ABSTRACT
The importance of balanced dietary habits, which include appropriate amounts of antioxidants to maintain the immune system, has become increasingly relevant during the current SARS-CoV-2/COVID-19 pandemic, because viral infections are characterized by high oxidative stress. Furthermore, the measures taken by governments to control the pandemic have led to increased anxiety, stress, and depression, which affect physical and mental health, all of which are influenced by nutritional status, diet, and lifestyle. The Mediterranean diet (MD), Atlantic diet (AD), and the Dietary Guidelines for Americans all provide the essential vitamins, minerals, and phenolic compounds needed to activate enzymatic and nonenzymatic antioxidant responses. However, viral pandemics such as the current COVID-19 crisis entail high oxidative damage caused by both the infection and the resultant social stresses within populations, which increases the probability and severity of infection. Balanced dietary patterns such as the MD and the AD are characterized by the consumption of fruit, vegetables, legumes, olive oil, and whole grains with low intakes of processed foods and red meat. For a healthy lifestyle in young adults, the MD in particular provides the required amount of antioxidants per day for vitamins D (0.3–3.8 μg), E (17.0 mg), C (137.2–269.8 mg), A (1273.3 μg), B-12 (1.5–2.0 μg), and folate (455.1–561.3 μg), the minerals Se (120.0 μg), Zn (11.0 mg), Fe (15.0–18.8 mg), and Mn (5.2–12.5 mg), and polyphenols (1171.00 mg) needed to maintain an active immune response. However, all of these diets are deficient in the recommended amount of vitamin D (20 μg/d). Therefore, vulnerable populations such as elders and obese individuals could benefit from antioxidant supplementation to improve their antioxidant response. Although evidence remains scarce, there is some indication that a healthy diet, along with supplemental antioxidant intake, is beneficial to COVID-19 patients.
Keywords: antioxidants, balanced diet, Mediterranean diet, Atlantic diet, viral infections, COVID-19
Introduction
Recommended, balanced dietary patterns are meant to provide vital antioxidants for the proper maintenance of the human organism. The Mediterranean diet (MD) and Atlantic diet (AD), as well as the American dietary guidelines—considered in this work as the Dietary Guidelines for Americans [American diet (AmD)]—are popular, Western dietary patterns that provide all of the required macro and micronutrients needed to keep an organism in optimal balance and counteract oxidative damage (1–3). Moreover, the antioxidant intake needed to combat oxidative stress is meant to be achieved by following these dietary patterns (4). During a pandemic, these diets can also act to diminish the negative effects of a viral infection (5). There is growing evidence suggesting that for certain antioxidants, including selenium, zinc, and vitamins D and E, their consumption above the currently recommended levels can improve immune functioning and resistance to infection (6–9).
Throughout history, humans have been subject to pandemics caused by pathogenic viruses that were, ultimately, contained by the immune response; however, a number of viral outbreaks such as coxsackievirus, Zika, HIV, Chikungunya, and influenza remain threats. Respiratory infections, including the 1918 Spanish influenza (H1N1), the swine flu of 2009, and the current SARS-CoV-2 (COVID-19) (10–12) illnesses, serve to remind us of our vulnerability to viral pandemics. As part of reducing these threats, the proper intake of antioxidants through food plays a fundamental role in maintaining our immune system in an optimal antioxidant state (12–14).
High oxidative stress characterizes viral pandemics and affects the antioxidant response. One characteristic of viral infections is the tremendous production of reactive oxygen species (ROS) in both infected and healthy people during a health crisis (12, 15). Individuals who become ill due to viral pathogenesis are in constant oxidative stress, which decreases their likelihood of overcoming the infection (12, 16, 17). Furthermore, the oxidative environments caused by the socio-psychological stresses to which the individual is subjected to decreases the antioxidant response in both healthy and ill people (18). Accordingly, a primary goal during viral pandemics is to maintain both an oxidative balance (16) and the protective antioxidant defense system to reduce the risk of contagion or overcome the infection (12, 19).
Whether the regular intake of vitamins, minerals, and phytochemicals such as phenolic compounds that are supplied by a balanced diet is adequate during special oxidative stress situations such as pandemics remains unclear (20, 21). Moreover, antioxidant requirements vary according to age, health status, and stress condition (22–24). Consequently, the objectives of this review are to discuss the roles of the leading Western, balanced dietary patterns (MD, AD, and AmD) and to provide information regarding antioxidant intake and its suitability given the age group and physical/mental state of the population. We also discuss the role of antioxidant supplementation in confronting viral pandemics based on scientific evidence.
Current Status of Knowledge
Balanced dietary patterns and health
According to the WHO (25), global life expectancy is 72 y, lower for men (69.8 y) than women (74.2 y). As the body ages, its nutritional requirements change. This is primarily due to a decrease in the absorption of both macronutrients and micronutrients (vitamins, minerals, antioxidants). Antioxidants, in particular, are crucial for producing coenzymes, enzymes, and catalysts in oxidation-reduction reactions. The WHO has promoted the concept of a “healthy lifespan” with the aim to increase health throughout the lifespan (26), and age-related oxidative stress can be related to age-related health conditions such as cardiovascular disease, diabetes, and cancer (27). To address these health problems, nutritional experts have taken a closer look at different dietary models such as the AD and MD that have been shown to improve healthy lifespans. The MD has historically been associated with health benefits for diseases such as diabetes and cancer and is based on the traditional cuisine of the countries of the Mediterranean coastline. It is abundant in vegetables, fruits, whole grains, legumes, nuts and seeds, and olive oil. The AD is very similar to the MD and includes seasonal, local, fresh, and minimally processed foods (1, 3, 28, 29). Other health organizations such as the AHA have also established guidelines to promote a healthy, balanced diet, especially for the American population (2). One study has suggested that a reduction of 10–50% of total calorie intake, without causing malnutrition, could modulate mitochondrial activity and lower ROS production and hence slow the aging rate and extend lifespan (27).
The MD is abundant in vegetables, fruits, cereals, minimally processed legumes, and cold-pressed olive oil as a primary source of fat, along with seasonal products, honey consumed several times a week, dairy products, poultry, fish in moderate amounts, red meat in small quantities, and moderate wine consumption (28, 29). According to Davis et al. (29), the MD provides 203.5 ± 66.3 mg/d vitamin C and ∼344.9 mg/d flavonoids, thereby providing a good source of antioxidants.
The AmD is similar to the MD recommendations; however, there is a difference in the servings and frequency, which can be important regarding the quantity of antioxidants provided by the diet, as shown in Figure 1A, C. Alcohol recommendation from the AHA is moderate, even for red wine, and they state that there is no research demonstrating a cause-effect link between wine and better health, though some researchers indicate the contrary (2, 30). Currently, it is unclear whether the associated beneficial effects of red wine are more a matter of a healthy lifestyle, which includes physical activity, a large consumption of fruit and vegetables, and wine in moderation (2, 31).
FIGURE 1.
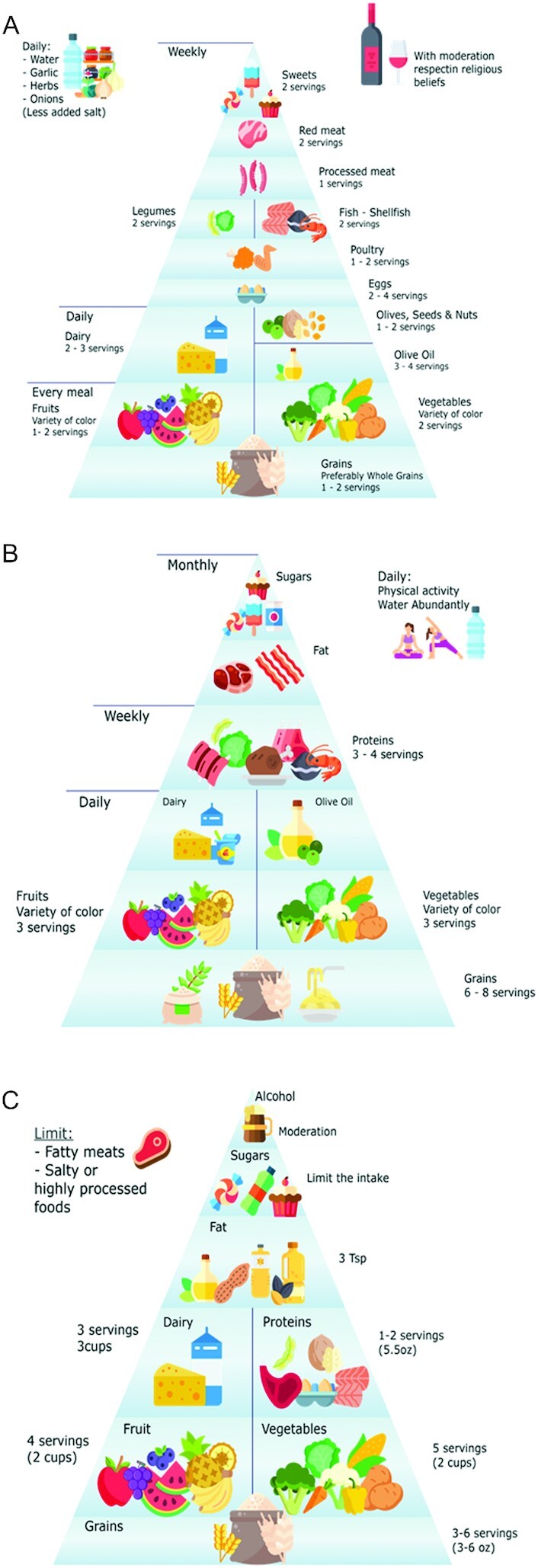
Food pyramids for the Mediterranean diet (A), Atlantic diet (B), and the Dietary Guidelines for Americans (C). Tsp: teaspoon. Panels A–C adapted with permission from references 28, 30, and 2, respectively.
The AD is closely related to the MD. It encompasses different regions, lifestyles, climate, and geography and is principally found among the countries located along the Atlantic and Mediterranean coasts of Europe (3). As illustrated in Figure 1A, B, the amount and frequency of fruits, vegetables, grains, dairy, protein, and fat consumed is the same for the MD and AD; however, the number of servings varies in the AD and provides more nutrients. Another difference concerns a more significant intake of fish in the AD, and it does not consider wine consumption as part of the diet (3, 32). The AHA guidelines and dietary patterns of the AD and MD diets discourage the use of ultraprocessed foods, refined sugars, and saturated fats, and promote the consumption of whole grains (rich in dietary fiber) (2, 3, 28, 29).
Vitamins, minerals, and phytochemicals provided by a balanced dietary pattern
The adage “let your food be your medicine” describes the ability of some foods to cure or prevent illnesses. At the end of the first quarter of the 20th century, these health properties were associated with vitamins, although the importance of some minerals for health was already known (33). During the final decades of the 20th century, the “biological antioxidant theory” claimed that some vitamins (E, D, C, and others) and minerals (selenium, zinc, and iron) protected the cell from oxidative damage triggered by infections (34). The association between oxidative stress, the consumption of foods rich in phenolic compounds, and the prevention of some nontransmissible diseases also emerged (35). Aside from antioxidant properties, the antimicrobial activity of polyphenols has also been highlighted (36). Currently, the scientific community has recommended the intake of vitamins, minerals, and natural compounds from a balanced diet to design effective antioxidant therapies for COVID-19 patients (15, 37); however, the dietary requirements among populations can vary.
Vitamins, minerals, and phenolic antioxidants activate the enzymatic and nonenzymatic antioxidant response. Vitamin C is perhaps the most water-soluble antioxidant that acts as a reducing agent and is significantly present in nature, though synthetic forms are available (38). Vitamin E, a potent liposoluble antioxidant in the form of α-tocopherol and tocotrienols, is considered one of the most active micronutrients that modulates the immune function and acts directly in protecting cell integrity (39). Vitamin A and related retinols modify the immune system through the expression of essential antibodies that eliminate viruses (40). Specifically, vitamin A has been related to improving lower respiratory tract infections in children; thus, in developing countries, its addition to some foods is mandatory (41). Liposoluble vitamin D also exhibits potent activity against respiratory diseases. This vitamin can be obtained from food products such as fish oil and can be synthesized under exposure to short periods of sunlight (6). Vitamin D is also involved in the synthesis of the antimicrobial peptides defensin and cathelicidin, which provide natural defenses against potential microbiological pathogens (42). Deficiencies of liposoluble vitamins are a concern because their absence in the diet can increase the risk of chronic diseases, autoimmune disorders, and infections (43). Other vitamins, such as those from the B complex, have also been suggested to positively affect the immune system, particularly vitamin B-9 (folate) and vitamin B-12 (cobalamin). Their deficiency dramatically inhibits the production of nucleic acids, proteins, and the activity of immune cells and other metabolic processes responsible for immunity (44, 45).
The enzymatic antioxidant response requires specific metals to act as cofactors to initiate protective activity. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) are the key enzymes responsible for the innate antioxidant response in aerobic organisms (38). Due to the presence of metals, these enzymes catalyze the breakdown of ROS and, in most cases, yield water and oxygen, which results in cell detoxification (46). Zinc, manganese, and copper modulate the activity of SOD, which converts the superoxide radical (O2−•) into hydrogen peroxide (H2O2) and molecular oxygen (O2) (16). Zinc activates antimicrobial mechanisms such as polymorphonuclear cells, macrophages, and natural killer cells (9). To reduce peroxides to water and molecular oxygen, CAT requires manganese as a cofactor (38). Likewise, selenium is crucial for the action of GPx, which breaks down lipid peroxides into alcohol and peroxides into water (47).
Phenolic compounds form part of the nonenzymatic antioxidant response. Polyphenols are a broad group of biologically active molecules present in plants (especially fruits and vegetables) with health-promoting benefits (48). The elemental phenolic composition consists of ≥1 aromatic ring and a hydroxyl group (OH). However, they can vary from single molecules to highly hydroxylated and complex polymers (49). Typically, polyphenols are divided into flavonoids and nonflavonoids, the former displaying interesting bioactive effects that rely on the structure-activity relation (50, 51). Thus, the hydroxylation pattern, that is, the number and position of OH and CH2 groups and their conjugation with other phenolic compounds such as phenolic acids and sugars, influences their antioxidant and antimicrobial activity (50–53). The reduction of free radicals by proton donation, the scavenging capacity of ROS, and the chelating activity of pro-oxidant metals are the antioxidant mechanisms underlying phenolic compounds and have been widely documented in previous studies. In addition, national institutions such as the FDA and Department of Agriculture in the United States and eBasis in the European Union have contributed to creating a database of the phenolic constituents in food and their effects on human well-being (49).
Viral Pandemics Associated with Respiratory-like Illnesses
Respiratory-related viral infections have affected the global population at different times. The oldest documented viral pandemic associated with a respiratory tract illness killed >30 million people in ∼6 mo (during 1918 and 1919) (24). Likewise, from 2009 to late 2010, another outbreak claimed over 18,449 lives (54). In both instances, influenza A H1N1, an RNA virus belonging to the Orthomyxoviridae family, was the underlying cause. Symptoms of these viral infections varied from fever, gastrointestinal problems, and cough to severe complications including pneumonia and acute respiratory distress syndrome leading to organ failure, encephalopathy, and death (55). In February 2020, the WHO announced a new threat that was rapidly spreading across the globe (56, 57). This global emergency was caused by SARS-CoV-2, an RNA virus belonging to the Coronaviridae family (56). Previously, viruses from this same family, namely severe acute respiratory syndrome (SARS)-CoV and the Middle East respiratory syndrome (MERS)-CoV, which occurred in 2002 and 2012, respectively, caused deadly pneumonia outbreaks (56, 58). As with influenza, SARS-CoV-2 is highly contagious and primarily transmitted through human-to-human contact. Some of its symptoms are difficult to distinguish from regular influenza, which might have contributed to its rapid spread in many countries (57, 58). To combat this threat, therapeutic drugs, vaccines, and the development of collective, acquired immunity are considered as possible solutions (16); however, for all of these scenarios, their implementation is a race against time (10). Therefore, one decisive measure that can be taken now is to stimulate the antioxidant response to reduce risk.
Social oxidative stress during viral pandemics
Stress is an inevitable part of human life and is a biological and psychological response to specific situations. However, exposure to prolonged periods of stress can provoke an adverse effect on mental health and decrease the antioxidant response (59). Stressful stimuli combined with a weak nutritional profile (e.g., a high fat intake and low antioxidants) can cause an oxidative imbalance, which manifests as an increase in the generation of ROS, provoking a burst of oxidative stress. This oxidative stress can cause oxidative damage to lipids, proteins, and DNA (17) and lead to alterations that can affect brain functioning. The brain, in particular, is highly sensitive to oxidative damage due to its consumption of large amounts of oxygen and the production of free radicals, which can raise the risk of mental disorders such as depression, anxiety, schizophrenia, bipolar disorder, or psychosis (60). Moreover, Filipović et al. (61) have suggested that stress can disrupt the redox homeostasis of the organism, thus diminishing immunity.
As the COVID-19 pandemic advances, governments have taken restrictive measures such as quarantines and social distancing to slow the viral spread. However, in managing a pandemic, one should not ignore the emotional toll that can produce severe mental health effects. Social isolation can lead to increased anxiety, boredom, stress, and depression due to negative emotions such as fear, anger, irritability, frustration, insomnia, mood disorders, and loneliness (62, 63). Episodes of anxiety and depression can be intensified by other factors such as fear of being infected, social exclusion, or stigmatization for having the virus, uncertainty about employment, and anguish over being separated from family and friends. For the latter, social networks have played a vital role and are an invaluable tool that have allowed people to maintain communication with others. However, studies have also shown that these social networks can influence people's behavior and create a climate of global nervousness due to rumors and misinformation—something that also occurred during the Zika and Ebola epidemics (64, 65). Furthermore, having greater access to news can become overwhelming and, in some instances, can consist of inaccurate content or fake news, which can increase negative feelings such as despair, sadness, and anxiety (64).
Public health specialists have suggested that the COVID-19 pandemic will generate unparalleled psychological distress in the global population (66). This idea is supported by studies on the psychological effects of previous pandemics, which have not shown encouraging results. The reviews by Brooks et al. (67) and Shah et al. (68) indicated that psychological responses to other pandemics and epidemics, including SARS, H1N1 influenza, MERS, equine influenza, and Ebola, were associated with a high prevalence of depressive and posttraumatic stress symptoms. Although these symptoms can decrease after an outbreak, in some cases they had effects lasting ≤3 y after the health emergency. In the specific case of SARS, there are reports that nearly 18% of the population in Hong Kong reported symptoms related to posttraumatic stress, anxiety, and depression (69). Slightly higher rates have been reported for Toronto, where ∼30% of individuals had symptoms of posttraumatic stress and depression (70). Regarding the COVID-19 pandemic, current reports thus far indicate a 7% prevalence of posttraumatic stress symptoms within the affected population in China (71).
Therefore, it is essential to emphasize the mental well-being of a population through the promotion of healthy lifestyles. One focus could be on the consumption of healthier foods, because studies have associated psychological disorders with poor nutritional status due to inadequate nutrient intake (67, 68). There is a connection between the brain and the gastrointestinal tract, indicating that the composition of the gut microbiota can positively influence the brain (72). Moreover, the gastrointestinal microbiota appears to play an essential role in immune functioning and responding to stress episodes (73). Gubert et al. (74) have suggested that diets rich in bioactive compounds such as polyphenols, vitamins C, E, B-12, and B-9 (folate), carotenoids, and ω-3 fatty acids can modulate the detrimental effects of oxidative stress. They also suggested that healthy dietary patterns can increase the availability of macro and micronutrients in the gut due to changes in the composition and functionality of gut microbiota, which could reduce the risk of having mental health issues.
The Impact of a Balanced Diet During the COVID-19 Pandemic
COVID-19 principally affects the lower respiratory tract and, compared with SARS and MERS, is less virulent, with the latest reports indicating that ∼81% of cases present with mild symptoms and 1.2% are asymptomatic (75). The mortality rate is also lower compared with MERS (56). However, the principal concern is the high transmissibility (56). Although SARS-CoV-2 does not discriminate between populations, children and young adults (aged 18–30 y) are unlikely to suffer severe symptoms. In contrast, adult males (average age 47 y) display a high incidence of serious symptoms. For the elderly (aged >60 y) and patients with pre-existing conditions, infection has been shown to be quite severe, with a high mortality rate (76).
Currently, measures such as social distancing, isolation, and vigilant hygiene are recommended by the WHO (77). However, what is the required amount of antioxidants in a balanced diet to combat oxidative stress in COVID-19 patients is a question that has yet to be answered. Currently, antioxidant therapy in noncritical patients consists primarily of doses of vitamin C, D, and E that exceed normal intake levels (14, 78, 79). Furthermore, exploratory recommendations regarding nutritional interventions have been made that include the use of plant biomolecules such as polyphenols that were used during past viral epidemics (11, 15, 37, 80). However, if we consider the oxidative stress caused by the infection (even if it is asymptomatic) and the social stresses surrounding the pandemic, one must consider the following questions: Are traditional balanced diets adequate? Is our antioxidant status prepared to manage the stress? How can diets be improved based on age group and nutritional status?
As shown in Table 1, not all diets provide the recommended amount of vitamin D (20 μg/d). Only the MD appears to supply nearly all of the antioxidant requirements. Although it is important to consider that vitamin D can be synthesized following exposure to the sun, in the context of COVID-19, sun exposure can be affected by confinement measures. For the vitamins mentioned above, additional consumption should be considered. For vitamin C, the diets are all within the recommended ranges of 100 to 200 mg/d (103 mg/d for the AmD, 137.2–269.8 mg/d for the MD, and 84–175 mg/d for the AD). For minerals, the 3 diets have a selenium consumption that exceeds the recommendation of 55 μg/d (81), whereas for zinc, iron, and manganese, the diets meet the recommended requirements. In the case of polyphenols, all 3 diets exceed recommendations, although the AmD delivers the lowest amount of the 3: it contributes 498–662 mg/d (82), whereas the MD and AD have values of 1171 mg/d and 1011–1284 mg/d, respectively (71, 74).
TABLE 1.
Daily antioxidant intakes provided by the Mediterranean, Atlantic, and American (Dietary Guidelines for Americans) diets1
| Provided by the balanced diet | ||||
|---|---|---|---|---|
| Antioxidant | Recommended | MD | AD | AmD |
| Vitamins | ||||
| D,2 μg/d | 20.00 (42) | 0.30–3.80 (1) | 0.80–3.40 (87) | 3.75–7.50 (31) |
| E, mg/d | 15 (88) | 17.00 (89) | 3.50–11.25 (87) | 5.80–7.80 (90) |
| C, mg/d | 100–200 (91) | 137.20–269.81 (29) | 84–175 (87) | 103.00 (31) |
| A,3 μg/d | 300–1300 (92) | 1273.3 (93) | 1404 (94) | 600–770 (95) |
| B-12, μg/d | 0.9–2.8 (92) | 1.50–2.00 (96) | 2.30–3.70 (87) | 1.80–2.80 (97) |
| Folate, μg/d | 150–600 (92) | 455.10–561.30 (31) | 157–259 (87) | 190.00 (98) |
| Minerals | ||||
| Se, μg/d | 55 (81) | 120.00 (4) | 26.40–59.80 (30) | 93–134 (99) |
| Zn, mg/d | 8–11 (97) | 11.00 (89) | 7.98–13.30 (87) | 9.60 (31, 100) |
| Fe, mg/d | 8–18 (97) | 15.00–18.00 (89) | 10.30–14.90 (87) | 15.30–15.50 (101) |
| Mn, mg/d | 1.60–2.30 (97) | 5.21–12.48 (102) | 3.00 (103) | 2.80 (31) |
| Polyphenols, mg/d | 396–593 (104)4 | 1171.00 (82) | 1011–1284 (105) | 498–662 (106) |
Numbers in parentheses denote references. AD, Atlantic diet; AmD, American diet; MD, Mediterranean diet.
As cholecalciferol, 1 μg cholecalciferol = 40 IU vitamin D, assuming minimal sunlight exposure (107).
RAE (retinol activity equivalents) = 1 μg retinol, 12 μg β-carotene, 24 μg α-carotene, or 24 μg β-cryptoxanthin. The RAE for dietary provitamin A carotenoid is 2-fold greater than retinol equivalents (REs), whereas the RAE for preformed vitamin A is the same as RE (107).
Recommendation to favor the immune system through gut microbiota.
A randomized pilot study with HIV patients was conducted to evaluate the feasibility of the MD to reduce dyslipidemia and, hence, cardiovascular risk. Findings from the HIV population could help us better understand the adequacy of this diet for the broader community (83). The results indicated that the phytochemicals and nutrients provided by the MD greatly improved systolic blood pressure compared with the low-calorie diet. Another study established the relation between microbial agents, including cytomegalovirus, herpes simplex, hepatitis C virus, and transmissible spongiform encephalopathies, and obesity. In that study, the researchers discovered that these viruses can alter host metabolism, which could then translate into obesity. The MD was shown to be useful in reducing the risk of obesity and infection (82).
Although studies have reported the benefits of the MD, there are no conclusive data on the need to supplement a balanced diet for either healthy individuals or those at risk, such as elders, during a complicated episode such as the current pandemic. Thus far, there have been no reports regarding the influence of balanced dietary patterns on COVID-19 patients.
However, there are studies that indicate changes in the eating habits of different populations during the COVID-19 pandemic. Among these changes there has been an increase in the consumption of legumes, vegetables, and fruit (84–86); the WHO recommends consumption of these foods during quarantine as an important part of a healthy diet. In particular, studies have reported that the populations of northern and central Spain and Italy have adhered to the MD specifically because of its capacity to strengthen the immune system given the high contributions of micronutrients and antioxidants the diet provides (85, 86). Similarly, another important factor observed in these studies is that families have had more time to cook, which might have improved diets in general (108). However, a study in Spain showed an increase of 539 kcal more than the recommended amount during confinement (109). This increase was related to a higher intake of snacks such as chips or cookies (110), sugary drinks (84), fried foods (111), and sweets (86). This increased consumption of unhealthy, comfort foods has likely helped to reduce stress and boredom caused by the pandemic (108).
Oxidative Stress in Viral Infections and the Role of Dietary Antioxidants
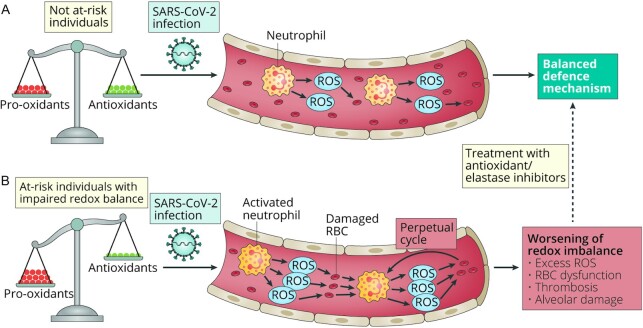
The enzymatic and nonenzymatic actors acting against oxidative stress boost the immune response during viral infections. The imbalance between oxidant production and antioxidant defenses determines the oxidative damage, including lipid peroxidation and DNA oxidation, leading to ROS production and inflammation via IL-6 production, that occurs during viral infections, such as COVID-19 (17, 112). In this sense, the respiratory viral infections have been associated with inhibition of the master redox-sensitive transcription factor nuclear factor erythroid 2–related factor 2 (NRF2) which is an emergent regulator of cellular resistance to oxidants. In fact, patients with COVID-19 have revealed high neutrophil infiltration in pulmonary capillaries that can result in exacerbated ROS release, and in the worst scenarios lead to RBC dysfunction, thrombosis, and alveolar damage (Figure 2) (113, 114) The deregulation of redox balance can have a more pronounced impact on vulnerable populations, namely those with a poor diet, obesity, or noncommunicable diseases (diabetes, cardiovascular diseases) (115). Thus, Laforge et al. (113) recently suggested the increment of free radical scavengers as a beneficial strategy against the aforementioned pathological responses.
FIGURE 2.
SARS-CoV-2 infection can lead to neutrophilia-induced ROS release. A) In not at-risk individuals, an excess of reactive oxygen species (ROS) is counterbalanced by an increase in antioxidant defenses. (B) In subjects with impaired redox balance, ROS production is not properly controlled, leading to RBC membrane peroxidation, which in turn perpetuates neutrophil activation. Excessive oxidative stress might be responsible for the alveolar damage, thrombosis, and RBC dysregulation seen in COVID-19. Antioxidants and elastase inhibitors could have therapeutic potential. Reprinted by permission from reference 113.
The promising free radical scavengers are the dietary antioxidants, and several studies in vitro and in vivo, and epidemiological evidence have suggested the importance of nutritional status in viral infections. Deficiencies in selenium and vitamin E in the diet have been shown to increase virulence in viral infections, which has led to increased severity (12, 13). Similarly, studies performed under controlled conditions using mouse models have evaluated coxsackievirus infection that induced myocarditis in 2 mouse groups: 1 that was deficient in selenium and vitamin E and 1 that received the recommended amounts. The results demonstrated that the subsequent pathologies were significantly higher in the undernourished mice. Overall, it was shown that virulence could be dramatically increased if antioxidant intake was inadequate. Similarly, there is in vitro evidence indicating that selenium and vitamin E are more effective in protecting Jurkat cell cultures than other antioxidants such as vitamin C, phenolics, glutathione, and N-acetylcysteine against ROS and lipid peroxidation by assisting in performance of detoxifying enzymes (47). Moreover, recently a combination of antioxidants has been purposed as a therapeutic to be used to target neutrophils in patients with severe COVID-19 (112, 113). In addition, Sgarbanti et al. (16) reported that ROS oxidized vitamin E, decreasing its activity. Thus, combining vitamin E with other antioxidants, namely vitamin C, protects vitamin activity. Moreover, epidemiological reviews discussing the importance of vitamins in viral respiratory diseases have highlighted the relevance of micronutrients in overcoming viral infections and other health conditions (41, 42, 116).
Multiple reviews have also focused on the effect of tea catechins concentrated in green tea and the oxidized tea flavins from black tea on antiviral and anti-inflammatory activities (117–119). The mechanism and characteristics of phenolic compounds to fight viral infections and their potential to be used as emerging therapeutic drugs have been reviewed (120, 121) and compared with commonly used antivirals (16, 55). Although phenolic compounds trigger the immune response, either by reducing ROS, activating enzymatic responses, or inactivating pathogens, the mechanism of absorption and the mode of action in human plasma remains unclear (21). Nonetheless, recent findings have suggested that phenolics affect immune functioning via microbiota modulation (122, 123). Polyphenols in the colon undergo several chemical reactions, including hydrolysis, reduction, decarboxylation, demethylation, and dehydroxylation, which modify their structure; hence, their bioactivity and the microbial composition in the intestine are altered (124). Furthermore, and in agreement with recent reviews (122, 104), a meta-analysis indicated that immunomodulatory bacteria, for example, Lactobacillus, Bifidobacterium, and Clostridium species, were stimulated at levels of phenolic intake of 396 mg/d, 540 mg/d, and 593 mg/d, respectively (104). However, phenolic concentrations outside these ranges did not lead to adverse effects on the microbiota.
Reinforced Balanced Diets, Aging, and Physical and Mental Status
Supplementation has been shown to improve health status synergistically with a balanced diet (125). For example, zinc plays an essetntial role in human health. In the case of individuals with zinc deficiency, supplementation improves the antiviral response and, in healthy individuals, helps to inhibit viral replication or the symptoms it causes (126). A meta-analysis by Wang et al. (127) found that zinc supplementation reduced episodes of the common cold by ≤53% in healthy children aged <10 y. This supplementation can also significantly reduce the duration of illness in cases of pneumonia (128). Similarly, Shaker et al. (129) have suggested that zinc or concurrent zinc and vitamin A therapy assist in fighting acute upper respiratory infections by improving the immune status in children aged between 2 and 12 y. Single supplementation of zinc improved the respiratory infections. Kurugöl et al. (130) and Singh et al. (131) have demonstrated that intake >15 mg/d reduced the duration of cold symptoms in children and healthy individuals, respectively. In contrast, Wang et al. (127) indicated that administering zinc during an infection could create a microenvironment that is more favorable for pathogen growth, and excessive zinc intake over prolonged periods could cause toxicity and diminished copper absorption (132). Regarding selenium supplementation, Rayman (99) and Broome et al. (133) have suggested that selenium improves the immune response to viruses in individuals with deficiencies and has an antiviral effect. In the Broome et al. study (133), clinical trials showed a selenium intake between 50 μg/d and 100 μg/d in adults in the United Kingdom eliminated viruses more rapidly than in a placebo group. Steinbrenner et al. (134) also reviewed supplementation with selenium ≤200 μg/d and found it could be used as an adjuvant therapy in the treatment of type A influenza virus. Moreover, asthmatic adults displayed clinical improvement upon supplementation with selenium (135). Regarding iron intake, Richard et al. (136) and Gera and Sachdev (137) clinically investigated and systematically reviewed the idea that iron supplementation reduced the risk of respiratory infection in children. The first authors indicated that in children in Peru aged 0.5–15 y the combination of iron and zinc provided protection against malaria. The latter authors systematically reviewed the oral or parenteral iron supplementation or fortified formula milk or cereals, and indicated a positive effect in reducing individual illnesses, including respiratory tract infection, although a risk of diarrhea was considered. Additionally, a literature review conducted by Maggini et al. (138) has suggested that iron can enhance or protect individuals from infection by bacteria and viruses, with its action depending on the level of intake.
For phenolic content, especially the catechins from tea, it has been observed that the equivalent of 10 cups (250 mL)/d (100 mg epigallocatechin‐3‐gallate per cup) had a positive effect in adults with influenza (118, 139). Likewise, supplementation with encapsulated juices from fruits and vegetables was shown to reduce the incidence of the common cold in 20% of adults (140).
Additional factors to consider regarding antioxidant intake for nutritional interventions during viral pandemics are age, and physical and psychological variables. Aside from nutrition, aging is a factor that influences antioxidant intake and the latter should be increased over time (141). Immunosenescence is the loss of the capacity of the immune system to effectively protect the organism and affects the ability of the elderly to overcome respiratory viral infections. Accordingly, an increase in antioxidant consumption has been shown to decrease the morbidity and mortality of this age group (141). In one study, influenza-infected mice with increased vitamin E intake (500 mg/kg) were able to significantly reduce their viral titers in the older group whereas little change was detected in the younger group (39). Likewise, in humans, it has been observed that supplementation with antioxidants positively improves the reaction against viral infections in the very young and elderly; however, in young adults no significant results have been observed (125, 45). A meta-analysis by Martineau et al. (116), demonstrated that vitamin D supplementation in humans (from 0 to 93 y of age) in daily doses <800 IU/d, 800–1999 IU/d, and ≥2000 IU/d resulted in reduced risk of acute respiratory tract infections, and the authors suggested that dietary supplementation should be adopted. For vitamin C, a study conducted in an elderly population found that consumption of 121 ± 54 mg/d and 118 ± 34 mg/d for men and women, respectively, decreased the incidence of influenza (87). In general, vitamin C supplementation above dietary recommendations decreases the incidence and negative impact of the symptoms during viral infections in both animal models and humans (6, 39, 125, 141).
A review has reported that obese (BMI ≥30 kg/m2) individuals need an antioxidant intake that is 2–3 times greater than that needed by nonobese individuals (43). If we consider the obese to be a particularly high-risk group during the COVID-19 pandemic, it could be due to their poor antioxidant status (142).
In regard to psychological status, in a study by Godos et al. (143), foods rich in phenolic compounds from the MD were provided to depressed adults. They reported that intake phenolic acids and flavonoids, especially anthocyanins, were inversely associated with depression.
Despite the lack of information regarding nutritional interventions and dietary supplementation during the COVID-19 pandemic, some authors have highlighted their importance. For instance, the recovery and mortality rates of COVID-19 patients in 2 cities in Wuhan, China—one with a high selenium intake from diet (3.13 ± 1.91 mg/kg for females and 2.21 ± 1.14 mg/kg for males) and other with low rates (0.5 mg/kg)—were investigated. The results indicated that the recovery rate was higher and the mortality rate lower (P ˂ 0.0001) in the high selenium–intake city in comparison to the low selenium–intake city (144). In another study investigating the efficacy of antioxidant supplementation in COVID-19 patients, 2840 IU vitamin A, 1.2 mg β-carotene, 205 mg vitamin C, 75 IU vitamin E, 18 μg selenium, and 5.7 mg zinc were administered daily. Results from this study are expected to be obtained at the end of 2020 (145). Similarly, Caccialanza et al. (146) provided 25-hydroxyvitamin D [25(OH)D] to noncritical COVID-19 patients if they were shown to be deficient. They adjusted the concentration of vitamin D in the blood with an intake of 50,000 UI/wk if 25(OH)D was <20 ng/mL, and an intake of 25,000 UI/wk if 25(OH)D was ≥20 and <30 ng/mL.
Others authors have reported similar supplementations to treat COVID-19 patients, with 24 g vitamin C (78), 200 g zinc (79), and 200,000–300,000 IU vitamin D (57) per day, which were observed to be effective. In regard to phenolic compound intake, a systematic review of the role of flavonoids against COVID-19 (147) found that these phytochemicals inhibited the viral infective cycle by deactivating the involved proteins. Thus, they are capable of synergistically acting with others drugs against COVID-19. Although this is the first study, to the best of our knowledge, comparing the antioxidant value of principal Western dietary patterns, others authors have suggested nutritional interventions and antioxidant supplementation during the current COVID-19 crisis, and perhaps for future pandemics (147–149).
Conclusions
The current SARS-CoV-2/COVID-19 pandemic has caused severe infections and social disruptions that can result in high oxidative stress among populations. High ROS production can lead to decreased immunity in the organism and, thus, increased vulnerability to viral infections. To fight this imbalance, obtaining natural antioxidants from food is crucial. Usually, a traditional, balanced diet is meant to provide all of the nutritional requirements and antioxidants needed to maintain well-functioning antioxidant enzymatic and nonenzymatic responses that support the immune system. In particular, the MD has been shown to contain most of the essential micronutrients and phenolics required for normal functioning. However, the requirements for antioxidant intake, especially among high-risk populations, are different. Evidence suggests that supplementation with minerals, vitamins, and phenolics has favorable results, especially in vulnerable adults, the obese, and individuals with depression. The effects of high oxidative environments resulting from social distress during a pandemic have been poorly considered. In general, studies linking dietary patterns and pandemics are scarce. Consequently, it is essential to research the effects of a balanced and supplemented diet on the physiological and psychological well-being of individuals.
Acknowledgments
We are grateful to Annunziata D'Alessandro, Manuela Vaz Velho, the American Heart Association, and Springer Nature for permitting the adaptation of the informative material. All copyright permissions have been obtained and are available upon request.
The authors’ responsibilities were as follows—all authors: contributed equally to the present work and read and approved the final manuscript.
Notes
Supported by the Agencia Nacional de Investigación y Desarrollo (ANID) [CONICYT] for scholarship no. 21171483, and by financial support from FCT (Fundação para a Ciência e a Tecnologia) under the framework of the project PTDC/SAU-NUT/30322/2017.
Author disclosures: The authors report no conflicts of interest.
All authors contributed equally to the present work.
Abbreviations used: AD, Atlantic diet; AmD, American diet; CAT, catalase; CoV, coronavirus; GPx, glutathione peroxidase; MD, Mediterranean diet; MERS, Middle East respiratory syndrome; ROS, reactive oxygen species; SARS, severe acute respiratory syndrome; SOD, superoxide dismutase; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Igor Trujillo-Mayol, Departamento de Ingeniería de Alimentos, Universidad del Bío-Bío, Chillán, Chile.
María Guerra-Valle, Departamento de Ingeniería de Alimentos, Universidad del Bío-Bío, Chillán, Chile.
Nidia Casas-Forero, Departamento de Ingeniería de Alimentos, Universidad del Bío-Bío, Chillán, Chile.
M Madalena C Sobral, LAQV/REQUIMTE, Laboratório de Bromatologia e Hidrologia, Departamento de Ciências Químicas, Faculdade de Farmácia da Universidade do Porto, Porto, Portugal.
Olga Viegas, LAQV/REQUIMTE, Laboratório de Bromatologia e Hidrologia, Departamento de Ciências Químicas, Faculdade de Farmácia da Universidade do Porto, Porto, Portugal; Faculdade de Ciências da Nutrição Alimentação da Universidade do Porto, Porto, Portugal.
Julio Alarcón-Enos, Laboratorio de Síntesis y Biotransformación de Productos Naturales, Facultad de Ciencia, Universidad del Bío-Bío, Chillán, Chile.
Isabel Mplvo Ferreira, LAQV/REQUIMTE, Laboratório de Bromatologia e Hidrologia, Departamento de Ciências Químicas, Faculdade de Farmácia da Universidade do Porto, Porto, Portugal.
Olívia Pinho, LAQV/REQUIMTE, Laboratório de Bromatologia e Hidrologia, Departamento de Ciências Químicas, Faculdade de Farmácia da Universidade do Porto, Porto, Portugal; Faculdade de Ciências da Nutrição Alimentação da Universidade do Porto, Porto, Portugal.
References
- 1. Azzini E, Polito A, Fumagalli A, Intorre F, Venneria E, Durazzo A, Zaccaria M, Ciarapica D, Foddai M, Mauro Bet al. Mediterranean diet effect: an Italian picture. Nutr J. 2011;10(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Heart Association . What is a healthy diet?. Recommended serving infographic [Internet]. 2020; [cited 2020 May 1]. Available from: https://cpr.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/what-is-a-healthy-diet-recommended-serving-infographic
- 3. Velho MV, Pinheiro R, Rodrigues AS. The Atlantic diet – origin and features. Int J Food Stud. 2016;5(1):106–19. [Google Scholar]
- 4. Tosti V, Bertozzi B, Fontana L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol. 2018;73(3):318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. [Internet]2019;9:3160. doi:10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa H. Evidence that vitamin D supplementation could reduce the risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu D, Meydani S. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14(4):283–9. [DOI] [PubMed] [Google Scholar]
- 8. Viladomiu M, Hontecillas R, Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur J Pharmacol. 2016;785:87–95. [DOI] [PubMed] [Google Scholar]
- 9. Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9(6):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunasekera D. The next global pandemic. Chemistry in Sri Lanka. 2015;32(01):13–7. [Google Scholar]
- 11. Zhang Y, Nguyen A, Chun M, Lin Z, Ross J, Sun L. Ninety days in: a comprehensive review of the ongoing COVID-19 outbreak. Heal Sci J. [Internet]2020;14(2):706. doi:10.36648/1791-809X.14.2.706. [Google Scholar]
- 12. Beck MA, Levander OA. Dietary oxidative stress and the potentiation of viral infection. Annu Rev Nutr. 1998;18(1):93–116. [DOI] [PubMed] [Google Scholar]
- 13. Beck MA. The influence of antioxidant nutrients on viral infection. Nutr Rev. 2009;56(1):S140–6. [DOI] [PubMed] [Google Scholar]
- 14. Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S, Guido A, Sabatino A, Belliato M, Calvi Met al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang JA, Rui-Ying Z, Bai J. An anti-oxidative therapy for ameliorating cardiac injuries of critically ill COVID-19-infected patients. Int J Cardiol. 2020;312:137–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sgarbanti R, Amatore D, Celestino I, Marcocci M, Fraternale A, Ciriolo M, Magnani M, Saladino R, Caraci E, Palamara Aet al. Intracellular redox state as target for anti-influenza therapy: are antioxidants always effective?. Curr Top Med Chem. 2014;14(22):2529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwarz KB. Oxidative stress during viral infection: a review. Free Radic Biol Med. 1996;21(5):641–9. [DOI] [PubMed] [Google Scholar]
- 18. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. [DOI] [PubMed] [Google Scholar]
- 19. Ponton F, Wilson K, Cotter SC, Raubenheimer D, Simpson SJ. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 2011;7(12):e1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Del Bo C, Bernardi S, Marino M, Porrini M, Tucci M, Guglielmetti S, Cherubini A, Carrieri B, Kirkup B, Kroon Pet al. Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern?. Nutrients. 2019;11(6):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandez-Panchon MS, Villano D, Troncoso AM, García-Parrilla MC. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Crit Rev Food Sci Nutr. 2008;48(7):649–71. [DOI] [PubMed] [Google Scholar]
- 22. Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19(2):73–82. [DOI] [PubMed] [Google Scholar]
- 23. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phillips H, Killingray D, editors. The Spanish influenza pandemic of 1918–1919: new perspectives. London: Routledge; 2003. [Google Scholar]
- 25. World Health Organization (WHO) . Global health observatory. [Internet]. 2016; [cited 2020 May 1]. Available from: https://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/.
- 26. Peng C, Wang X, Chen J, Jiao R, Wang L, Li YM, Zuo Y, Liu Y, Lei L, Ying Ket al. Biology of aging and the role of dietary antioxidants. Biomed Res Int. 2014;2014:831841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo J, Mills K, le Cessie S, Noordam R, van Heemst D. Ageing, age-related diseases and oxidative stress: what to do next?. Ageing Res Rev. 2020;57:100982. [DOI] [PubMed] [Google Scholar]
- 28. D'Alessandro A, Lampignano L, De Pergola G. Mediterranean diet pyramid: a proposal for Italian people. A systematic review of prospective studies to derive serving sizes. Nutrients. 2019;11(6):1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet: a literature review. Nutrients. 2015;7(11):9139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jennings A, Tang J, Gillings R, Perfecto A, Dutton J, Speakman J, Fraser W, Nicoletti C, Berendsen A, Groot Let al. Changing from a Western to a Mediterranean-style diet does not affect iron or selenium status: results of the New Dietary Strategies Addressing the Specific Needs of the Elderly Population for Healthy Aging in Europe (NU-AGE) 1-year randomized clinical trial in elderly Europeans. Am J Clin Nutr. 2020;111(1):98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price CT, Langford JR, Liporace FA.. Essential nutrients for bone health and a review of their availability in the average North American diet. Open Orthop J(2012);6:143–9.22523525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calvo-Malvar M del M, Leis R, Benítez-Estévez AJ, Sánchez-Castro J, Gude F.A randomised, family-focused dietary intervention to evaluate the Atlantic diet: the GALIAT study protocol. BMC Public Health 2016;16:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neswald E, , Smith DF, Thoms U. Setting nutritional standards: theory, policies, practices. Rochester (NY): University of Rochester Press; 2017. [Google Scholar]
- 34. Witting LA. Vitamin E and lipid antioxidants in free-radical-initiated reactions. In: Pryor W, editor. Free radicals in biology. Academic Press;1980. pp.; 295–319. [Google Scholar]
- 35. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sánchez E, Nabavi SF, Nabavi SM. Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol Res. 2017;196:44–68. [DOI] [PubMed] [Google Scholar]
- 37. Naja F, Hamadeh R. Nutrition amid the COVID-19 pandemic: a multi-level framework for action. Eur J Clin Nutr. 2020;74:1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Hernández-Carlos B. Antioxidant compounds and their antioxidant mechanism. In Shalaby E, editor. Antioxidants. London (UK): IntechOpen; 2019; [cited 2020 May 20]. Available from: https://www.intechopen.com/books/antioxidants/antioxidant-compounds-and-their-antioxidant-mechanism. [Google Scholar]
- 39. Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71(4):487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Semba RD. The role of vitamin A and related retinoids in immune function. Nutr Rev. 2009;56(1):S38–48. [DOI] [PubMed] [Google Scholar]
- 41. Semba RD. Vitamin A and immunity to viral, bacterial, and protozoan infections. Proc Nutr Soc. 1999;58(3):719–27. [DOI] [PubMed] [Google Scholar]
- 42. Bartley J. Vitamin D, innate immunity, and upper respiratory tract infection. J Laryngol Otol. 2010;124(5):465–9. [DOI] [PubMed] [Google Scholar]
- 43. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment, and prevention. Rev Endocr Metab Disord. 2017;18(2):153–65. [DOI] [PubMed] [Google Scholar]
- 44. Mikkelsen K, Apostolopoulos V. Vitamin B12, folic acid, and the immune system. In: Mahmoudi M, Rezaei N, editors. Nutrition and immunity. Cham: Springer; 2019. pp. 103–14. 10.1007/978-3-030-16073-9_6. [DOI] [Google Scholar]
- 45. Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22(1):115–25. [DOI] [PubMed] [Google Scholar]
- 46. Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. [DOI] [PubMed] [Google Scholar]
- 47. Saito Y, Yoshida Y, Akazawa T, Takahashi K, Niki E. Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem. 2003;278(41):39428–34. [DOI] [PubMed] [Google Scholar]
- 48. Lule SU, Xia W. Food phenolics, pros and cons: a review. Food Rev Int. 2005;21(4):367–88. [Google Scholar]
- 49. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, Novellino E, Santini A. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33(9):2221–43. [DOI] [PubMed] [Google Scholar]
- 50. Wang T, Li Q, Bi K. Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci. 2018;13(1):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–84. [DOI] [PubMed] [Google Scholar]
- 52. Nemzer BV, Yashin AY, Vedenin AN, Yashin YI, Yashunsky DV, Nifantiev NE, Kalita D. Selected powerful natural antioxidants: structure, food sources, antioxidant activities, and important health benefits. J Food Res. 2019;8(1):60. [Google Scholar]
- 53. Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23(2):174–81. [DOI] [PubMed] [Google Scholar]
- 54. Rajasekaran D, Palombo EA, Yeo TC, Ley DLS, Tu CL, Malherbe F, Grollo L. Evidence of synergistic activity of medicinal plant extracts against neuraminidase inhibitor resistant strains of influenza viruses. Adv Microbiol. 2014;04(16):1260–77. [Google Scholar]
- 55. Uchide N, Ohyama K, Toyoda H. Current and future anti-influenza virus drugs. Open Antimicrob Agents J. 2010;2(1):34–48. [Google Scholar]
- 56. Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspective. Int J Antimicrob Agents. 2020;55:105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wamilawansa SJ. Global epidemic of coronavirus – Covid-19: what can we do to minimize risks?. Eur J Biomed Pharm Sci. 2020;7(3):432–8. [Google Scholar]
- 58. Arshad A, Baloch M, Ahmed N, Arshad A, Iqbal A. The outbreak of coronavirus disease 2019 (COVID-19)—an emerging global health threat. J Infect Public Health. 2020;13(4):644–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schiavone S, Colaianna M, Curtis L. Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress. Curr Pharm Des. 2015;21(11):1404–12. [DOI] [PubMed] [Google Scholar]
- 60. Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. 2014;12:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Filipović D, Todorović N, Bernardi RE, Gass P. Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive- and anxiety-like symptoms. Brain Struct Funct. 2017;222(1):1–20. [DOI] [PubMed] [Google Scholar]
- 62. Orrù G, Ciacchini R, Gemignani A, Conversano C. Psychological intervention measures during the COVID-19 pandemic. Clin Neuropsychiatry. 2020;17(2):76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Usher K, Bhullar N, Jackson D. Life in the pandemic: social isolation and mental health. J Clin Nurs. 2020;29:2756–7. [DOI] [PubMed] [Google Scholar]
- 64. Sharma MK, Anand N, Vishwakarma A, Sahu M, Thakur PC, Mondal I, Sing P, Suma N, Biswas A, Archana Ret al. Mental health issues mediate social media use in rumors: implication for media based mental health literacy. Asian J Psychiatry. 2020;53:102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huremović D. Brief history of pandemics. In: Huremović D, editor. Psychiatry of pandemics: a mental health response to infection outbreak. Cham: Springer;2019. pp.; 7–36. [Google Scholar]
- 66. Sritharan J, Sritharan A. Emerging mental health issues from the novel coronavirus (COVID-19) pandemic. J Heal Med Sci. 2020;3(2):157–62. [Google Scholar]
- 67. Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shah K, Kamrai D, Mekala H, Mann B, Desai K, Patel RS. Focus on mental health during the coronavirus (COVID-19) pandemic: applying learnings from the past outbreaks. Cureus. 2020;12(3):e7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu KK, Chan SK, Ma TM. Post-traumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS). J Traum Stress. 2005;18(1):39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu N, Zhang F, Wei C, Jia Y, Shang Z, Sun L, Wu L, Sun Z, Zhou Y, Wang Yet al. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatry Res. 2020;287:112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tabrizi A, Khalili L, Homayouni-Rad A, Pourjafar H, Dehghan P, Ansari F. Prebiotics, as promising functional food to patients with psychological disorders: a review on mood disorders, sleep, and cognition. NeuroQuantology. 2019;17(6):1–9. [Google Scholar]
- 73. Rieder R, Wisniewski PJ, Alderman BL, Campbell SC. Microbes and mental health: a review. Brain Behav Immun. 2017;66:9–17. [DOI] [PubMed] [Google Scholar]
- 74. Gubert C, Kong G, Renoir T, Hannan AJ. Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol Dis. 2020;134:104621. [DOI] [PubMed] [Google Scholar]
- 75. Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, Megawati D, Hayati Z, Wagner A, Mudatsir M. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13(5):667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tu H, Tu S, Gao S, Shao A, Sheng J. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. 2020;81(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). [Internet]. 2020; [cited 2020 Jun 27]. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 78. Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hernández A, Papadakos PJ, Torres A, González DA, Vives M, Ferrando C, Baeza J. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Rev Esp Anestesiol Reanim. 2020;67(5):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Institute of Medicine . Dietary reference intakes for vitamin A, vitamin K. arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 82. Saura-Calixto F, Goñi I. Antioxidant capacity of the Spanish Mediterranean diet, Food Chem. 2006;94(3):442–7. [Google Scholar]
- 83. Stradling C, Thomas N, Taylor S, Das S, Ross J, Taheri S. ‘Best foods for your heart’: a pilot randomized controlled trial of dietary intervention to reduce cardiovascular risk in HIV dyslipidemia. Atherosclerosis. 2016;255:5. [Google Scholar]
- 84. Pietrobelli A, Pecoraro L, Ferruzzi A, Heo M, Faith M, Zoller T, Antoniazzi F, Piacentini G, Fearnbach S, Heymsfield S. Effects of COVID‐19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity. 2020;28(8):1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attiná A, Cinelli G, Leggeri C, Caparello G, Barrea L, Scerbo Fet al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rodríguez-Pérez C, Molina-Montes E, Verardo V, Artacho R, García-Villanova B, Guerra-Hernández E, Ruíz-López M. Changes in dietary behaviours during the COVID-19 outbreak confinement in the Spanish COVIDiet study. Nutrients. 2020;12(6):1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vaquero MP, Sánchez-Muniz FJ, Carbajal A, Carmen García-Linares M, Camino García-Fernández M, Trinidad García-Arias M. Mineral and vitamin status in elderly persons from Northwest Spain consuming an Atlantic variant of the Mediterranean diet. Ann Nutr Metab. 2004;48(3):125–33. [DOI] [PubMed] [Google Scholar]
- 88. Maras JE, Bermudez OI, Qiao N, Bakun PJ, Boody-Alter EL, Tucker KL. Intake of α-tocopherol is limited among US adults. J Am Diet Assoc. 2004;104(4):567–75. [DOI] [PubMed] [Google Scholar]
- 89. Mesías M, Seiquer I, Navarro MP. The Mediterranean diet and mineral composition. In: Preedy V, Watson RR, editors. The Mediterranean diet: an evidence-based approach. Amsterdam: Elsevier;2015. pp. 185–98. [Google Scholar]
- 90. Bailey RL, Fulgoni VL, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet. 2012;112(5):657–63. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Carr AC, Maggini S. Vitamin C, and immune function. Nutrients. 2017;9(11):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. National Academies of Sciences . Dietary Reference intakes (DRIs): recommended dietary allowances and adequate intakes, vitamins. [Internet]. 2011; [cited 2020 May 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56068/table/summarytables.t2/?report=objectonly.
- 93. Castañé S, Antón A. Assessment of the nutritional quality and environmental impact of two food diets: a Mediterranean and a vegan diet. J Cleaner Prod. 2017;167:929–37. [Google Scholar]
- 94. Esteve-Llorens X, Darriba C, Moreira M, Feijoo G, González-García S. Towards an environmentally sustainable and healthy Atlantic dietary pattern: life cycle carbon footprint and nutritional quality. Sci Total Environ. 2019;646:704–15. [DOI] [PubMed] [Google Scholar]
- 95. National Institutes of Health (NIH) . Vitamina A. [Internet]. 2020; [cited 2020 May 20]. Available from: https://ods.od.nih.gov/factsheets/VitaminA-DatosEnEspanol/
- 96. Llamas FP, Roldán CM. Concepto de dieta prudente. Dieta Mediterránea. Ingestas recomendadas. Objetivos nutricionales. Guías alimentarias. Manual practico de Nutricón y Salud. 2013:1–18. [Google Scholar]
- 97. National Academies of Sciences . Dietary reference intakes (DRIs): recommended dietary allowances and adequate intakes, elements. [Internet]. 2019; [cited 2020 May 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545442/table/appJ_tab3/?report=objectonly.
- 98. National Institutes of Health (NIH) . Folate. [Internet]. 2020; [cited 2020 May 20]. Available from: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/.
- 99. Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–68. [DOI] [PubMed] [Google Scholar]
- 100. Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eicher-Miller HA, Fulgoni VL, Keast DR. Contributions of processed foods to dietary intake in the US from 2003–2008: a report of the Food and Nutrition Science Solutions Joint Task Force of the Academy of Nutrition and Dietetics, American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. J Nutr. 2012;142(11):2065S–72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Filippini T, Cilloni S, Malavolti M, Violi F, Malagoli C, Tesauro M, Bottecchi I, Ferrari A, Vescovi L, Vinceti M. Dietary intake of cadmium, chromium, copper, manganese, selenium, and zinc in a northern Italy community. J Trace Elem Med Biol. 2018;50:508–17. [DOI] [PubMed] [Google Scholar]
- 103. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) . Scientific opinion on dietary reference values for manganese. EFSA J. 2013;11(11):3419. [Google Scholar]
- 104. Ma G, Chen Y. Polyphenol supplementation benefits human health via gut microbiota: a systematic review via meta-analysis. J Funct Foods. 2020;66:103829. [Google Scholar]
- 105. Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, Tjonneland A, Kyro C, Fagherazzi G, Boutron-Ruault Met al. Dietary polyphenol intake in Europe: the European prospective investigation into cancer and nutrition (EPIC) study. Eur J Nutr. 2016;55(4):1359–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Burkholder-Cooley N, Rajaram S, Haddad E, Fraser GE, Jaceldo-Siegl K. Comparison of polyphenol intakes according to distinct dietary patterns and food sources in the Adventist Health Study-2 cohort. Br J Nutr. 2016;115(12):2162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. National Academies of Sciences , Dietary Reference intakes (DRIs): recommended dietary allowances and adequate intakes vitamins. [Internet]. 2011; [cited 2020 May 20]. Available from, https://www.ncbi.nlm.nih.gov/books/NBK56068/table/summarytables.t2/?report=objectonly.
- 108. Ruiz-Roso MB, de Carvalho P, Mantilla-Escalante D, Ulloa N, Brun P, Acevedo-Correa D, Ferreira W, Martorell M, Tschoepke M, de Oliveira Let al. Covid-19 confinement and changes of adolescent's dietary trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients. 2020;12(6):1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Batlle-Bayer L, Aldaco R, Bala A, Puig R, Laso J, Margallo M, Vasquez-Rowe I, Antó J, Fullana-i-Palmer P. Environmental and nutritional impacts of dietary changes in Spain during the COVID-19 lockdown. Sci Total Environ. 2020;748:141410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Carroll N, Sadowski A, Laila A, Hruska V, Nixon M, Ma D, Haines J, on behalf of the Guelph family health study. The impact of COVID-19 on health behavior, stress, financial and food security among middle to high income Canadian families with young children. Nutrients. 2020;12(8):2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reyes-Olavarría D, Latorre-Román P, Guzmán-Guzmán I, Jerez-Mayorga D, Caamaño-Navarrete F, Delgado-Floody P. Positive and negative changes in food habits, physical activity patterns, and weight status during covid-19 confinement: associated factors in the Chilean population. Int J Environ Public Health. 2020;17(15):5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nasi A, McArdle S, Gaudernack G, Westman G, Melief C, Rockberg J, Ares R, Kouretas D, Sjolin J, Mangsbo S. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol Rep. 2020;7:768–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, Benoliel J, Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20:515–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tan BL, Norhaizan ME, Liew WPP. Nutrients and oxidative stress: friend or foe?. Oxid Med Cell Longev. 2018;2018:art. ID 9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde Aet al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Furushima D, Ide K, Yamada H. Effect of tea catechins on influenza infection and the common cold with a focus on epidemiological/clinical studies. Molecules. 2018;23(7):1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ide K, Kawasaki Y, Kawakami K, Yamada H. Anti-influenza virus effects of catechins: a molecular and clinical review. Curr Med Chem. 2016;23(42):4773–83. [DOI] [PubMed] [Google Scholar]
- 119. Zu M, Yang F, Zhou W, Liu A, Du G, Zheng L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012;94(3):217–24. [DOI] [PubMed] [Google Scholar]
- 120. Li W-F, Chik WI, Wang D-Y, Pan L-T. Plant phenolic compounds as potential lead compounds in functional foods for antiviral drug discovery. Curr Org Chem. 2017;21(18). [Google Scholar]
- 121. Costa SS, Couceiro J, Silva IC V, Malvar DDC, Coutinho MAS, Camargo LMM, Muzitano M, Vanderlinde F. Flavonoids in the therapy and prophylaxis of flu: a patent review. Expert Opin Ther Pat. 2012;22(10):1111–21. [DOI] [PubMed] [Google Scholar]
- 122. Ding S, Jiang H, Fang J. Regulation of immune function by polyphenols. J Immunol Res. 2018;2018:1264074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vamanu E. Polyphenolic nutraceuticals to combat oxidative stress through microbiota modulation. Front Pharmacol. 2019;10:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57(15):6485–501. [DOI] [PubMed] [Google Scholar]
- 125. Han SN, Meydani M, Wu D, Bender BS, Smith DE, Viña J, Cao G, Prior R, Nikbin S. Effect of long-term dietary antioxidant supplementation on influenza virus infection. J Gerontol A Biol Sci Med Sci. 2000;55(10):B496–503. [DOI] [PubMed] [Google Scholar]
- 126. Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G.. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang MX, Win SS, Pang J. Zinc supplementation reduces common cold duration among healthy adults: a systematic review of randomized controlled trials with micronutrients supplementation. Am J Trop Med Hyg. 2020;103:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Acevedo-Murillo JA, García León ML, Firo-Reyes V, Santiago-Cordova JL, Gonzalez-Rodriguez AP, Wong-Chew RM. Zinc supplementation promotes a Th1 response and improves clinical symptoms in fewer hours in children with pneumonia younger than 5 years old. a randomized controlled clinical trial. Front Pediatr. 2019;7:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shaker S, Fathy H, Abdelall E, Said A. The effect of zinc and vitamin A supplements in treating and reducing the incidence of upper respiratory tract infections in children. Natl J Physiol Pharm Pharmacol. 2018;8(7):1010–7. [Google Scholar]
- 130. Kurugöl Z, Akilli M, Bayram N, Koturoglu G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr. 2006;95(10):1175–81. [DOI] [PubMed] [Google Scholar]
- 131. Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev. 2015;2015(4):CD001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wani AL, Parveen N, Ansari MO, Ahmad MF, Jameel S, Shadab G. Zinc: an element of extensive medical importance. Curr Med Res Pract. 2017;7(3):90–8. [Google Scholar]
- 133. Broome CS, McArdle F, Kyle JAM, Andrews F, Lowe NM, Hart CA, Arthur J, Jackson M. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80(1):154–62. [DOI] [PubMed] [Google Scholar]
- 134. Steinbrenner H, Al-Quraishy S, Dkhil M, Wunderlich F, Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv Nutr. 2015;6(5):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Shaheen SO, Sterne JAC, Thompson RL, Songhurst CE, Margetts BM, Burney PGJ. Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med. 2001;164(10 I):1823–8. [DOI] [PubMed] [Google Scholar]
- 136. Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75(1):126–32. [DOI] [PubMed] [Google Scholar]
- 137. Gera T, Sachdev HPS. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ. 2002;325(7373):1142–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Maggini S, Pierre A, Calder PC. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10(10):1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Satheesh BN, Suriyakala PC, Anjaneyulu V, Senthil RD. Green tea catechin loaded nanodelivery systems for the treatment of pandemic diseases. Asian J Pharm Clin Res. 2019, 12;(5):1–7. [Google Scholar]
- 140. Roll S, Nocon M, Willich SN. Reduction of common cold symptoms by encapsulated juice powder concentrate of fruits and vegetables: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2011;105(1):118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Han SN, Meydani SN. Vitamin E and infectious diseases in the aged. Proc Nutr Soc. 1999;58(3):697–705. [DOI] [PubMed] [Google Scholar]
- 142. Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57:759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Godos J, Castellano S, Ray S, Grosso G, Galvano F. Dietary polyphenol intake and depression: results from the Mediterranean healthy eating, lifestyle and aging (MEAL) study. Molecules. 2018;23(5):999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP, Association between regional selenium status and reported outcome of COVID-19 cases in China, Am J Clin Nutr. 2020. 111:1297–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sahebnasagh A, Saghafi F, Avan R, Khoshi A, Khataminia M, Safdari M, Habtemariam S, Rezai H, Mohammad S. The prophylaxis and treatment potential of supplements for COVID-19. Eur J Pharmacol. 2020;887:173530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S, Guido A, Sabatino A, Belliato M, Calvi Met al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Russo M, Moccia S, Spagnuolo C, Tedesco I, Russo GL. Roles of flavonoids against coronavirus infection. Chem Biol Interact. 2020;328:109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14(4):367–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Quiles JL, Rivas-García L, Varela-López A, Llopis J, Battino M, Sánchez-González C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19?. Environ Res. 2020;191:110053. [DOI] [PMC free article] [PubMed] [Google Scholar]