Abstract
Purpose
To share challenges and opportunities for antimicrobial stewardship programs based on one center’s experience during the early weeks of the coronavirus disease 2019 (COVID-19) pandemic.
Summary
In the spring of 2020, New York City quickly became a hotspot for the COVID-19 pandemic in the United States, putting a strain on local healthcare systems. Antimicrobial stewardship programs faced diagnostic and therapeutic uncertainties as well as healthcare resource challenges. With the lack of effective antivirals, antibiotic use in critically ill patients was difficult to avoid. Uncertainty drove antimicrobial use and thus antimicrobial stewardship principles were paramount. The dramatic influx of patients, drug and equipment shortages, and the need for prescribers to practice in alternative roles only compounded the situation. Establishing enhanced communication, education, and inventory control while leveraging the capabilities of the electronic medical record were some of the tools used to optimize existing resources.
Conclusion
New York City was a unique and challenging environment during the initial peak of the COVID-19 pandemic. Antimicrobial stewardship programs can learn from each other by sharing lessons learned and practice opportunities to better prepare other programs facing COVID-19 case surges.
Keywords: antimicrobial stewardship, COVID-19, drug utilization, electronic medical record, SARS-CoV-2
KEY POINTS
The COVID-19 pandemic has strained healthcare systems, including pharmacy departments and antimicrobial stewardship programs.
Rapid changes in patient volume, shifts in diagnostics, therapeutic uncertainty, and practice changes have challenged antimicrobial stewardship programs.
Pharmacists working as part of antimicrobial stewardship programs can prepare for these emergency situations by establishing enhanced communication channels, exploring optimal ways to disseminate information and educational material, developing antimicrobial stewardship alerts that are specific to the type of emergency or using existing alerts to prioritize workflow, and leveraging the electronic medical record when possible.
After the first case of coronavirus disease 2019 (COVID-19) was identified in the United States, New York City quickly became a global hotspot of the COVID-19 pandemic, with over 212,000 cases and over 55,000 hospitalizations reported as of July 2, 2020.1 At the peak of the initial COVID-19 case surge in New York City in late March and early April 2020, about 1,700 new hospitalizations occurred each day, leading to significant stress on the healthcare system. Antimicrobial use in hospitalized patients in New York City was high, with reports that approximately 70% of patients received antibacterial treatment.2-4 However, unlike patients with influenza, in whom the prevalence of bacterial coinfection commonly ranges between 11% and 35%, patients with COVID-19 appear to present with lower rates of bacterial coinfection, ranging from 3% to 8% at the time of hospital admission.2,5-8 Despite this, a majority of patients in published studies received broad-spectrum antibacterial therapy, likely due to an initial paucity of data and difficulty in differentiating between bacterial superinfections and COVID-19.4,6,9,10 Interestingly, a recent small retrospective study of 48 patients with COVID-19 from Switzerland found no difference in rates of mortality or delayed hospital-acquired infections when comparing those who received antibiotics prior to intensive care unit (ICU) admission and those who did not.11
With prolonged hospitalization, mechanical ventilation, and use of immunomodulatory agents, the risk of secondary bacterial infections increases. A high index of suspicion for infection and initiation of broad-spectrum antibacterial therapy may be necessary in at-risk patients to limit poor outcomes resulting from unrecognized infections. However, in practice, as with rates of bacterial coinfection at initial presentation, rates of healthcare-associated infections in patients with COVID-19 appear lower than rates of antibiotic use. For example, a retrospective cohort study of 191 patients with COVID-19 in Wuhan, China, found that 95% of patients received antibiotics and 30% received corticosteroids. However, the researchers reported a secondary infection rate of only 15%, albeit with a much higher rate (50%) in nonsurvivors.12 A recent meta-analysis encompassing over 3,300 patients with COVID-19 identified a secondary bacterial infection rate of 14.3%.13
The use of antibiotics in patients with COVID-19 appears to be out of proportion to the reported rates of bacterial infections and may not improve outcomes. Antimicrobial stewardship programs (ASPs) are well equipped to improve antibiotic prescribing, critically evaluate the literature to optimize anti-infective treatment, and assess appropriateness of therapy to minimize adverse events. In the setting of COVID-19, ASPs have faced several new challenges.
Problem
Our ASP was simultaneously confronted with a new infectious disease fraught with diagnostic and therapeutic uncertainties, a dramatic increase in critically ill patients, drug and equipment shortages, and prescribers practicing in alternative roles—all of which contributed to increased antimicrobial use.
A surge in admissions of critically ill patients strained the hospital’s intensive care unit (ICU) bed and personnel capacities. To match the increased ICU demand, specialized ICUs focused on areas such as neurosurgery and cardiac and general surgery/trauma housed patients with COVID-19 almost exclusively. Furthermore, our hospital quickly converted additional general medical and pediatric wards and operating/recovery spaces into “pop-up” ICUs. All available healthcare workers, including out-of-state volunteers, were deployed to staff these units. Many of these physicians, pharmacists, and nurses were not familiar with our hospital-specific ASP tools, such as the local antibiogram; empiric antibiotic guidelines for community-acquired pneumonia, ventilator-associated pneumonia, and bacteremia; and established antimicrobial de-escalation practices based on stewardship. The surge in the critically ill patient census and corresponding increase in broad-spectrum antibiotic use led to longer lists of patients about whom the ASP team was alerted to provide postprescription review. This situation, combined with drug supply disruptions and shortages, requests for the initiation and monitoring of the investigational antiviral drug remdesivir or off-label treatment agents, and the need for creation and dissemination of treatment guidelines for patients with COVID-19 in a rapidly changing environment, led to strains on ASP resources. An additional challenge was a shortage of swabs used for collecting nasopharyngeal (NP) specimens,14 as the ASP has historically relied upon rapid multiplex polymerase chain reaction (PCR) testing for respiratory pathogens to streamline and optimize antibiotic use. Furthermore, diagnostic test results suggestive of infection (eg, procalcitonin [PCT] elevation) were challenging to interpret, and diagnostic stewardship was difficult at this time given a lack of robust data defining the course of COVID-19 and its complications.
Background
The ASP at NewYork-Presbyterian Hospital is primarily comprised of 7 infectious diseases (ID) pharmacists, 6 ID and/or infection prevention and control physicians, and 2 clinical microbiology labs. The ASP serves an academic medical hospital system in New York City that encompasses 6 acute care hospitals, 2 medical schools, and about 2,600 beds. The core activities of the ASP include preprescription approvals for restricted anti-infectives, postprescription review, optimization of therapy based on rapid diagnostic results, development and maintenance of clinical guidelines, dose optimization, and prescriber education.
The ASP had to adapt quickly to the COVID-19 pandemic (Table 1). Usual activities and practices were insufficient to meet the needs of a changing population. We had to differentiate where we could ease antimicrobial stewardship principles from where they needed tightening. We also had to evaluate job responsibilities, redistribute resources, and prioritize based on more critical needs. We needed to enhance communication with each other, with prescribers, and with pharmacy and hospital administrators.
Table 1.
Challenges Encountered and Strategies Developed and/or Used to Assist Antimicrobial Stewardship Program Activities During COVID-19 Pandemic
| Challenge | Antimicrobial Stewardship Strategies |
|---|---|
| Rapid increase in patient volume and change in patient mix | Restructured responsibilities of infectious diseases pharmacists |
| Developed COVID-19 scoring system to prioritize antimicrobial stewardship patient reviews | |
| Developed COVID-19 clinical summary in a central location within the electronic medical record to streamline clinical monitoring | |
| Increased infectious diseases physician oversight in patient care | |
| Increased communication with infectious diseases physicians | |
| Lack of information about a new disease | Reviewed literature daily and shared findings |
| Enhanced communication with medical personnel nationally and internationally | |
| Revised treatment guidelines continuously and communicated changes |
|
| Allowed more empiric broad-spectrum therapy as clinically indicated, with de-escalation or discontinuation in the absence of laboratory-confirmed infectious disease other than COVID-19 | |
| Need for education | Developed treatment guidelines |
| Enhanced communication and education dissemination to prescriber and pharmacy groups | |
| Implemented new antimicrobial restrictions as needed to quickly change practice; restrictions removed as needed with guideline changes | |
| Included attestations and informational links within prescriber ordering of experimental agents | |
| Changes in drug availability | Enhanced communication with pharmacy personnel responsible for drug procurement |
| Implemented antimicrobial restrictions to increase or decrease use quickly | |
| Enhanced daily and/or weekly inventory tracking of key agents | |
| Changes in diagnostic supplies and personal protective equipment availability | De-escalated or modified antimicrobial regimens to prefer use of agents administered daily (instead of 3 times daily) when possible |
| Relied more on standard culture and susceptibility testing than on rapid diagnostic tools requiring nasopharyngeal swabs | |
| Increased use of experimental agents | Developed order sets for experimental and off-label treatment agents |
| Established infectious diseases pharmacist point person for experimental agents to streamline processes | |
| Collaborated with infectious diseases physicians to maximize resources and, where possible, used clinical trial staff expertise | |
| Developed electronic medical record antimicrobial stewardship alerts to enhance monitoring (eg, new orders, abnormal laboratory results) | |
| Dissemination of clinical trial inclusion/exclusion criteria |
Analysis and resolution
Rapid increase in patient volume and change in patient mix
Due to the rapid growth in demand, usual ASP pharmacist review of patient lists by nursing units was reevaluated and restructured based on patient and ICU volumes. The ID physician service selected liaisons who could join a portion of daily rounds for the newly established ICUs. The ASP pharmacists, who were fielding antimicrobial approvals and conducting postprescription review for these units, kept in close contact with the ID physician liaisons. In addition, we assigned 1 ASP pharmacist at each main campus (2 in total) to do less postprescription review while focusing more on investigational agents (eg, remdesivir emergency use and procurement) and coordinating with the ID clinical trials groups.
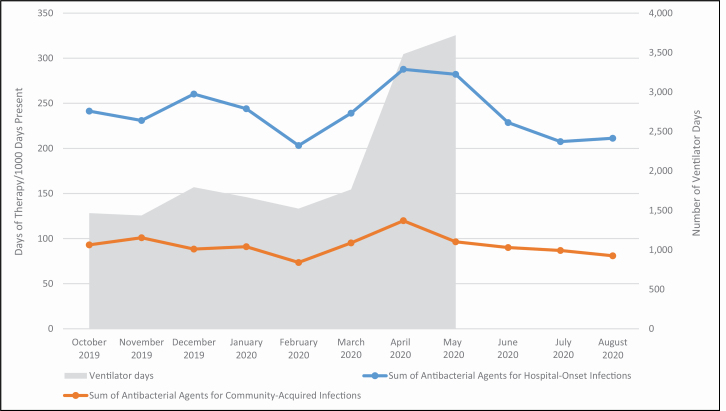
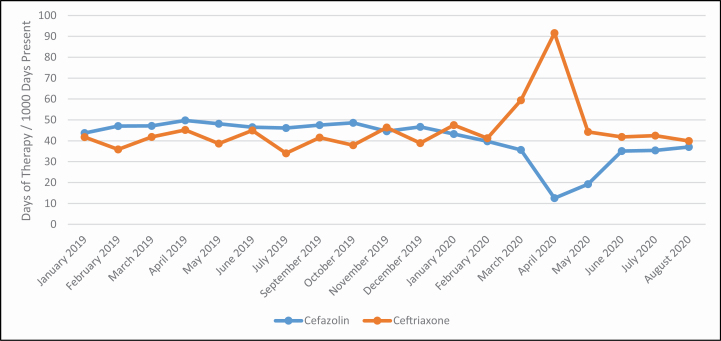
With the rapid increase in COVID-19 cases and increase in mechanically ventilated patients we observed notable shifts in the use of antibiotics (Figure 1). These patients tended to be older and to have multiple comorbidities, including hypertension, diabetes, and obesity.3,15 Acute kidney injury was common, with about one-third of patients requiring renal replacement therapy. We recognized that these patients were critically ill but did not have risk factors for early multidrug-resistant bacterial infections, such as recent hospitalization and use of antibiotics, and therefore discouraged use of broad-spectrum antipseudomonal agents. Despite significant increases in ventilator days during this time, the use of broad-spectrum antibiotics did not increase to the same degree due to our efforts (see Figure 2). As medical patients largely replaced surgical patients throughout the healthcare system, use of first-generation cephalosporins decreased and use of third-generation cephalosporins increased (see Figure 3). As length of stay became more prolonged in critically ill patients, particularly those requiring mechanical ventilation, the ASP team began to receive an increasing amount of antimicrobial requests for broad-spectrum antibiotics as well as antifungal agents to treat secondary healthcare-associated infections. Subsequent de-escalation of antibiotic and antifungal therapy in the absence of identified infection became a focus of our ASP and may have been key in limiting the impact of empiric therapy on development of antimicrobial-resistant infections.
Figure 1.
Changes in antibiotic use relative to increases in ventilator days at NewYork-Presbyterian Hospital. Antibacterial agents used for treatment of hospital-onset infections included amikacin, aztreonam, cefepime, ceftazidime, gentamicin, imipenem/cilastatin, meropenem, piperacillin/tazobactam, and tobramycin. Antibacterial agents used for treatment of community-acquired infections included ceftriaxone, cefuroxime, ciprofloxacin, ertapenem, and levofloxacin.
Figure 2.
Trends in cefazolin and ceftriaxone days of therapy at NewYork-Presbyterian Hospital.
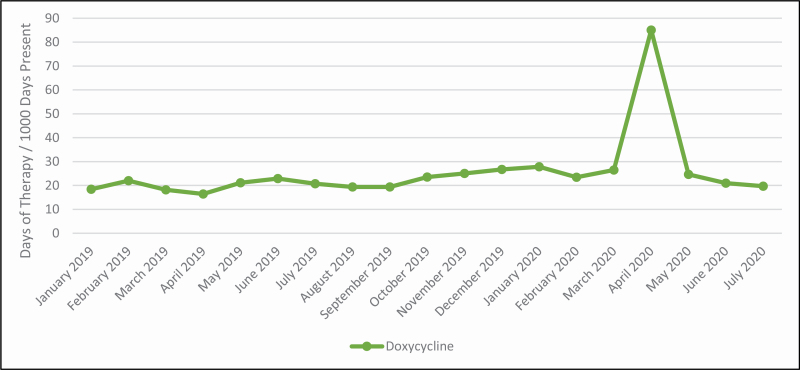
Figure 3.
Trend in doxycycline days of therapy at NewYork-Presbyterian Hospital.
We leveraged electronic medical record (EMR) tools when possible to prioritize daily patient review as the ASP lists became lengthy. In addition to new alerts about patients with PCR results positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), alerts were developed to guide use of COVID-19–specific therapies and modified throughout the pandemic as needed. Alerts were created to address situations including hydroxychloroquine initiation, QT interval prolongation in patients receiving hydroxychloroquine with or without azithromycin, lack of QT interval documentation in patients receiving hydroxychloroquine, duration of hydroxychloroquine use greater than 5 days, new remdesivir orders, remdesivir orders without daily liver function test results, and remdesivir use in patients with elevated liver function test results. Similarly, we streamlined daily clinical monitoring by creating a COVID-19 clinical summary dashboard in a central location in the EMR. This summary, which included vital signs, oxygen requirements, laboratory results, inflammatory markers, and medications received, facilitated evaluation of trends and streamlined clinical monitoring.
Lack of information about a new disease
When patients were admitted with COVID-19, prescribers frequently initiated empiric antibiotics for community-acquired pneumonia, since initial chest radiographs often revealed pulmonary infiltrates (75% of patients), patients were often severely ill, and there was uncertainty about bacterial coinfections.4,15 Furthermore, as the disease later progressed to respiratory failure, the need for central venous catheters for administration of vasopressors, prolonged hospitalization, and the use of immunomodulatory therapies added to the risk of secondary bacterial or fungal infections and the need to initiate empiric broad-spectrum antimicrobials later during hospitalization.16,17 Diagnostic workups were often difficult to interpret, especially in the setting of rising inflammatory markers. As a result, uncertainty drove antimicrobial use. Antimicrobial steward-ship principles in this setting remained critically relevant. Treatment recommendations included withholding antibiotics in the absence of laboratory results suggestive of bacterial infection (eg, elevated white blood cell count and percentage of neutrophils), using the most narrow-spectrum agent when possible, obtaining clinically indicated specimens for culturing when possible, tailoring antibiotics to culture results, and discontinuing antibiotics if they had not provided a benefit after short courses (ie, 5 days). These types of interventions have previously been shown to reduce antibiotic days of therapy without negatively impacting patient outcomes.18,19 When such tools were available, we also encouraged use of rapid diagnostic tools such as the T2Candida Panel (T2 Biosystems, Lexington, MA) to shorten durations of empiric antifungal therapy. We tried to reinforce stewardship principles through the ID consult service, when approving restricted antimicrobials, and through postprescription review. Frequent prospective antimicrobial audits with one-on-one education were crucial to successful stewardship efforts during the early COVID-19 pandemic response.20,21
As one of the first areas in the United States to deal with the pandemic, New York and its hospitals and clinicians had to overcome a paucity of data regarding COVID-19 treatment. The uncertainty about the disease course and effective treatments, as well as the rapid influx of information from observational studies (including from non–peer-reviewed preprint publications), called for rapid and critical evaluation of the emerging data. The ASP pharmacists partnered with the ID physicians to review literature daily and share findings. Both ASP pharmacists and ID physicians reached out to medical contacts nationally and internationally to share experiences with COVID-19 populations and treatments. We developed an internal COVID-19 treatment guideline that also included antibacterial treatment recommendations in the context of COVID-19.
COVID-19 treatment recommendations evolved every few weeks—from limited use of hydroxychloroquine (with or without azithromycin) to more widespread hydroxychloroquine use, with subsequent recommendations for remdesivir use, limited use of steroids, and then more widespread use of steroids. In addition, every patient was always considered for a clinical trial. The ASP pharmacists took the lead in developing a multidisciplinary guideline that included criteria for the use of each agent. Treatment guidelines were agreed upon by practitioners in multiple specialties, including ID, critical care, pulmonology, and rheumatology. One challenge was an inability to quickly disseminate and integrate the guideline into daily practice, especially when opportunities for education were limited by time and physical space constraints as well as social distancing requirements. In addition, the recommendations did not easily translate into clinical decision support within the EMR. To overcome this, we were able to rapidly implement restrictions for various therapies as a method of oversight, to drive prescribing, and to provide an additional opportunity for discussion with prescribers to share changes in treatment guidelines. Finally, we included prescriber attestations and links for emergency use criteria for COVID-19 treatments such as remdesivir in the EMR to inform medical staff of contraindications and monitoring requirements at the point of prescribing.
Changes in drug availability
Antibiotic use was also impacted by drug supply disruptions and shortages of key agents that were in high demand, including intravenous azithromycin and doxycycline. Keeping medical staff abreast of treatments and toxicities also shifted antibiotic use. For example, azithromycin use was decreased in favor of doxycycline for atypical pneumonia coverage during the period of increased hydroxychloroquine use in order to avoid additive cardiotoxicity (see Figure 3). Restrictions were placed quickly and selectively to limit use of agents like azithromycin as treatment guidelines evolved, as drug inventory changed, and to avoid unnecessary toxicities.
Drug supply shortages required constant communication with pharmacy and medical staff. Daily and weekly inventory tracking of primary anti-infective and immunomodulatory agents was key in ensuring that supply was distributed appropriately among our hospitals with the rapid influx of patients. Furthermore, preprescription approval was useful in ensuring appropriate use of agents in short supply and for educating medical staff about changing treatment recommendations and the need for monitoring of experimental and off-label treatments. There was at least weekly communication with clinical pharmacists, pharmacy operations personnel, and the pharmacy administration regarding changing treatment recommendations and drug procurement.
Changes in diagnostic supplies and personal protective equipment availability
In order to prioritize swabs for use in the diagnosis of COVID-19, we had to significantly curtail the routine use of the respiratory pathogen panel and Staphylococcus aureus nasal PCR testing when initiating intravenous vancomycin, which limited our detection of other respiratory viral pathogens and S. aureus colonization. As such, we had to rely on standard culture and susceptibility testing and biomarkers such as PCT. Prior to the COVID-19 pandemic, PCT monitoring was used at our institution to promote de-escalation and discontinuation of antibiotics prescribed for respiratory infections. In the setting of COVID-19, PCT levels may be elevated in the absence of a bacterial infection due to the presence of certain cytokines, including interleukin-6.22 PCT levels should be interpreted carefully in the broader clinical context of COVID-19 pneumonia, as elevations may represent bacterial coinfection or immune dysregulation or be a marker of severity of illness. However, low or normal PCT levels may be useful for encouraging withholding or early discontinuation of empiric antibiotics in non–critically ill patients.
A lack of some rapid diagnostic tools and difficulty in interpreting PCT levels compelled us to rely more on routine culture and susceptibility testing. With prescribers practicing in alternative roles, we frequently reminded medical teams to send routine cultures as part of fever workups. We were forced to accept use of unnecessarily broad-spectrum antibiotics for a longer duration than usual as we waited for culture results; this required us to check back on the status of a patient regularly in order to evaluate clinical response as we awaited results. While the true risk of infection by antimicrobial-resistant pathogens in hospitalized patients with COVID-19 is unknown, initial empiric antibiotics should be narrow-spectrum agents and target community-associated respiratory infections unless patients have traditional risk factors for infection by multidrug-resistant pathogens.23,24 The standard fever and respiratory diagnostic workup should not be abandoned.
While we had historically encouraged de-escalation from ceftriaxone (daily) to cefazolin (3 times daily) for susceptible gram-negative infections whenever possible, we did not do so during the early response to the COVID-19 pandemic in order to minimize the frequency of healthcare worker entry into isolation rooms. Similarly, our ASP relaxed restrictions on the use of levofloxacin, an agent whose use is typically highly restricted for gram-negative infections but favored as a once-daily antibiotic in preference to frequently dosed β-lactams for culture-directed therapy.25,26 Finally, difficulties obtaining thorough allergy histories from critically ill patients affected antibiotic use for patients with reported β-lactam allergies. Antibiotic choices were limited to broader-spectrum agents (ie, aztreonam, vancomycin, and levofloxacin) in the absence of tools such as graded drug challenges, which were difficult to conduct given isolation precautions and personal protective equipment requirements coupled with the need for frequent room entry for close patient monitoring.
Increased use of experimental agents
The lack of established effective treatments for COVID-19 led to increased use of emergency use authorization (EUA) drugs like remdesivir and enrollment of patients in clinical trials. In order to streamline processes around clinical trials, we established an ASP clinical pharmacist point person to interact more closely with the ID clinical trials group. This person was responsible for communicating clinical trial inclusion and exclusion criteria to the ASP and pharmacy teams, and updates to ongoing clinical trial enrollment were disseminated to the clinical pharmacy staff weekly.
We developed order sets in the EMR for each EUA agent. Within these order sets specific criteria for use were provided, including daily monitoring attestations to ensure hospital compliance with regulatory requirements. If specific dosing information for COVID-19 treatment was available (as for steroids), then a new drug entry specific to COVID-19 that defaulted to the recommended dosing was created. In conjunction with creation of these order sets, the ASP team created alerts specific to the monitoring of EUA agents to ensure safety and to identify potential adverse events for reporting to regulatory authorities.
Discussion
The long-term impact of SARS-CoV-2 and of the aforementioned changes in clinical care remain unknown. The use of broad-spectrum antibiotic therapy, coupled with strains on antimicrobial stewardship resources, may increase the prevalence of multidrug-resistant organisms. Our need to increase our prospective review capacity forced us to reevaluate ASP workload and the workloads of our physician colleagues each week. As the ID physician group modified its consult service structure to include multiple ID consult teams, it was not possible to have an ID pharmacist physically present on each team. Daily communication with each primary and ID consult team was necessary to review opportunities identified from prospective reviews. During the initial peak of the pandemic and despite the extra hours of effort daily, we came to the realization that the volume of patients did not allow for daily review of every patient. We learned to stagger our reviews and redistribute workload with our pharmacy colleagues as needed.
It is important for all ASP leaders to reflect on what has the potential to change during a rapid influx of patients and to evaluate best practices for communication within their organization. The period of initial COVID-19 response was a time of rapid change for us, especially early in the pandemic, as we figured out what worked best for our organization. Social distancing prohibited us from face-to-face contact but weekly, and in some cases daily calls or online meetings allowed us to share information efficiently and effectively. We were able to leverage our EMR to help us with daily workflows, to educate prescribers, and to drive practice changes quickly. Not all institutions will have similar capabilities related to prescriber ordering and ASP alerts. Becoming familiar with information technology capabilities and establishing contacts with information technology personnel prior to emergencies will be important for ASP teams to maximize efficiency during the COVID-19 pandemic and future emergencies.
Moving forward, antimicrobial stewardship efforts will become even more critical. As our understanding of and access to treatments and vaccines for COVID-19 expand, it is our hope that fewer patients will require intubation or patients will have shorter durations of mechanical ventilation and require less use of immunomodulatory agents, leading to fewer bacterial complications and correspondingly lower antimicrobial utilization.
Conclusion
The early response to the COVID-19 pandemic did not change our antimicrobial stewardship principles but instead changed our antimicrobial stewardship team’s practices. It is necessary for antimicrobial stewardship teams to adapt to a challenging and rapidly changing environment. The degree of uncertainty about the novel coronavirus and the lack of effective antivirals weighed into decision-making. Leveraging existing resources, strategies, and communication were key in optimizing care. ASPs play a critical role in helping hospitals respond to the unique challenges posed by COVID-19. As the COVID-19 pandemic continues, there is a strong need to study the impact of antimicrobial decision-making on outcomes, particularly antimicrobial resistance. In the months since we got past the initial peak of the pandemic in New York City and retrospectively analyzed our practices during that stressful time, we continued to identify opportunities for improvement. Here we have offered reflections on the challenges we faced, the valuable tools that helped us along the way, and opportunities for improvement with the goal of assisting other ASPs facing challenges due to COVID-19 case surges.
Disclosures
The authors have declared no potential conflicts of interest.
References
- 1. City of New York. COVID-19: Data. https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed July 2, 2020.
- 2. Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10(5):394-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sepulveda J, Westblade LF, Whittier S, et al. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J Clin Microbiol. doi: 10.1101/2020.05.05.20080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clancy CJ, Nguyen MH. COVID-19, superinfections and antimicrobial development: what can we expect?. Clin Infect Dis. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC). COVID-19 report: 08 April 2020. https://media.tghn.org/medialibrary/2020/04/ISARIC_Data_Platform_COVID-19_Report_8APR20.pdf. Accessed July 2, 2020.
- 11. Buetti N, Mazzuchelli T, Priore EL, et al. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J Infect. doi: 10.1016/j.jinf.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. City of New York. 2020 health alert #10: COVID-19 updates for New York City face mask use policy, swab shortage, reporting COVID-19 related deaths and crisis communication resources. https://www1.nyc.gov/assets/doh/downloads/pdf/han/alert/2020/covid-19-update-04112020.pdf. Accessed July 2020.
- 15. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020;382(21):2012-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurtzhalts KE, Sellick JA Jr, Ruh CA, et al. Impact of antimicrobial stewardship on outcomes in hospitalized veterans with pneumonia. Clin Ther. 2016;38(7):1750-1758. [DOI] [PubMed] [Google Scholar]
- 19. Bolten BC, Bradford JL, White BN, et al. Effects of an automatic discontinuation of antibiotics policy: a novel approach to antimicrobial stewardship. Am J Health-Syst Pharm. 2019;76(suppl 3):S85-S90. [DOI] [PubMed] [Google Scholar]
- 20. Doernberg SB, Abbo LM, Burdette SD, et al. Essential resources and strategies for antibiotic stewardship programs in the acute care setting. Clin Infect Dis. 2018;67(8):1168-1174. [DOI] [PubMed] [Google Scholar]
- 21. Cole KA, Rivard KR, Dumkow LE. Antimicrobial stewardship interventions to combat antibiotic resistance: an update on targeted strategies. Curr Infect Dis Rep. 2019;21(10):33. [DOI] [PubMed] [Google Scholar]
- 22. Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013;28(3):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aliberti S, Cilloniz C, Chalmers JD, et al. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax. 2013;68(11):997-999. [DOI] [PubMed] [Google Scholar]
- 24. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160. [DOI] [PubMed] [Google Scholar]
- 25. Shah S, Barton G, Fischer A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc. 2015;16(2):147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veiga RP, Paiva JA. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit Care. 2018;22(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]