Abstract
Background and Aim
The aim of this systematic review was to quantify the association between frailty and COVID-19 in relation to mortality in hospitalised patients.
Methods
Medline, Embase, Web of Science and the grey literature were searched for papers from inception to 10 September 2020; the search was re-run in Medline up until the 9 December 2020. Screening, data extraction and quality grading were undertaken by two reviewers. Results were summarised using descriptive statistics, including a meta-analysis of overall mortality; the relationships between frailty and COVID-19 mortality were summarised narratively.
Results
A total of 2,286 papers were screened resulting in 26 being included in the review. Most studies were from Europe, half from the UK, and one from Brazil; the median sample size was 242.5, median age 73.1 and 43.5% were female. In total, 22/26 used the Clinical Frailty Scale; reported mortality ranged from 14 to 65%. Most, but not all studies showed an association between increasing frailty and a greater risk of dying. Two studies indicated a sub-additive relationship between frailty, COVID-19 and death, and two studies showed no association.
Conclusions
Whilst the majority of studies have shown a positive association between COVID-19-related death and increasing frailty, some studies suggested a more nuanced understanding of frailty and outcomes in COVID-19 is needed. Clinicians should exert caution in placing too much emphasis on the influence of frailty alone when discussing likely prognosis in older people with COVID-19 illness.
Keywords: COVID-19, frailty, hospital-related mortality, systematic review, older people
Key points
Frailty is being used to assess the risk of dying from COVID-19.
Emerging studies demonstrate a complex relationship between frailty and COVID-19-related deaths.
Clinicians should exert caution in placing too much emphasis on the influence of frailty in older people with COVID-19.
Researchers should ensure that frailty scales are used as designed when planning and reporting future research.
Introduction
The COVID-19 pandemic has had a disproportionate impact upon older people. An emerging feature of the clinical response has been to use the frailty construct to estimate likely outcomes or direct treatment escalation planning [1,2]. Frailty is a state of increased vulnerability to poor resolution of homeostasis after a stressor event, which increases the risk of adverse outcomes, including delirium, disability and death [3–5].
Where frailty has previously been studied in the critical care context, lower levels of frailty have been associated with better outcomes [6]. This data may have informed the decision by the National Institute of Clinical Excellence to encourage the use of the Clinical Frailty Scores (CFSs) when considering critical care escalation in older people with COVID-19 illness [2]. At the time of the NICE guidance being issued, there had been no studies validating such an approach in the context of COVID-19. Since, a number of studies have assessed outcomes from COVID-19 in older people, using various frailty scales.
The aim of this review was to synthesise emerging findings by quantifying the association between frailty and COVID-19 illness in relation to mortality in hospitalised patients.
Methods
The full systematic review protocol has been published elsewhere (PROSPERO ID: CRD42020200445) [7]; the only change to the protocol was the extension of the search period (see below).
Search strategies
Medline, Embase and Web of Science databases were searched with exploded MeSH headings and relevant keywords, restricted to English language. Databases were searched from inception to 10 September 2020, and references were managed using Endnote software. The reference lists of included full-texts were hand-searched for additional papers. Indicative search terms are displayed below; these were modified accordingly for each database.
‘Frail*’
AND
COVID-19 ((‘COVID-19’ OR ‘COVID-2019’ OR ‘severe acute respiratory syndrome coronavirus 2’ OR ‘severe acute respiratory syndrome coronavirus 2’ OR ‘2019-nCoV’ OR ‘SARS-CoV-2’ OR ‘2019nCoV’ OR (Wuhan AND coronavirus)).
Grey literature was accessed by searching: Open Grey, medRxiv, and bioRxiv.
A focused search was re-run in Medline on the 9 December 2020 to seek out more recent studies.
Inclusion criteria
Studies published from inception to 9 December 2020.
Original peer-reviewed articles, pre-prints, conference proceedings and letters to the editor reporting primary data, in any language.
Studies reporting mortality as related to frailty in individuals diagnosed with COVID-19 in acute hospital settings.
Frailty identified using a recognised frailty instrument.
Participants with a positive diagnosis of COVID-19 (Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Ribonucleic acid Polymerase Chain Reaction (RNA-PCR) positive or specialist clinical opinion).
Participants aged 18 years or older.
Exclusion criteria
Studies not involving humans.
Articles not reporting primary data.
Studies in which COVID-19 was self-diagnosed.
Study quality assessment
Two independent reviewers (TDC and KW or SC and JvO) assessed the study quality using the Newcastle-Ottawa Quality Assessment Scale (NOS). The NOS scale uses a star system assess the validity of studies in the domains of the selection and comparability of cohorts, and the ascertainment of either the exposure or outcome of interest. This gives rise to quality ratings:
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain
Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
A maximum of 2 stars were possible in the selection domain, as cohorts were not matched for frailty.
Data extraction
Two reviewers (TDC and KW/IR or SC and JvO) identified and exported articles identified by the search strategy into EndNote reference software; duplicates were deleted. Independent title and abstract screens were conducted by TDC, KW or SC identifying articles for full-text extraction. Full-text screening was used to identify a final list of included studies. Relevant data were extracted by two independent researchers (JB and TDC, or SC and JvO) from the included studies into a pre-established extraction form.
Analysis
Summary statistics for age were combined, after converting medians/IQRs into means and standard deviations using Wan’s method [8]. Overall mortality was summarised using meta-analysis, with heterogeneity assessed using the I-squared statistic [9]. A meta-analysis summarising the effect of frailty on COVID-19 mortality was planned but the heterogeneity in study designs, frailty tools used and reporting of mortality made this impossible.
Ethics
No ethical approval was required for this work.
Results
The initial searches identified 2,276 records of which 650 were duplicates, leaving 1,626 papers for review. After scrutinising the titles and abstracts against the eligibility criteria, 36 papers were retained for full-text review; a further 10 papers were identified on re-running the search in Medline, leading to 26 papers being included for data abstraction (Figure 1).
Figure 1 .
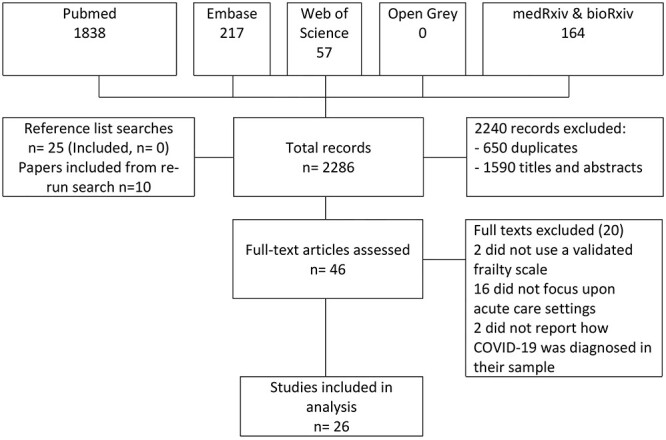
Study selection
The summary characteristics are shown in Table 1.
Table 1 .
Summary characteristics of retained studies examining frailty and COVID-19-related outcomes
| Author | Country | Setting | Sample size | Age, mean (SD) *unless otherwise stated | Proportion female (%) | Proportion white (%) | Frailty measure | COVID diagnosis | NOS grading |
|---|---|---|---|---|---|---|---|---|---|
| Apea [16] | UK | Five acute hospitals | 1996 | 62.2 (17.4) | 39 | 35 | CFS | PCR | 6 |
| Aw [21] | UK | Acute hospital | 677 | 81.1 (8.1) | 46 | 81 | CFS | PCR | 6 |
| Baker [22] | UK | Acute hospital | 316 | 72.7 (17.1) | 45 | 96 | CFS | PCR | 6 |
| Brill [23] | UK | Acute hospital | 410 | 81.1 (8.1) | 65 | 60 | CFS | PCR | 4 |
| Chinnadurai [24] | UK | Acute hospital | 215 | 72.0 (16.4) | 38 | 87 | CFS | PCR | 5 |
| Cobos-Siles [25] | Spain | Acute hospital | 656 | 82.7 (10.5) | 43 | Not stated | CFS | PCR | 6 |
| Conway [26] | UK | Acute hospital | 71 | 70.7 (16.6) | 42 | 99 | CFS | PCR | 4 |
| Crespo [10] | Spain | Renal transplant cohort, acute hospital | 16 | 59.7 (12.6) | 6 | Not stated | Fried | PCR | 4 |
| Davis [27] | UK | Acute hospital | 222 | 82 (range 56–99) | 67 | Not stated | CFS | PCR | 5 |
| De Smet [28] | Belgium | General hospital | 81 | 70.3 (20.1) | 59 | Not stated | CFS | PCR | 6 |
| Doglietto [11] | Italy | Patients with COVID undergoing surgery | 41 | 82.7 (10.5) | 56 | Not stated | CFS | PCR | 4 |
| Frost [29] | UK | Seven acute hospitals | 749 | 85.3 (6.8) | 32 | Not stated | CFS | PCR | 6 |
| Hagg [17] | Sweden | Acute hospital | 250 | 81.0 (8.6) | 52 | Not stated | CFS/HFRS | PCR/clinical | 7 |
| Hewitt [18] | Italy/UK | 11 acute hospitals (10 England, 1 Italy) | 1,564 | 76.0 (5.2) | 42 | Not stated | CFS | PCR/clinical | 6 |
| Hoek [30] | The Netherlands | Multi-centre—solid organ transplant recipients | 23 | 60.7 (15.0) | 22 | 61 | CFS | PCR | 4 |
| Knights [31] | UK | General hospital | 108 | 69.3 (16.3) | 39 | 76 | CFS | PCR | 7 |
| Kundi [15] | Turkey | All acute hospitals in Turkey | 18,234 | 74.1 (7.4) | 53.4 | Not stated | HFRS | PCR | 7 |
| Marengoni [12] | Italy | COVID-19 special hospital | 165 | 69.3 (14.5) | 39 | Not stated | CFS | PCR/clinical | 7 |
| Mendes [13] | Switzerland | COVID-19 special hospital | 235 | 86.3 (6.5) | 57 | 100 | CFS | PCR/clinical | 6 |
| Miles [32] | UK | Acute hospital | 217 | 59 | 38 | Not stated | CFS | PCR | 6 |
| Owen [19] | UK | Acute hospital | 301 | 68.7 (15.6) | 44 | Not stated | CFS | PCR/clinical | 6 |
| Poco [14] | Brazil | COVID-19 special hospital | 711 | 66 (−) | 43 | Not stated | CFS | PCR/clinical | 6 |
| Rawle [33] | UK | Acute hospital | 134 | 80.0 (6.8) | 46 | 76 | CFS | PCR | 4 |
| Steinmeyer [20] | France | Acute hospital | 94 | 85.5 (7.5) | 55 | Not stated | FIND | PCR | 5 |
| Tehrani [34] | Sweden | Acute hospital | 255 | 66 (17) | 41 | Not stated | CFS (7 point scale) | PCR | 7 |
| Thompson [35] | UK | Acute hospital | 470 | 78.8 (8.3) | 46 | 83 | CFS | PCR | 6 |
Thirteen of the 26 studies were from the UK, 13 from other European countries and one from Brazil. All studies reported findings from acute hospitals (secondary care), with Crespo et al. [10] reporting specifically on renal transplant recipients, Doglietto on surgical patients [11] and three studies reported from COVID-19 dedicated hospitals (Marengoni [12], Mendes [13], Poco [14]); all the other studies reported outcomes for general, acute medical care. All studies described outcomes in people with clinically diagnosed and PCR-confirmed COVID-19, with the exception of Miles (contemporaneous matched controls), Owen and Aw (clinical and PCR positive versus clinically positive only) and Doglietto (historical matched controls). Most of the studies were fair–good quality according to the NOS (Table 1).
The median sample size was 242.5 (IQR 108–656); the largest study reported on over 18,000 participants from Turkey (Kundi) [15,16]. Overall, the median age of included participants was 73.1 years (IQR 69.3–81.1), and 43.5% were female. Where reported, the majority of studies reflected white participants, except for Apea [16], which had a majority of non-white participants. Frailty was assessed using the CFS in 22 studies, one used the Hospital Frailty Risk Score (HFRS), one used both CFS and HFRS, one Fried’s frailty phenotype and one used the Frail Non-Disabled survey (FIND). Infection causing COVID-19 illness was confirmed using clinical features and a positive PCR in all studies though Hagg [17], Hewitt [18], Marengoni [12], Mendes [13], Owen [19], Poco [14] and Steinmeyer [20] also included people with clinical diagnoses but negative PCR tests.
Mortality was varied widely across the studies, ranging from 14 to 65%; studies reported mortality at different time points (5–60 days), some reported in-hospital deaths only and others all deaths in and outside of the hospital over the followed period. A descriptive summary is shown in Table 2 and a meta-analysis summarising overall mortality in Figure 2 (random effects were used as heterogeneity was high, I-squared 97.3%, P = 0.000).
Table 2 .
Descriptions of mortality outcomes
| Author | Frailty measure used | Overall cohort mortality (%) | Follow up (days unless otherwise stated) | Associations of frailty with mortality |
|---|---|---|---|---|
| Mortality reported using HRs (95% CI) | ||||
| Apea [16] | CFS | 28.7 | 30 | Covariates in adjusted analysis: age, sex, ethnicity, smoking, BMI and IMD |
| CFS 1–2: reference category | ||||
| CF 3–4: 1.61 (0.82–3.16) | ||||
| CFS 5–6: 1.84 (0.93–3.64) | ||||
| CSF 8–9: 3.25 (1.49–7.06) | ||||
| Aw [21] | CFS | 40.0 | 34 | Covariates in adjusted analysis: age, sex, ethnicity, IMD, previous hospital admissions in 2019 and NEWS-2 |
| CFS 1–3: reference category | ||||
| CFS 4 1.30 (0.76–2.21) | ||||
| CFS 5 1.19 (0.70–2.03) | ||||
| CFS 6 2.13 (1.34–3.38) | ||||
| CFS 7–9 1.79 (1.12–2.88) | ||||
| Sensitivity analyses: association between frailty and mortality was similar when cases were confined to RT-PCR positive cases. | ||||
| Chinnadurai [24] | CFS | 40.0 | 5 (IQR 2–10) | Comparing CFS ≥5 to CFS <5, the unadjusted HR for death was 3.45; 95% CI: 1.76–6.79; P < 0.001) |
| Hagg [17] | CFS/HFRS | 24 | 25 | Covariates in adjusted analysis: age, sex, comorbidities, HFRS and acute kidney injury |
| CFS ≥5 vs. CFS < 5 HR 1.85 (0.97–3.52) | ||||
| HFRS HR 1.00 (0.91–1.10), adjusted for age and sex | ||||
| No modelling undertaken on non-COVID controls | ||||
| Hewitt [18] | CFS | 27.2 | 28 | Covariates in adjusted analysis: age, sex, smoking, C-reactive protein, diabetes, coronary artery disease, hypertension, renal function |
| CFS 1–2: reference category | ||||
| CFS 3–4: 1.55 (1.00–2.41) | ||||
| CFS 5–6: 1.83 (1.15–2.91) | ||||
| CFS 7–9: 2.39 (1.50–3.81) | ||||
| Marengoni [12] | CFS | 25.6 | To death or discharge. Max: 40 | Covariates in adjusted analysis: age, sex, primary education, number of chronic diseases |
| For each 1 point increase in the CFS score, the HR for death was 1.30 (1.05–1.62) | ||||
| Mendes [13] | CFS | 32.3 | To death or discharge: Mean 12.8 (SD 7.6) | Covariates in adjusted analysis: sex |
| For each 1 point increase in the CFS score, the HR for death was 1.46 (1.24–1.70) | ||||
| Comparing CFS ≥5 to CFS <5, adjusted for sex, the HR for death was 4.39 (1.60–12.02) | ||||
| Miles [32] | CFS | 51.2 | 60 | Covariates used in the adjusted analysis included age, sex, ethnicity, IMD |
| For each 1 point increase in the CFS score, the HR for death was 1.88 (1.37–2.59) | ||||
| The different associations with frailty according to COVID-19 status was confirmed by demonstrating an interaction term (HR 0.51, 95% CI 0.37 to 0.71) | ||||
| Owen [19] | CFS | 42.9 | 30 | Covariates in adjusted analysis: age, sex, acuity and comorbidities. Compares results in those with PCR confirmed COVID-19 only. |
| CFS 1–3: reference | ||||
| CFS 4–5: 2.12 (0.86–5.18) | ||||
| CFS 6: 1.69 (0.67–4.28) | ||||
| CFS 7–8: 2.36 (0.96–5.76) | ||||
| CFS 9: 11.97 (3.70–38.72) | ||||
| CFS Not Recorded 2.14 (0.89–5.13) | ||||
| In COVID-19 positive individuals, the interaction between COVID-19 status and CFS suggests a sub-additive relationship. | ||||
| Mortality reported using odds ratios | ||||
| Cobos-Siles [25] | CFS | 19.5 | 33 | Comparing mild to very severely frail older people, the odds ratio for death was 8.73 (95% CI 1.37–55.46) |
| De Smet [28] | CFS | 23.5 | 48 | Covariates included in adjusted analysis: age, LDH, RT-PCR |
| For each 1-point increase in CFS, the odds of being dead at follow up increased by 1.75 (5% CI 1.1–3.4) | ||||
| Kundi [15] | HFRS | 18.2 | In-hospital mortality – maximum follow-up possible 103 days | Covariates included in the adjusted analysis: age, sex and comorbidities For each 1 point increase in the HFRS, in-hospital mortality risk increased by an odds ratio of 1.036 (1.029–1.043) |
| By HFRS categories, the adjusted risk of in-hospital mortality compared to low HFRS category (HFRS <5) was: intermediate (HFRS 5–15) 1.482 (1.334–1.646); high (HFRS >15) 2.084 (1.799–2.413) | ||||
| Rawle [33] | CFS | 64.9 | The risk of death was associated with an odds ratio of 2.68 (96% CI 1.26–6.49) for each 1 point increase in CFS. | |
| Tehrani [34] | CFS (7 point scale) | 27.5 | 60 | Covariates used in the adjusted analysis included age, and chronic kidney disease For each 1 point increase in the 7 point CFS scale, the odds ratio for death was 2.07 (1.47–2.92) |
| Thompson [35] | CFS | 36.0 | 30 | Median CFS was significantly higher in non-survivors (6 IQR 4–7 vs. 3 IQR 2–5 for survivors. In the multivariate analysis adjusting for age, hypertension, cancer, CRP, platelet count, acute kidney injury and > 50% total lung field infiltrates, frailty was not a significant predictor. |
| Other descriptions of mortality as an outcome | ||||
| Baker [22] | CFS | 25.6 | 28 | Patients who died without ventilatory support had a median (IQR) CFS score of 7 (6–7). |
| Brill [23] | CFS | 42.2 | 28 | People aged 80+ that died were more frail (median (IQR) CFS 6 (5, 7) vs. 5 (4, 6), P = 0.002 |
| Conway [26] | CFS | 14.1 | Maximum 14 days | Patients who died had higher CFS scores (5.75 v 3.36, P = 0.005) |
| Crespo [10] | Fried | 50.0 | 14 | Mortality if Fried >0 was 5/7 (62.5%) |
| Davis [27] | CFS | 42.8 | 30 | Frailty was associated with a higher chance of dying (46.1% mortality when CFS ≥ 5 vs 32.7 when CFS ≥ 4, P = 0.02) |
| Doglietto [11] | CFS | 19.5 | No data on CFS associated mortality (used as a case-mix adjuster) | |
| Frost [29] | CFS | 40.1 | 30 | Univariate difference in CFS score (median and IQR): |
| at 72-hours: 3 (2–6) alive versus 6 (4–7) deceased | ||||
| at 30-days: 3(2–5) versus alive 5 (3–6) deceased | ||||
| Hoek [30] | CFS | 21.7 | Mean CFS was 5.8 in those that died | |
| Knights [31] | CFS | 31.5 | 30 | Median CFS was higher in patients over 65 who died (5, IQR 4–6) than in survivors (3.5, IQR 2–5) P < 0.01). |
| Poco [14] | CFS | 37 | Median length of stay 11 | Mortality among older adults CFS ≥ 5 = 58% vs. CFS < 5 = 42%; P = 0.003 |
| Steinmeyer [20] | FIND | 18.1 | Mean length of stay 12.0 (SD 5.5; range 2–31) | In a univariate Cox regression, frailty was not associated with mortality (25% mortality among ‘robust’ individuals vs 15% mortality among ‘frail’ and ‘dependant’. |
CI, confidence interval; BMI, body mass index; IMD, index of multiple deprivation.
Figure 2 .
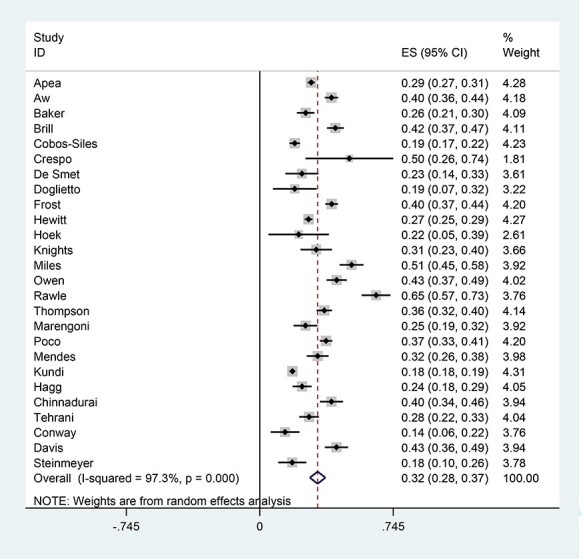
Random effects meta-analysis showing overall mortality (note high heterogeneity)
Nine studies reported mortality over time using hazard ratios (HRs) to describe the effect of frailty (Apea, Aw, Chinnadurai, Hagg, Hewitt, Marengoni, Mendes, Miles, Owen); all used the CFS and with the exception of Chinnadurai, adjusted for important baseline covariates. However, these studies used different cut-points of the CFS, as well as different covariates, which meant combining the results in a meta-analysis was not possible. These studies did, however, all show an increase in the risk of dying with increasing levels of frailty, although Miles and Owen both found a ‘sub-additive’ effect in the most severe frailty groups, in which the increased risk of dying was less than might have been expected.
Six studies (Cobos-Siles, De Smet, Kundi, Rawle, Tehrani, Thompson) reported mortality risk as odds ratios, some using the CFS others the HFRS, again with a range of covariates and different CFS categories. It was not possible to combine these data, however, with the exception of Thompson et al., they all showed an increased odds of dying with increasing levels of frailty.
The remaining 11 studies used a range of frailty measures in different ways to describe some aspect of COVID-19-related mortality, such that combining results would not be clinically meaningful. Most of these studies with the exception of Steinmeyer et al. tended reported an association between increased risk of dying and increase levels of frailty.
In summary, the majority of studies found a positive association between increasing frailty and COVID-19-related mortality—but not all. Miles and Owen both found an interaction between frailty and PCR testing that attenuated the expected mortality associated with increasing frailty. Steinmeyer et al. and Thompson et al. found that frailty was not a significant predictor in an adjusted analysis.
Discussion
Summary
This systematic review identified 26 studies assessing the influence of frailty on COVID-19-related mortality in hospitalised patients. The overall quality of the studies was reasonable, and the majority of studies showed that in older people hospitalised with COVID-19 illness that frailty was associated with COVID-19-related mortality. However, this was not consistent across all cohorts, with some showing a more complex interaction between frailty and COVID-19 status: two studies found a sub-additive interaction with frailty) i.e. that the mortality seen in severely frail older people was not as high as expected and that excess mortality was observed in those relatively fitter). This may relate to a selection effect, as policy and practice during the pandemic emphasised avoiding hospitalisation in many settings (e.g. national lockdowns). Patients with higher frailty scores are more likely to represent care-home residents, in whom COVID-19 illness might be managed in the community [36]. Treatment effects varied over time, for example, greater or lesser use of critical care or treatment escalation plans, or the introduction of ‘new’ treatments such as Dexamethasone, which could have affected outcomes. Less frail patients may have had more aggressive treatment than those with increased levels of frailty, and this practice may have changed over time and varied between centres. Taken together, whilst the bulk of the studies find the ‘expected’ relationship between frailty and COVID-19 mortality, our findings suggest a more nuanced understanding of frailty and outcomes in COVID-19 is needed.
Strengths and weakness
This review was methodologically robust according to the quality of reporting of meta-analyses and PRISMA reporting guidelines. It is possible that in this new field, emerging studies not yet published may have been missed, although we searched pre-print collections in an effort to minimise this risk, as well as updating the search in December 2020. The British Geriatrics Society has agreed to host a live update of this review so that future studies can be incorporated into the analysis (https://www.bgs.org.uk/covidfrailty). Whilst the individual papers included in the review were of fair-good quality, frailty (its operationalisation and reported cut-points) and mortality were reported variably across the studies, making meta-analysis and comparisons difficult.
Most of the studies were from Europe—mostly the UK—which may limit generalisability to other health systems. We focused upon studies reporting outcomes for hospitalised patients, so we cannot make any comment about COVID-19-related risk in the wider population, in particular in care homes or population samples.
We did not examine other risk scores designed to predict outcomes from COVID-19, such as those looking at comorbidities or biomarkers [29,37–39], as these are separate constructs from frailty. In clinical practice, both physiological risk scores and frailty risk scores would be used together to inform prognostication, and future work might compare the relative merits of combined risk scoring.
We focused upon mortality, but outcomes such as function, cognition or quality of life are equally, if not more important, especially for older people [40]. However, in this relatively early stage of the COVID-19 pandemic, we anticipated that there would be very few studies reporting such outcomes, though this will be an important area upon which to focus in the future.
Relationship to existing literature
The CFS appears to perform similarly to other predictors of mortality in the context of COVID-19, such as the Palliative Performance Scale [38], but perhaps less well than the 4C Mortality Score, developed and validated specifically in COVID-19 cohorts [39].
Whilst mortality in hospital may be related to frailty, wider determinants of health have an important impact upon country-specific survival rates. Paradoxically, 1% decrease in pre-existing all-cause mortality is associated with a 4.1% increase in the COVID-19 death rate in those ≥60 years of age, thought to be related to an unhealthy survivor effect i.e. longevity at the price of dependency and increased susceptibility to COVID-19 (e.g. care home populations) [41].
Implications for research
Larger, more robust studies examining the relationship between COVID-19 and frailty are needed to resolve the limitations of the existing papers. Future studies should preserve the integrity of frailty scales so that comparisons can be made across studies [42] and should take account of the apparent interaction between frailty and COVID-19 testing [19,32].
Implications for clinical practice
Clinicians should exert caution in placing too much emphasis on the influence of frailty alone when discussing likely prognosis in older people with COVID-19 infection. No tool should be used in isolation to direct clinical care, though frailty scores can form part of a more holistic assessment to inform a shared decision-making discussion. Frailty can be useful in identifying the risk of complications such as delirium—increasingly being recognised as a high-risk scenario [20,43,44]—and further frailty or deconditioning [45]. Updated clinical guidance on frailty and COVID, as well as other resources are available here, https://www.criticalcarenice.org.uk/, and the British Geriatrics Society will maintain a live web-repository of COVID and frailty studies here: https://www.bgs.org.uk/covidfrailty.
Supplementary Material
Contributor Information
Theodore D Cosco, Department of Gerontology, Simon Fraser University, Vancouver, Canada.
John Best, Department of Gerontology, Simon Fraser University, Vancouver, Canada.
Daniel Davis, University College London, London, UK.
Daniele Bryden, Sheffield Teaching Hospitals, Sheffield, UK.
Suzanne Arkill, University Hospitals of Leicester, Leicester, UK.
James van Oppen, Department of Health Sciences, University of Leicester, University Road, Leicester LE1 7RH, UK.
Indira Riadi, Department of Gerontology, Simon Fraser University, Vancouver, Canada.
Kevin R Wagner, Simon Fraser University, Vancouver, Canada.
Simon Conroy, Department of Health Sciences, University of Leicester, University Road, Leicester LE1 7RH, UK.
Declaration of Sources of Funding
Daniel Davis is funded through a Wellcome Intermediate Clinical Fellowship (WT107467). Theodore D Cosco is funded through a Michael Smith Foundation for Health Research Scholar Award (SCH-2020-0490). James van Oppen is funded through a National Institute for Health Research Doctoral Research Fellowship (NIHR300901).
Declaration of Conflicts of Interest
None.
References
- 1. Maltese G, Corsonello A, Di Rosa M et al. Frailty and COVID-19: a systematic scoping review. J Clin Med 2020; 9: 2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Institute for Clinical Excellence . COVID-19 rapid guideline: critical care in adults. NICE guideline. 2020 March 2020([NG159]). [PubMed]
- 3. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (London, England) 2013 Mar 02 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hubbard RE, Peel NM, Samanta M, Gray LC, Mitnitski A, Rockwood K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing 2017; 2017: 1–6. [DOI] [PubMed] [Google Scholar]
- 5. Wou F, Gladman JRF, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing 2013; 42: 776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muscedere J, Waters B, Varambally A. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med 2017; 43: 1105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosco T, Davis D, Conroy S. Frailty and mortality outcomes for patients with COVID-19: a rapid systematic review and meta-analysis of hospitalised cohorts. PROSPERO; CRD42020200445. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200445 (18 October 2020, date last accessed).
- 8. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 10. Crespo M, Pérez-Sáez MJ, Redondo-Pachón D et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant 2020; 20: 2883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doglietto F, Vezzoli M, Gheza F et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg 2020; 155: 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marengoni A, Zucchelli A, Vetrano DL et al. Beyond chronological age: frailty and multimorbidity predict in-hospital mortality in patients with coronavirus disease 2019. J Gerontol A Biol Sci Med Sci 2020; 20: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendes A, Serratrice C, Herrmann FR et al. Predictors of in-hospital mortality in older patients with COVID-19: the COVIDAge study. J Am Med Dir Assoc 2020; 21: 1546, e3–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poco PCE, Aliberti MJR, Dias MB et al. Divergent: age, frailty, and atypical presentations of COVID-19 in hospitalized patients. J Gerontol A Biol Sci Med Sci 2020. doi: 10.1093/gerona/glaa280. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kundi H, Cetin EHO, Canpolat U et al. The role of frailty on adverse outcomes among older patients with COVID-19. J Infect 2020; 28: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Apea VJ, Wan YI, Dhairyawan R et al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. medRxiv 2020; 2020.06.10.20127621. 10.1101/2020.06.10.20127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hagg S, Jylhava J, Wang Y et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc 2020; 21: 1555–59.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hewitt J, Carter B, Vilches-Moraga A et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020; 5: e444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Owen RK, Conroy SP, Taub N et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing 2020. 10.1093/ageing/afaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinmeyer Z, Vienne-Noyes S, Bernard M et al. Acute care of older patients with COVID-19: clinical characteristics and outcomes. Geriatrics 2020; 5: 65. doi: 10.3390/geriatrics5040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aw D, Woodrow L, Ogliari G, Harwood R. Association of frailty with mortality in older inpatients with Covid-19: a cohort study. Age Ageing 2020; 49: 915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baker KF, Hanrath AT, Schim vander loeff I et al. COVID-19 management in a UK NHS foundation trust with a high consequence infectious diseases Centre: a detailed descriptive analysis. medRxiv 2020; 2020.05.14.20100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brill SE, Jarvis HC, Ozcan E et al. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. BMC Med 2020; 18: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chinnadurai R, Ogedengbe O, Agarwal P et al. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatrics [Observational Study] 2020; 20: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cobos-Siles M, Cubero-Morais P, Arroyo-Jiménez I et al. Cause-specific death in hospitalized individuals infected with SARS-CoV-2: more than just acute respiratory failure or thromboembolic events. Intern Emerg Med 2020; 15: 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conway J, Gould A, Westley R et al. Clinical characteristics and progression of COVID-19 confirmed cases admitted to a single British clinical Centre-a brief case series report. Int J Clin Pract 2020; 31: e13807. [DOI] [PubMed] [Google Scholar]
- 27. Davis P, Gibson R, Wright E et al. Atypical presentations in the hospitalised older adult testing positive for SARS-CoV-2: a retrospective observational study in Glasgow. Scotland Scott Med J 2020; 11: 36933020962891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Smet R, Mellaerts B, Vandewinckele H et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc 2020; 21: 928–32.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradley P, Frost F, Tharmaratnam K, NW-CORR Collaborators, Wootton DG. The utility of established prognostic scores in COVID-19 hospital admissions: a multi-centre prospective evaluation of CURB-65, NEWS2, and qSOFA. medRxiv 2020; 2020.07.15.20154815. 10.1101/2020.07.15.20154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoek RAS, Manintveld OC, Betjes MGH et al. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int 2020; 33: 1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knights H, Mayor N, Millar K et al. Characteristics and outcomes of patients with COVID-19 at a district general hospital in surrey. UK Clinical Medicine 2020; 20: e148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miles A, Webb TE, Mcloughlin BC et al. Outcomes from COVID-19 across the range of frailty: excess mortality in fitter older people. Eur Geriatr Med 2020; 11: 851–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rawle MJ, Bertfield DL, Brill SE. Atypical presentations of COVID-19 in care home residents presenting to secondary care: a UK single centre study. Aging Med 2020; 3: 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tehrani S, Killander A, Åstrand P, Jakobsson J, Gille-Johnson P. Risk factors for death in adult COVID-19 patients: frailty predicts fatal outcome in older patients. Int J Infect Dis 2021; 102: 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thompson J, Meghani N, Powell B et al. Patient characteristics and predictors of mortality in 470 adults admitted to a district general hospital in England with Covid-19. Epidemiology and Infection 2020; 148: E285. doi: 10.1017/S0950268820002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. British Geriatrics Society . COVID-19: Managing the COVID-19 pandemic in care homes for older people. London; Guidance: https://www.bgs.org.uk/resources/covid-19-managing-the-covid-19-pandemic-in-care-homes (29th April 2020, date last accessed).
- 37. Ji D, Zhang D, Xu J et al. Prediction for progression risk in Patients with COVID-19 pneumonia: the CALL Score. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fiorentino M, Pentakota SR, Mosenthal AC, Glass NE. The palliative performance scale predicts mortality in hospitalized patients with COVID-19. Palliat Med 2020; 34: 1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knight SR, Ho A, Pius R et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C mortality score. BMJ 2020; 370: m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akpan A, Roberts C, Bandeen-Roche K et al. Standard set of health outcome measures for older persons. BMC Geriatr 2018; 18: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Altringer L, Zahran S, Prasad A. The longevity-frailty hypothesis: evidence from COVID-19 death rates in Europe. medRxiv 2020; 2020.04.14.20065540. 10.1101/2020.04.14.20065540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J 2020 Sep; 23: 210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marengoni A, Zucchelli A, Grande G, Fratiglioni L, Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing 2020; 49: 923–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zazzara MB, Penfold RS, Roberts AL et al. Probable delirium is a presenting symptom of COVID-19 in frail, older adults: a cohort study of 322 hospitalised and 535 community-based older adults. Age Ageing 2021; 50: 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flaatten H, Beil M, Guidet B. Prognostication in older ICU patients: mission impossible? Br J Anaesth 2020; 125: 655–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


