telomeres (Figure 1B; Supplementary Table S2). Consistent with these findings, lymphocyte count was positively correlated with TeSLA mTL (p = .03; Figure 1C; Supplementary Table S2). This correlation was not significant among non-COVID-19 patients (Figure 1D; Supplementary Table S2). Patterns for COVID-19 patients and non-COVID-19 patients differed significantly with regard to the proportion of short telomeres (interaction: COVID-19 status × Proportion short telomeres: p = .0013; p < .02 for interaction with TeSLA mTL). Finally, for COVID-19 patients, the correlation of lymphocyte count with SB mTL showed a pattern similar to the TeSLA data (Supplementary Figure S3), but did not reach statistical significance (Supplementary Table S3).
Figure 1.
Lymphocyte count in relation to TeSLA telomere length parameters. Proportion of telomeres <2 kb in COVID-19 patients (A) and non-COVID-19 patients (B) and mean telomere length by TeSLA (TeSLA mTL) in COVID-19 patients (C) and non-COVID-19 patients (D). See Supplementary Table S2 for the statistical models.
Discussion
Three well-established findings form the foundation for our model. First, the plummeting number of T cells is the principal cause of COVID-19 lymphopenia (1–3). Second, TL parameters in peripheral blood mononuclear cells (PBMCs) largely reflect TL parameters in all leukocyte lineages, including T cells (10). Third, the shortest telomeres in the TL distribution—which can be detected by TeSLA—are better indicators than mTL of telomere-related biological endpoints, including cell viability (12,13). We note, however, that thresholds of telomeres shorter than a finite value (e.g., <3, <2, and <1.6 kb) are arbitrary. We regard these thresholds as probabilities rather than distinct TL cutoffs, that is, the likelihood of a biological endpoint, such as cessation of replication, increases as cell telomeres approach these thresholds.
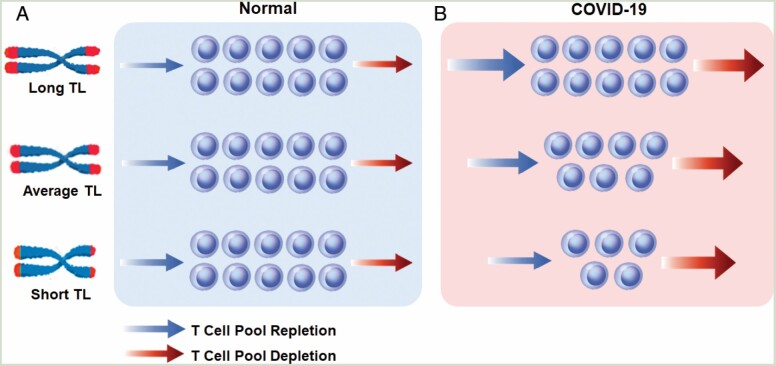
The T-cell blood pool is a component of the T-cell hierarchy, whose structure and composition are complex and change with age (18,19). For simplicity, we consider the T-cell blood pool as a homogeneous entity (Figure 2). In this highly simplified scenario, the size of the T-cell blood pool reflects the balance between T-cell depletion—due to senescence/death and sequestration out of the circulation—and T-cell repletion through proliferation. As T-cell proliferation is TL dependent (5,6), individuals with extremely short telomeres because of rare detrimental mutations are unable to maintain their T-cell blood pool (20). In the general population, however, TL variation shows no relation with lymphocyte count (21), presumably because of the low turnover of T cells (Figure 2A) (22). This might not apply in the face of COVID-19, since rapid T-cell proliferation is probably critical for offsetting the development of, and recovery from COVID-19 lymphopenia. We note, however, that the exact etiology of COVID-19 lymphopenia remains incomplete (2). For instance, the “vanishing” T cells from the circulation might reflect a massive inflammatory response that consists of “recruitment” of T cells to engage SARS-CoV-2 in the lung and the vascular endothelium. However, no excessive lymphocytic infiltrate is observed in the lungs of patients with severe COVID-19. That said, massive T-cell proliferation, which is TL dependent, must happen to avoid severe T-cell lymphopenia in response to the plummeting numbers of T cells in the circulation.
Figure 2.
Depletion and repletion of the T-cell blood pool under normal condition and during COVID-19. Under normal, “steady-state” condition (A), persons with shorter telomeres maintain their T-cell blood pool. However, in the face of SARS-CoV-2 infection (B), persons with shorter telomeres might lag in their ability to increase T-cell production (increase in the pace of the T-cell pool repletion—arrow) to match increased T-cell loss due to the infection (increase in the pace of T-cell pool depletion—arrow). This nonsteady condition would result in shrinking of the T-cell pool in proportion to the shorter telomeres. Telomeres are displayed as red caps at the ends of the chromosomes.
Accordingly, we hypothesized that when infected with SARS-CoV-2, persons with short telomeres lag in their T-cell proliferative response, resulting in a deficit in their T-cell blood pool (Figure 2B) (7). Our results support this hypothesis. They also suggest that COVID-19 exposes the limited TL-dependent replicative reserves of T cells in older persons with short telomeres. Notably, while telomerase activity fails to offset telomere shortening in T cells as they proliferate and differentiate into memory T cells, the reverse transcriptase lengthens telomeres in memory B cells (6,7). These findings might explain in part why COVID-19 lymphopenia is principally T-cell lymphopenia and not B-cell lymphopenia.
Data from children with COVID-19 further support our model. Children, who are otherwise healthy, typically show a mild clinical course when infected with SARS-CoV-2. Whereas lymphopenia is a major prognostic feature of COVID-19 in adults, it is a minor aspect of little prognostic value in children with COVID-19 (23–25). The average LTL in children is ~2–3 kb longer than in adults (8,17); hence, children have the TL-dependent replicative capacity to accelerate rapidly T-cell production and offset even a drastic T-cell loss due to SARS-CoV-2 infection.
As LTL shortens by ~0.025 kb/year during adulthood (26), LTL is shorter by about 1 kb in an 80-year-old senior than a 40-year-old person and by 1.5 kb than in a 20-year-old person. This means that the senior has a considerable replicative disadvantage compared to the younger adults, which might contribute to the propensity of seniors to severe COVID-19 lymphopenia (3). That said, from birth onwards, LTL is highly variable (SD ~0.7 kb for a given age) across individuals (8,17), and heritability, estimated at ~70% for LTL and ~30% of age-dependent LTL attrition (27), is a major source of this variation. Given the wide interindividual TL variation in the general population, TL of some young adults in their 30s and 40s is as short as TL in older persons in their 70s and 80s (8). These young adults, we propose, may be susceptible to COVID-19 lymphopenia despite their young age. In addition, some studies suggest that that in response to the SARS-CoV-2 infection, factors in the innate immune response suppress the T-cell response, explaining in part the plummeting number of T cells in severe COVID-19 (28). However, these studies have not explained the propensity of older adults to have severe COVID-19 lymphopenia and die from the disease.
We acknowledge limitations of this study. First, the cross-sectional design provides only associative data that do not prove causality. Reverse causation, however, seems unlikely; a low lymphocyte count would not rapidly shorten TL in COVID-19 patients. Second, we build our model on T-cell TL dynamics, but measured TL in PBMCs. Although interindividual differences in TL tend to outweigh differences in TL for cell types within an individual (9–11), further insight and support for the hypothesis will be gained by isolating T cells and examining their TL parameters in COVID-19 patients. Third, the detection of the inverse correlation between lymphocyte count and the proportion of very short telomeres required use of TeSLA, a novel and expensive approach available in few laboratories worldwide. Nonetheless, the correlation between lymphocyte count and SB mTL showed a pattern similar to that generated by TeSLA mTL. As TeSLA and SB results are correlated, we anticipate that the TL–COVID-19 connection we highlight might also be captured using SB measurements in larger cohorts. Fourth, we studied a small cohort of older persons of European ancestry, all surviving the disease for at least 15 days. This most vulnerable group to severe COVID-19 is not representative of the general population, but studying its members has provided the optimal setting to examine the TL-lymphopenia connection in this disease (7). In addition, TL dynamics of hematopoietic cells are complex, poorly understood, and partially contribute to COVID-19 severity and its clinical outcomes.
In conclusion, in a small study of older adults, we tested a model that links lymphopenia in older COVID-19 patients with PBMC TL parameters. Our findings show that comparatively short PBMC telomeres, as expressed in the shortest telomeres of the TL distribution, are associated with diminished lymphocyte count in older persons infected with SARS-CoV-2. These findings point the way for larger investigations in the general adult population, focusing on T-cell TL parameters and their role in COVID-19 lymphopenia. Finally, as severe lymphopenia also features in SARS (29) and Middle East respiratory syndrome (30), the biological template we propose might be relevant not only to the present pandemic but also future outbreaks and pandemics by other β-coronaviruses.
Supplementary Material
Acknowledgments
We thank the staff of the Geriatric Department of the University Hospital of Nancy for their contribution in the conduction of this study. We also thank the staff of the “Centre de Ressources Biologiques-CRB” of the University Hospital of Nancy for their help in the preparation of the biological samples.
Funding
This study has been supported by the French National Research Agency (ANR), Translationnelle: N°ID RCB: 2014-A00298-39: 2014–2017 and partially supported by the French PIA project “Lorraine Université d’Excellence ,” reference ANR-15-IDEX-04-LUE, and the Investments for the Future program under grant agreement no. ANR-15-RHU-0004. A.A.’s research is supported by National Institutes of Health grants R01HL134840 and U01AG066529 and Norwegian Institute of Public Health grants 262700 and 262043.
Conflict of Interest
None declared.
Author Contributions
A.B.: conceptualization, data curation, supervision, validation, and writing original draft; T.-P.L.: methodology, data analysis, writing review, and editing; S.T.: methodology, data analysis, writing review, and editing; C.L.: data analysis, validation, writing review, and editing; S.V.: data curation, methodology, supervision, validation, writing review, and editing; S.G.: investigations, data curation, supervision, writing review, and editing; M.-N.U.: conceptualization, validation, writing review, and editing; C.P.-G.: investigations, data curation, supervision, writing review, and editing; D.L.: conceptualization, validation, and writing original draft; E.S.: conceptualization, validation, and writing original draft; and A.A.: conceptualization, methodology, validation, supervision, and writing original draft.
References
- 1. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diao B, Wang C, Tan Y, et al. . Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao Q, Meng M, Kumar R, et al. . Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002;2(9):699–706. doi: 10.1038/nri890 [DOI] [PubMed] [Google Scholar]
- 6. Patrick M, Weng NP. Expression and regulation of telomerase in human T cell differentiation, activation, aging and diseases. Cell Immunol. 2019;345:103989. doi: 10.1016/j.cellimm.2019.103989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aviv A. Telomeres and COVID-19. FASEB J. 2020;34(6):7247–7252. doi: 10.1096/fj.202001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steenstrup T, Kark JD, Verhulst S, et al. . Telomeres and the natural lifespan limit in humans. Aging (Albany NY). 2017;9(4):1130–1142. doi: 10.18632/aging.101216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018;373(1741):pii: 20160436. doi: 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol. 2010;38(10):854–859. doi: 10.1016/j.exphem.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniali L, Benetos A, Susser E, et al. . Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9 [DOI] [PubMed] [Google Scholar]
- 13. Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15(8):3709–3718. doi: 10.1091/mbc.e04-03-0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai TP, Zhang N, Noh J, et al. . A method for measuring the distribution of the shortest telomeres in cells and tissues. Nat Commun. 2017;8(1):1356. doi: 10.1038/s41467-017-01291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimura M, Stone RC, Hunt SC, et al. . Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5(9):1596–1607. doi: 10.1038/nprot.2010.124 [DOI] [PubMed] [Google Scholar]
- 16. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2019. https://www.R-project.org/. Assessed February 10, 2021. [Google Scholar]
- 17. Factor-Litvak P, Susser E, Kezios K, et al. . Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics. 2016;137(4) :e20153927. doi: 10.1542/peds.2015-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davenport MP, Smith NL, Rudd BD. Building a T cell compartment: how immune cell development shapes function. Nat Rev Immunol. 2020;20:499–506. doi: 10.1038/s41577-020-0332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout Life. Immunity. 2018;48(2):202–213. doi: 10.1016/j.immuni.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner CL, Hanumanthu VS, Talbot CC Jr, et al. . Short telomere syndromes cause a primary T cell immunodeficiency. J Clin Invest. 2018;128(12):5222–5234. doi: 10.1172/JCI120216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mollica L, Fleury I, Belisle C, Provost S, Roy DC, Busque L. No association between telomere length and blood cell counts in older individuals. J Gerontol A Biol Sci Med Sci. 2009;64(9):965–967. doi: 10.1093/gerona/glp065 [DOI] [PubMed] [Google Scholar]
- 22. Vrisekoop N, den Braber I, de Boer AB, et al. . Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc Natl Acad Sci USA. 2008;105(16):6115–6120. doi: 10.1073/pnas.0709713105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma X, Liu S, Chen L, Zhuang L, Zhang J, Xin Y. The clinical characteristics of pediatric inpatients with SARS-CoV-2 infection: a meta-analysis and systematic review. J Med Virol. 2020. doi: 10.1002/jmv.26208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu H, Zhu H, Yuan C, et al. . Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3(6):e2010895. doi: 10.1001/jamanetworkopen.2020.10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zachariah P, Johnson CL, Halabi KC, et al. . Columbia Pediatric COVID-19 Management Group. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020;174(10):e202430. doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum—artifact or biology? Nucleic Acids Res. 2013;41(13):e131. doi: 10.1093/nar/gkt370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hjelmborg JB, Dalgård C, Möller S, et al. . The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52(5):297–302. doi: 10.1136/jmedgenet-2014-102736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou T, Tianjiao Su T, Mudianto T, Wang J. Immune asynchrony in COVID-19 pathogenesis and potential immunotherapies. J Exp Med. 2020;217(10):e20200674. doi: 10.1084/jem.20200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Booth CM, Matukas LM, Tomlinson GA, et al. . Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. J Am Med Assoc. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885 [DOI] [PubMed] [Google Scholar]
- 30. Ko JH, Park GE, Lee JY, et al. . Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect. 2016;73(5):468–475. doi: 10.1016/j.jinf.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.