Abstract
Objective:
We sought to determine if global cognitive function in patients with epilepsy (PWE) differs when electroencephalographic (EEG) abnormalities are present during concurrent neuropsychological (NP) evaluation.
Methods:
We explored the association between subclinical epileptiform discharges (sEDs) and interictal epileptiform discharges (IEDs) and global aspects of cognition in 79 consecutive patients with epilepsy who underwent continuous EEG monitoring during NP evaluation for diagnostic (15%) or presurgical (85%) purposes while on their standard antiseizure medication (ASM) regimens. As some researchers have suggested that the apparent link between IEDs and cognition represent epiphenomena of an underlying damaged neural substrate, we used functional status as a stratifying covariate to allow us to address this position.
Results:
EEG was abnormal in 68% of patients despite being on their standard ASM regimen. Epileptiform abnormalities (IEDs, sEDs, or both) were seen in isolation or coupled with diffuse or focal slowing in 38% of patients. Individuals with IEDs occurring during their NP evaluation demonstrated poorer scores in attention/working memory (forward and backward digit span), processing speed (symbol searching and coding) and speeded components of language (semantic fluency) tests compared to those with normal EEG tracings matched by their real-world, functional status. In two high functioning patients, performance was significantly better when these individuals were tested in the absence of IEDs, with performances appearing invalid when tested during periods of IED activity. No significant association was found between NP performance and non-epileptiform EEG abnormalities.
Significance:
A substantial proportion of epilepsy patients undergoing NP evaluation manifest concurrent EEG abnormalities, with epileptiform abnormalities associated with poorer global cognitive performance. As this pattern was observed regardless of functional status, this association appears to represent more than unrelated features coincidentally shared by the lowest functioning cohort. Coupled with our individual case data, our findings suggest that NP testing may be adversely affected by IEDs and sEDs going unrecognized in the absence of simultaneous EEG recordings, and set the stage for future studies to definitively establish this possible relationship.
Keywords: epileptiform activity, cognition, processing speed, attention, electrophysiology
1. INTRODUCTION:
Cognitive impairment is a common comorbidity in persons with epilepsy (PWE).1 Frequently, it is interwoven with the underlying neurobiologic substrate that drives the disease and is occasionally exacerbated by the selected antiepileptic treatment.2 Yet, aside from these chronic and subacute culprits, research suggests that acute fluctuations can also occur due to the effect of subclinical epileptiform electroencephalographic activity on cognition, a.k.a. transitory cognitive impairment (TCI).3–5 Nevertheless, much of this research literature is far from definitive,6,7 with the vast majority of studies attempting to associate poor cognitive scores from one study session with EEG abnormalities from another, often separated by days or weeks.4,8–10 Additionally, some have argued that poor cognition and epileptiform activity are resulting from a poor neural substrate, and that a causative role between them is actually illusory.11,12
Despite these limitations in study methodology, clinicians and researchers have speculated about TCI since the 1930s.13 Significant evidence has accumulated seeming to implicate both generalized and focal interictal epileptiform discharges (IEDs) and subclinical epileptiform discharges (sEDs) in cognitive dysfunction. IED effects have been proposed to account for delayed reaction times, impaired vigilance, attention, perception and compromised performance on visuospatial and verbal tasks,4,14–17 commonly with neuroanatomical specificity to the task.6,16
The repercussions of TCI transcend mere theoretical interest in the EEG laboratory. Patients with epileptic encephalopathies such as Continuous Spike Wave in Sleep, Landau-Klefner syndrome and Rasmussen encephalitis manifest cognitive improvement when reduction of their IED burden is achieved.18–20 Even in presumably benign epilepsy syndromes such as rolandic epilepsy, IEDs appear to take a significant toll on cognitive function.21,22 Studies have shown a negative effect of these epileptiform EEG discharges on school performance.23 Moreover, subclinical epileptiform EEG discharges were proven to have a negative influence on drivers24,25 and air-traffic controllers.26
Formal neuropsychological testing is an indispensable constituent of presurgical evaluation of PWE.27 It aims to identify the brain structures and systems that are predominantly affected by the disease and hence, provide useful lateralizing and localizing information in the quest to characterize the epileptogenic zone. Additionally, it is paramount in assessing functional reserve and assisting in decision making about surgical candidacy, elucidating the need for additional, costly and occasionally invasive evaluations, in the pre- (e.g. intracarotid amytal testing, fMRI) and peri-operative (e.g. stimulation mapping, electrocorticography) phase of the work up.
If TCI is so common and clinically impactful, why is it not routinely evaluated with continuous EEG monitoring in patients with refractory epilepsy undergoing pre-surgical neuropsychological evaluation? We have suggested neurocognitive data may be invalid if IEDs are occurring in our patients during testing, and argued that this could have significant ramifications for surgical planning and critical decisions based on neurocognitive status (e.g., school and work placements, capacity evaluations).28 Likewise, if IEDs do have a significant effect on cognition, then group outcome studies will be potentially obscured by its occurrence. In this study, we attempted to evaluate the prevalence of epileptiform activity in continuous EEG tracings recorded simultaneously with outpatient neuropsychological testing and to ascertain its association with global neuropsychological scores.
The ideal way to answer these questions is to have simultaneous EEG data obtained for several days prior to testing as well as during it, to time lock this data to the performance of each task, and to evaluate the same patients during the presence and absence of IEDs on equivalent tasks. Nevertheless, the state of clinical neuropsychological practice does not utilize simultaneous EEG at all nor does it attempt to measure the presence of IEDs/sEDs in any fashion. Additionally, it would be best to study patients on a continuous task variable during periods of IEDs and during their absence. However, while this continuous procedure would be ideal for studying the effects of IEDs on a single task, it does not lend itself to establishing the possible impact of IEDs on a standard clinical NP evaluation. The latter is made of individual component tasks, which are often lacking in equivalent versions. In this study, we are attempting to answer some fundamental, key questions in order to understand the potential prevalence of IEDs during NP testing, to understand their potential effect on global processes, and to set the stage for more ideal time-locked EEG studies in the context of the NP evaluation in the future:
1) How frequent are IEDs/sEDs and nonepileptiform discharges (non-EDs) occurring in epilepsy patients undergoing neuropsychological testing in the epilepsy surgery setting? Some have argued that IEDs/sEDs do not occur frequently in patients on their standard ASM regimens.29,30 They suggest that research arising from the setting of the epilepsy monitoring unit is actually capitalizing on greater IEDs/sED occurrence in patients whose medications have been withheld. Therefore, we will determine the frequency of occurrence of EEG abnormalities in a typical sample of epilepsy patients undergoing neuropsychological evaluation on their standard ASM regimen.
2) We predict that non-EDs will not be associated with global task performance across any functional groups based on negative findings from other studies.31,32
3) As some researchers have suggested that TCI is actually caused by something other than IEDs/sEDs (e.g., a poor neural substrate contributing to both IEDs/sEDs and cognitive dysfunction),12 we are examining whether IEDs/sEDs relate to cognitive performance in individuals of varying functional levels.
3a) We predict that IEDs/sEDs will be present in patients with epilepsy regardless of their functional status (i.e., high functioning patients will experience IEDs/sEDs just as frequently as low functioning patients, with functional status based on ability to work and live independently).
3b) We predict that patients experiencing IEDs/sEDs will perform worse than patients with normal EEG records who have been matched by “real-world” functional status on global measures of cognition. This pattern will be observed across all levels of functional status. Based on prior studies, we predict that even minimal evidence of IEDs will be sufficient to disrupt global function.4
If performance differs in the higher functioning individuals in a manner that covaries with the presence of IEDs/sEDs when all other key factors are kept equivalent, then it would seem highly worthwhile to prospectively study this relationship for a possible causative role.
Finally, we present differences in performance for two high-functioning patients when IEDs were present or absent from their EEG tracings. These within subject observations of performance change dependent upon the presence of IEDs more definitively establish a causative relationship between epileptiform activity and cognitive dysfunction.
2. METHODS:
2.1. Study population
This study was approved by the Emory University Institutional Review Board. The study population consisted of 79 consecutive adult PWE who underwent neuropsychological evaluation due to cognitive complaints or presurgical purposes with concurrent EEG monitoring between October 2013 and April 2016. Primary language for all patients included in the study was English. For each patient, demographic and epilepsy information was collected and cross-referenced with medical records. Subjects with comorbid psychiatric disease and those receiving psychotropic medications were included. Antiepileptic treatment was evaluated both as a total number of ASMs at the time of NP evaluation, as well as the presence of ASMs notorious for their deleterious cognitive side effects (including benzodiazepines, barbiturates, or carbonic anhydrase inhibitors).33,34
For two patients in the current study, we were able to explore performance differences in the presence and absence of IEDs.
2.2. Neurophysiological evaluation
All patients underwent conventional 21-channel scalp EEG (Brain Monitor headbox, Natus, WI) at the beginning of the NP evaluation and were monitored for its entire duration. Retrospective interpretation of the EEG recordings was performed by 2 board certified Clinical Neurophysiologists blinded to the NP results (IK and OT). Abnormalities were dichotomized into non-epileptiform (e.g. focal and/or diffuse slowing) and epileptiform (e.g. IEDs and/or seizures). IEDs were defined as spikes, sharp waves or spike/sharp-wave complexes, isolated or occurring serially, without evolution to seizures. Seizures were defined as any pattern lasting at least 10 seconds satisfying previously well-established criteria.35 All events were classified based upon location and frequency. For IED frequency an IED index was estimated based upon the percentage of the EEG record that included IEDs grouped into five categories (0%, <1%, 1-10%, >10-50% and >50%), akin to prior studies.36,37
2.3. Neuropsychological evaluation
Patients underwent complete NP evaluation by a board certified (DLD) and one board certification eligible (ES) neuropsychologist using standardized protocols. For the purposes of this study, due to sample size limitations and our initial focus on broader markers of cognitive dysfunction, we limited our evaluation to data more likely to be affected by epileptiform activity occurring anywhere in the brain rather than requiring focal dysfunction of a specific brain region. This would include broad functions such as processing speed, motor and psychomotor response time, and attention that are often impaired in general conditions such as a metabolic encephalopathy rather than cognitive constructs requiring localized, focal dysfunction (e.g., naming deficits likely result from specific lateralized temporal and frontal lobe network dysfunction rather than focal dysfunction occurring in other brain regions).
Attention and working memory was evaluated using digit span forwards and backwards from the 4th edition of the Wechsler Adult Intelligence Scale (WAIS-IV).38 Processing speed was assessed using WAIS-IV Symbol Search and Coding scaled scores. Generative fluency was assessed using the Controlled Oral Word Association (COWA) test and semantic fluency task (i.e., Animal Fluency) for initiation and selection of specific words,39 as these functions are often disrupted by transient processes (i.e., very sensitive to IEDs and changes in dosages and number of ASMs),34 while the quantitative performance score from the Boston Naming Test40 was used for a measure that was not likely to be affected by epileptiform activity. In general, total score has not proven significantly affected by epileptiform activity or drug effects although naming response time can be so disrupted.34 With the exception of the BNT, T scores were used as a common metric instead of raw scores for all NP measures. As the BNT is not normally distributed, raw scores were used instead as has been customary practice in the literature.27,41
2.4. Functional Ratings
All patients were rated regarding work/school status and their ability to perform activities of daily living (ADL) in an independent fashion. We classified each patient using the following Emory Level of Functioning criteria formula, which was designed to be hierarchical in level of independence: 1) Employed or attending school and independent with all ADLs, 2) Not employed nor attending school and independent with all ADLs (both higher and lower order ADLs), 3) Unemployed and not attending school and independent with lower ADLs only (e.g., basic self-care), or 4) Unemployed and not attending school and not independent with any ADLs.
2.5. Statistical design and analysis
Descriptive statistics were computed for the clinical, neurophysiological and neuropsychological characteristics of our cohort, with comparisons made across subgroups based on functional level using a series of ANOVAs. This included the relative frequency of occurrence of IEDs and seizures during testing, as well as non-ED EEG abnormalities.
To determine whether the occurrence of IEDs, sEDs, and non-EDs are associated with a poor neural substrate, we began by determining whether these discharges were distributed across all functional groups or primarily occurring in the lower groups. We accomplished this goal by using Fisher’s Exact Test to determine if the frequency of epileptiform and non-ED EEG activity differed across the four functional groups.
Next, we used two-way factorial univariate ANOVAs to determine whether global cognitive measures differed on the basis of functional status or when epileptiform or activity was present. We did not include non-EDs in these analyses as correlational metrics showed no significant relationship between non-EDs and global cognitive measures in our sample. Patients experiencing clinically recognized seizures less than 24-hours prior to completing the global NP tests to be examined were not included in this analysis (n=2). Our specific predictions were that functional status and epileptiform activity would prove to be independent contributors to these global cognitive measures, but there would be no interaction between these main effects. We used the BNT as a measure not expected to be affected by EEG activity based on prior studies. We used a false discovery rate (FDR) adjustment to control for multiple comparisons in these primary analyses.
Secondary analyses included the use of ANOVAs to determine whether performance on the global neuropsychological measures differed on the basis of the IED spike frequency index (IED index <1% vs. >1%) or on the basis of the broad location (focal vs. multifocal/generalized) of IEDs.
Overall, although this is the largest data set of PWE undergoing clinical neuropsychological testing with simultaneous EEG recordings while on their typical AED regimen, the number of subjects is still too low for multivariate exploration of more specific brain localization effects on given tasks or domains, even on a lobar basis.
3. RESULTS:
3.1. Clinical characteristics
Clinical characteristics are depicted in Table 1 for the entire sample. Seventy-nine patients underwent EEG monitoring during their NP evaluation (85% for presurgical purposes) while on standard medication regimens. They were mostly unemployed and with modest educational attainment. Three quarters of them had a good/fair functional status (levels 1 or 2). Most patients were diagnosed with epilepsy in their adolescence with a median disease duration of 16 years. They manifested on average 9 seizures per month and they were typically on 2 ASMs. Nearly half were on ASMs notorious for cognitive side effects and less than half were on additional, non-ASMs, central nervous system acting medications, typically to manage psychiatric comorbidities (e.g., anxiety, depression). No patient included in the study was experiencing psychosis. Less than half of all assessed patients had an active diagnosis of mood disturbance and/or anxiety, and approximately half of these patients were being actively treatment with psychotropic medications. The majority suffered from focal epilepsy, which was unitemporal or bitemporal in approximately 2/3 of them.
Table 1.
Clinical characteristics for our epilepsy sample based upon functional status.
| Demographic Characteristics | All Patients (N=79) | Level 1 (n=26) | Level 2 (n=32) | Level 3 (n=13) | Level 4 (n=8) | |
|---|---|---|---|---|---|---|
| Age (mean, SD) | 37.8 (14.9) | 35.5 (14.7) | 41.7 (16.6) | 34.9 (11.0) | 34.6 (11.7) | n.s. |
| Gender (female, n, %) | 52 (65.8) | 20 (76.9) | 23 (71.9) | 6 (46.2) | 3 (37.5) | n.s. |
| Handedness (right handed, n, %) | 72 (91.1) | 24 (92.3) | 30 (93.8) | 11 (84.6) | 7 (87.5) | n.s. |
| Education (years) | 12.8 (3.0) | 13.9 (3.4)δβ | 12.9 (2.7) | 11.5 (2.9)δ | 11.0 (1.5)β | F=3.30, p<.03 |
| Disease characteristics | ||||||
| Age of onset (mean, SD) | 17.6 (14.1) | 18.6 (12.7) | 21.8 (16.7)ρτ | 11.2 (7.6)ρ | 8.3 (7.6)τ | F=3.29 p<.03 |
| Duration of epilepsy (in years: mean, SD) | 19.9 (15.0) | 15.8 (11.1) | 19.9 (17.8) | 23.6 (13.2) | 26.4 (14.6) | n.s. |
| Seizure frequency (per month: mean, SD) | 8.9 (19.6) | 12.6 (31.9) | 6.3 (6.5) | 8.1 (7.8) | 7.0 (10.3) | n.s. |
| Number of AEDs (mean, SD) | 2.5 (1.0) | 2.2 (1.0) | 2.4 (0.8) | 2.9 (0.8) | 2.9 (1.3) | n.s. |
| Treatment with sedating AEDs (yes, n, %) | 36 (45.6) | 12 (46.2) | 13 (40.6) | 6 (46.2) | 5 (62.5) | n.s. |
| Treatment with other CNS acting drugs (yes, n, %) | 29 (36.7) | 7 (26.9) | 15 (42.9) | 5 (38.5) | 2 (25.0) | n.s. |
| Location of epilepsy (n, %) | ||||||
| Right temporal | 19 (24.1) | 11 (42.3) | 7 (21.9) | 0 (0.0) | 1 (12.5) | n.s. |
| Left temporal | 24 (30.4) | 6 (23.1) | 12 (37.5) | 4 (30.8) | 2 (25.0) | n.s. |
| Right frontal | 6 (7.6) | 2 (7.7) | 2 (6.3) | 1 (7.7) | 1 (12.5) | n.s. |
| Left frontal | 5 (6.3) | 0 (0.0) | 3 (9.4) | 2 (15.4) | 0 (0.0) | n.s. |
| Right parieto-occipital | 1 (1.3) | 1 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | n.s. |
| Left parieto-occipital | 3 (3.8) | 2 (7.7) | 1 (3.1) | 0 (0.0) | 0 (0.0) | n.s. |
| Bitemporal | 9 (11.4) | 2 (7.7) | 3 (9.4) | 3 (23.1) | 1 (12.5) | n.s. |
| Multifocal | 7 (8.9) | 0 (0.0)πρ | 1 (3.1) | 3 (23.1 )ρ | 3 (37.5)π | p<.05 |
| Generalized | 5 (6.3) | 2 (7.7) | 3 (9.4)ωμ | 0 (0.0)ω | 0 (0.0)μ | p<.05 |
Note. SD = standard deviation; ASMs = antiseizure medications. Functional Levels range from 1 to 4, with Level 1 representing the highest level of independent function and Level 4 the lowest. Sedating ASMs included benzodiazepines, phenobarbital, and the decarboxylase inhibitors (i.e., topiramate & zonisamide). Continuous variables were analyzed using an analysis of variance (ANOVA) while proportional counts were analyzed using Fisher’s Exact Test. Matching superscripts indicate that subgroups differed significantly at the specified level of significance.
The same demographic and disease-related variables were explored across subgroups. As our primary analyses are targeted at the subgroup level, we wanted to be aware of any subgroup differences (Table 1). Some subgroup differences were found, which would be expected given that such differences likely are either the cause or effect of subgroup placement. For example, groups 1 and 2 were more educated and had later ages of onset than groups 3 and 4. Group 4 was mainly comprised of individuals with severe conditions from birth frequently carrying comorbid diagnoses of developmental delay. However, subgroups did not differ with regards to gender, the use of ASMs or other psychotropic agents, the presence of active psychiatric conditions, or the use of ASMs with pronounced cognitive side effects.
3.2. Neurophysiological characteristics
Neurophysiological characteristics are depicted in Table 2. EEG was abnormal in 68% of patients. Sixty-one percent of patients (48/79) had non-ED abnormalities (e.g. diffuse or focal slowing). Epileptiform abnormalities were seen in isolation or coupled with diffuse or focal slowing in 38% (30/79) of all patients. Out of these, 6 patients had sEDs ranging from 1 up to 30 per session. For patients experiencing IEDs, these were most often of a focal nature (21/30; 70%) rather than a multifocal or generalized variety. The majority of IEDs where localized in either or both temporal regions. For nearly half of the patients experiencing IEDs, these were of a low frequency (spike-wave index of 1% or less). As hypothesized, the presence of IEDs was equally distributed across all four subgroups (n.s., Fisher’s Exact Test) rather than being overrepresented in subgroups 3 and 4 as believed by many researchers and clinicians on the basis of clinical lore.11,12 The presence of non-ED EEG abnormalities (e.g., diffuse or focal slowing) was also equally distributed across functional subgroups (n.s., Fisher’s Exact Test). The presence of both IEDs and non-ED abnormalities appeared substantial (38% and 68% respectively) in a PWE sample that was being evaluated while on their standard medication regimens.
Table 2:
Neurophysiological characteristics
| Neurophysiological characteristics | All subjects | Functional Levels | |||
|---|---|---|---|---|---|
| I | 2 | 3 | 4 | ||
| EEG findings (n, %) | N=79 | n=26 | n=32 | n=13 | n=8 |
| No abnormality | 25 (32) | 7 (9) | 13 (16) | 4 (5) | 1 (1) |
| Non-epileptiform abnormality only | 24 (30) | 9 (11) | 10 (13) | 3 (4) | 2 (3) |
| Epileptiform abnormality only | 6 (8) | 2 (3) | 4 (5) | 0 (0) | 0 (0) |
| Both | 24 (30) | 8 (10) | 5 (6) | 6 (8) | 5 (6) |
| Type of non-epileptiform abnormality (n, %) | |||||
| Diffuse slowing only | 14 (27) | 4 (8) | 4 (8) | 4 (8) | 2 (4) |
| Focal slowing only | 34 (67) | 14 (27) | 13 (25) | 3 (6) | 4 (8) |
| Both | 3 (6) | 0 (0) | 0 (0) | 2 (4) | 1 (2) |
| Type of epileptiform abnormality (n, %) | |||||
| Interictal epileptiform discharges only | 26 (81) | 8 (25) | 7 (22) | 6 (19) | 5 (15) |
| Electrographic seizures | 6 (19) | 1 (3) | 4 (13) | 0 (0) | 1 (3) |
| Distribution of interictal epileptiform discharges | |||||
| Unifocal | 21 (70) | 8 (27) | 7 (23) | 5 (17) | 1 (3) |
| Multifocal/Generalized | 9 (30) | 2 (7) | 2 (7) | 1 (3) | 4 (13) |
| Location of interictal epileptiform discharges | |||||
| Right temporal | 3 (12) | 3 (12) | 0 (0) | 0 (0) | 0 (0) |
| Left temporal | 6(24) | 1 (4) | 2 (8) | 2 (8) | 1 (4) |
| Right frontal | 3 (12) | 1 (4) | 1 (4) | 1 (4) | 0 (0) |
| Left frontal | 3 (12) | 0 (0) | 2 (8) | 1 (4) | 0 (0) |
| Right parieto-occipital | 1 (4) | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Bitemporal | 2 (8) | 0 (0) | 1 (4) | 0 (0) | 1 (4) |
| Multifocal | 4 (16) | 0 (0) | 0 (0) | 1 (4) | 3 (12) |
| Generalized | 3 (12) | 2 (8) | 1 (4) | 0 (0) | 0 (0) |
| Index of interictal epileptiform discharges | |||||
| <1% | 12 (48) | 6 | 1 | 2 | 3 |
| 1-10% | 7(28) | 1 | 3 | 3 | 0 |
| 10-50% | 5 (20) | 1 | 2 | 0 | 2 |
| >50% | 0 | 1 | 0 | 0 | |
Note. There were no significant differences in neurophysiological patterns across functional groups using Fisher’s Exact Test.
3.3. Neuropsychological characteristics
Neuropsychological characteristics are depicted in Table 3. At the group level, attention was mildly impaired in the substantial portion of patients, processing speed was borderline to low average, and generative fluency was mildly impaired to borderline. Most of the patients exhibited mild concurrent language deficits with visual naming in the borderline range. As hypothesized, subgroup differences in performance were clearly observed for most cognitive tests examined, as reflected by the main effect of functional subgroup contribution to NP performance seen in Tables 3 and Figure 1, which align to the expected ordinal functional subgroup hierarchy (i.e., those with a better functional status related to work and level of independence performed better on NP measures than those functioning poorly). The one exception was semantic fluency, which followed the same ordinal hierarchical pattern but did not remain significant after application of the FDR correction. We are reporting main effects for functional subgroup as there were no significant interactions between this variable and the presence of IEDs on any of the two-way factorial univariate ANOVAs performed.
Table 3:
Neuropsychological characteristics.
| Neuropsychological Performance | All Patients (N=79) | Level 1 (n=26) | Level 2 (n=32) | Level 3 (n=13) | Level 4 (n=8) | Main Effects | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Attention | ||||||||||
| Digit Span – Forward (T-Score, mean, SD) Omnibus F=6.33, p<001 |
39.8 (10.5) | 45.1 (12.0)αβγ | 40.3 (6.3)αλ | 37.8 (7.8)βμ | 23.8 (5.3)γλμ | F=10.79, p<.001 | ||||
| IED+ (n=9) | IED− (n=17) | IED+ (n=9) | IED− (n=23) | IED+ (n=6) | IED− (n=7) | IED+ (n=5) | IED− (n=3) | |||
| 42.4 (15.5) | 46.5 (10.0) | 39.3 (5.3) | 41.8 (6.3) | 32.3 (7.3) | 42.4 (4.6) | 22.6 (5.6) | 25.7 (5.1) | F=4.0,* p=.05 | ||
| Digit Span-Backward (T-Score, mean, SD) Omnibus F= 12.04, p<.001 |
40.7 (11.7) | 46.4 (9.5)ϕĜ | 43.5 (8.0)ϑÎ | 34.2 (10.2)ϕϑτ | 22.7 (87.5)ĜÎτ | F=18.3, p<.001 | ||||
| IED+ (n=9) | IED− (n=17) | IED+ (n=9) | IED− (n=23) | IED+ (n=6) | IED− (n=7) | IED+(n=5) | IED−(n=3) | |||
| 42.0 (10.7) | 49.1 (8.1) | 39.2 (11.6)Υ | 45.1 (5.4)Υ | 30.3 (13.0) | 37.6 (6.1) | 17.0 (2.1)ų | 27.7 (2.8)ų | F= 11.13, p=.001 | ||
| Processing Speed | ||||||||||
| Symbol Search (T-Score, mean, SD) Omnibus F=10.83, p<.001 |
38.0(10.4) | 41.5 (8.3)ϖθ | 40.7 (8.2)Οπ | 32.0 (9.8)ϖΟΣ | 22.3 (3.7)ΘπΣ | F=11.05, p<.001 | ||||
| IED+ (n=9) | IED− (n=17) | IED+ (n=9) | IED−(n=23) | IED+ (n=6) | IED− (n=7) | IED+ (n=5) | IED− (n=3) | |||
| 36.7ğ (8.9) | 44.8ğ (8.3) | 36.2 (7.9) | 42.2 (7.8) | 23.3ŝ (4.3) | 39.4ŝ (6.0) | 20.6ż (1.3) | 27.5ż (5.0) | F=17.00, p<.001 | ||
| Coding (T-Score, mean, SD) Omnibus F=13.35, p<.001 |
36.7(11.2) | 41.2 (8.6)ĀĦ | 40.5 (7.9)ιĚ | 31.0 (11.0)ÃιΔ | 22.4 (4.2)ĦĚΔ | F=16.00. p<.001 | ||||
| IED+ (n=9) | IED− (n=17) | IED+ (n=9) | IED− (n=23) | IED+ (n=6) | IED− (n=7) | IED+ (n=5) | IED− (n=3) | |||
| 35.9ŕ (9.4) | 44.1ŕ (6.9) | 36.6 (8.5) | 41.4 (6.8) | 18.7Ľ (8.9) | 38.0Ľ (8.8) | 17.4ã (5.4) | 26.0ã (9.6) | F=23.94, p<.001 | ||
| Generative Fluency | ||||||||||
| COWA (T-Score, mean,SD) Omnibus F= 8.12, p<001 |
33.8 (13.7) | 38.8 (14.8)ŔŨ | 36.8 (10.9)Òâ | 26.9 (10.9)ŔÒ | 17.3 (7.0)Ũâ | F=7.35, p<.001 | ||||
| IED+ (n=9) | IED− (n=17) | IED+ (n=9) | IED− (n=23) | IED+ (n=6) | IED− (n=7) | IED+ (n=5) | IED− (n=3) | |||
| 26.0v (13.6) | 45.6v (10.4) | 34.4(7.8) | 37.7(11.9) | 22.0Ç(7.4) | 31.0Ç (12.1) | 13.6ÿ(3.5) | 23.3ÿ (7.6) | F=12.93, p<.001 | ||
| Semantic Fluency (T-Score, mean, SD) Omnibus F= 3.53, p< 003 |
36.7(13.1) | 39.5 (13.5) | 38.4 (12.3) | 34.9 (12.9) | 23.4 (8.0) | F=2.57, p=.05* | ||||
| IED+ (n=9) | IED− (n=17) | IED+ (n=9) | IED− (n=23) | IED+ (n=6) | IED− (n=7) | IED+ (n=5) | IED− (n=3) | |||
| 31.0ç (15.0) | 44.1ç (10.4) | 34.1(15.0) | 40.1(11.1) | 29.5ò(13.2) | 39.4ò(11.7) | 20.0(6.2) | 29.0(8.5) | F= 8.80, p=.003 | ||
Note. IED+ = presence of interictal epileptiform discharges (IEDs & sEDs); IED− = absence of all interictal epileptiform discharges; SD = standard deviation. COWA = Controlled Oral Word Association Test. Two-way factorial univariate ANOVAs were conducted for each cognitive metric to determine the potential effects of functional level and IEDs on each cognitive metric, with a description of these analyses reported in the text. Subgroups that significantly differ from one another on the basis of post-hoc testing have matching superscripts.
Analyses with an asterisk did not survive FDR correction.
Figure 1. Graphical presentation of cognitive outcome on the basis of functional subgroup classification and the presence or absence of epileptiform activity (IEDs/sEDs) for each global cognitive metric.
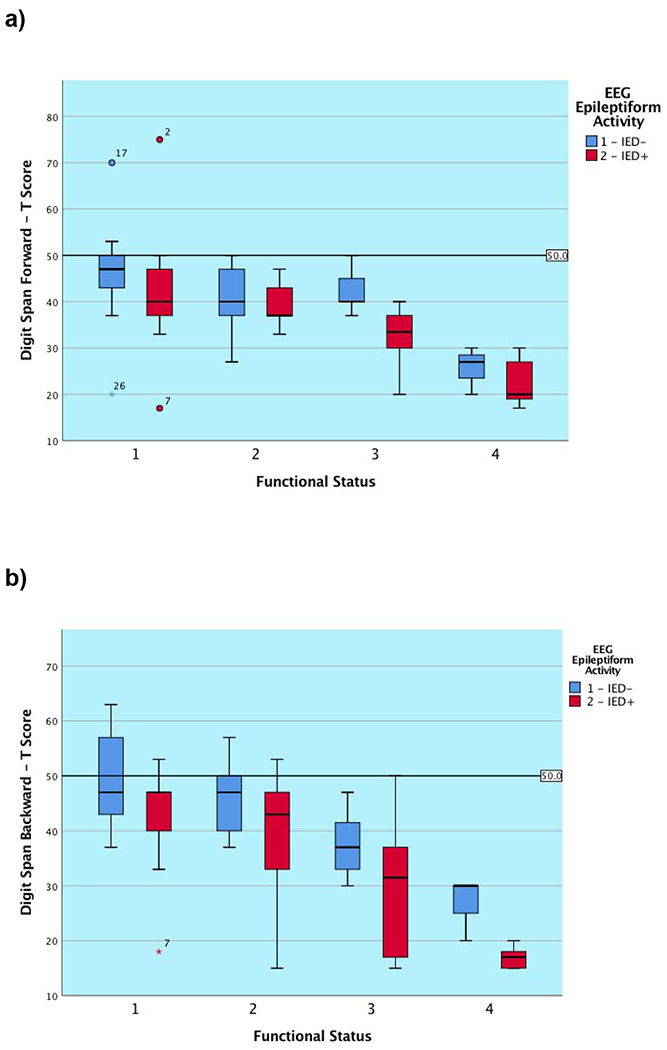
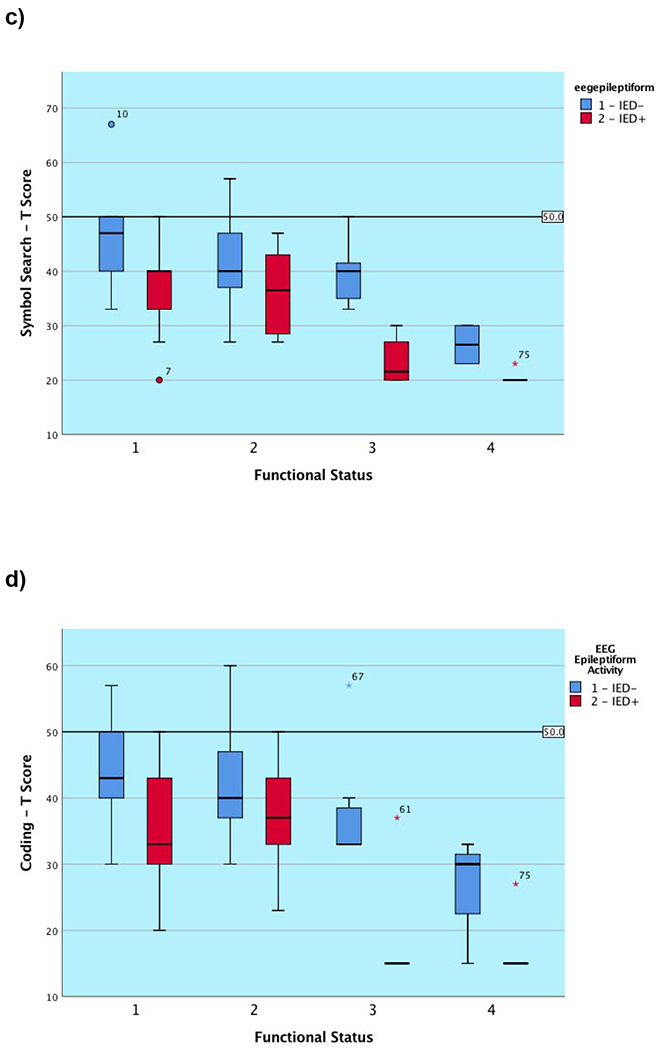
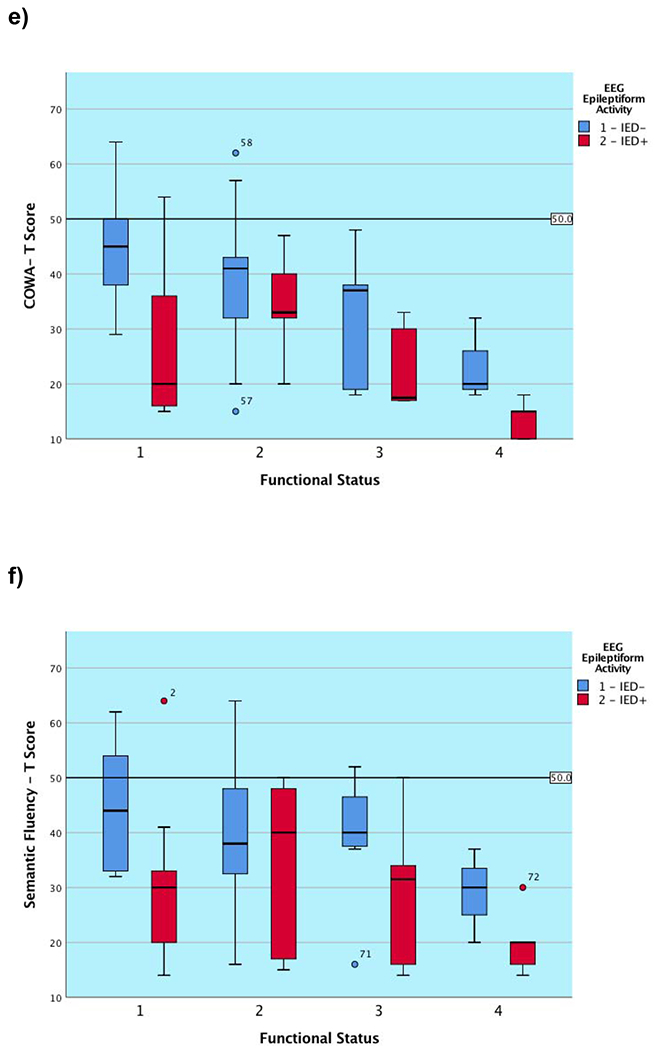
Note. IED− = Absence of interictal epileptiform discharges; IED+ = Presence of interictal epileptiform discharges (IEDs/sEDs). Two patients were excluded who experienced clinical seizures less than 24 hours prior to the test session.
3.4. Association of neurophysiological abnormalities with neuropsychological findings
Examining the main effects of the presence of IEDs (including sEDs) for each of our global neuropsychological measures and one control task (i.e., a language measure not expected to be influenced greatly by epileptiform activity) allowed us to determine if cognitive function differed by functional subgroups defined on the basis of the presence or absence of interictal and subclinical neurophysiological abnormalities. The subgroups formed by functional level and IED status did not differ with regards to psychiatric status or use of psychiatric medications or noxious ASMs.
We found a main effect for the presence of epileptiform activity in 5 of 6 (83.3%) measures (tapping primary and complex attention, cognitive processing speed, and motor speed). The one measure not significantly associated with the presence of epileptiform activity was performance on a measure of primary attention after the application of the FDR correction. There appeared to be a trend for patients across subgroups to perform worse on measures of primary attention when IEDs/sEDs were present, but this finding did not survive the application of the FDR correction factor, and appeared weaker than the remainder of our results. As noted, there were no interaction effects for these neurophysiological and functional rating variables on any outcome measure, again highlighting that the presence of EEG abnormalities is independent of functional status. Figure 1 demonstrates that the presence of IED/sED activity predicts that the subgroup experiencing it is likely to perform significantly more poorly on nearly all administered global tasks. For many of the subgroups, the graphical representation shows that those with epileptiform activity present during their simultaneous EEG performed worse than those without (ranging from approximately 0.5 to 1.75 SDs for each subgroup). In contrast, as predicted, we found no relationship between epileptiform or non-ED activity and the visual naming task. None of the functional subgroups differed as to the proportion of members with a psychiatric history, nor was there a subgroup difference when examining the patients on the basis of whether IEDS/sEDs were present.
Secondary analyses demonstrated patients with multifocal/generalized distribution of IEDs had lower T scores on symbol search (F=6.94, p=0.01), compared to those with unifocal IEDs. No other differences between multi- and uni-focal IEDs were observed on the remaining global cognitive measures. The frequency of IEDs, as expressed by spike index, was not associated with poorer neurocognitive function. We were unable to assess the effects of localized, focal seizure occurrence both due to sample size limitations and due to our focus on neurocognitive measures that are affected by more global dysfunction.
3.5. Cognitive performance differences for two epilepsy patients related to the presence of IEDs
Data for two patients from our sample were available that allowed for a closer analysis of performance differences in the presence or absence of IEDs. Patient A required testing over two dates and was also on our EMU for additional video-EEG monitoring. This allowed us to test him on more than one occasion with a measure of attention, processing speed, and a performance validity test in the presence of EEG monitoring. Patient B was included in a short case series previously published in this journal.28
Patient A was a 28 year-old, African-American, right-handed male with a long-standing history (seizure onset = age 2 years) of focal dyscognitive seizures who was being evaluated for possible epilepsy surgery. The patient underwent video-EEG monitoring, which demonstrated left temporal lobe seizure onset. 3T MRI of the brain was read as normal, while FDG PET scan exhibited regional hypometabolism of the left temporal region (medial > lateral). The patient ultimately underwent a left stereotactic laser amygdalohippocampotomy, and has been seizure free for more than 2 years.
During his baseline neuropsychological evaluation, patient A exhibited occasional left frontotemporal spike and slow wave discharges and occasional bifrontal spike wave discharges. Discharges were rated as occurring during 1–10% of the clinical record. This patient exhibited moderate deficits in attention, failed a performance validity measure, and exhibited moderate deficits in auditory/verbal learning and memory, verbal generative fluency, and naming ability. He received a level 2 functional rating, as he was living independently, but was not working at the time of his evaluation. We were able to repeat these measures approximately one-month and one-year later. The patient was undergoing additional video-EEG monitoring at the one-year date, allowing us to repeat a few of the same cognitive measures. At that time, he was not experiencing any IEDs or sEDs, and he demonstrated normal performance validity test performance and improved performance (1 to 2 SD gains) on the re-administered measures of attention, verbal generative fluency, and processing speed.
Patient B, an individual thoroughly described in a prior case series,28 was a right-handed, Caucasian male in his late 20s undergoing evaluation of new onset focal seizures with impaired awareness. Video-EEG monitoring captured three of this patient’s characteristic spells, with all of these events associated with rhythmic theta activity over the left anterior TL region, which evolved in morphology and frequency, consistent with electrographic seizures. Interictally, the patient experienced occasional left temporal sharp wave activity (F7, T3), seen throughout the recording. The 3T MRI of the brain was notable for left mesial temporal sclerosis (MTS). FDG PET was notable for decreased uptake in the lateral and medial TL regions.
During neuropsychological testing, patient B was noted to experience occasional left TL IEDs during the morning of his evaluation (spikes occurred infrequently, with just a few spikes per minute), as well as a brief run of interictal theta activity (lasting for 1–2 min in duration). At one point, during the morning session, the patient reported that he felt funny, although he never became unresponsive or confused. Neither the staff nor the patient noted any overt clinical seizure activity. He reported that his last clinical event had been several days earlier.
During the morning session, when the patient exhibited IEDs, he failed a PVT and also exhibited a severely impaired list learning performance. In contrast, during the afternoon, when not experiencing epileptiform activity, the patient performed normally on a harder word-pair learning task than he had failed during the morning. He also exhibited a normal contextual learning performance. Visual memory was decreased during the morning as well but less so during the afternoon. The patient’s processing speed was also better in the afternoon, although there was no significant change in verbal generative fluency or attention.
An initial report from the attending epileptologist suggested that the patient’s ambulatory EEG had been normal, although this reading was later revised as indicated above to include the presence of IED activity during the morning session of testing. Prior to receiving this update, we began to think that the inconsistent memory patterns, which included failure on a performance validity test, must reflect poor task engagement. This interpretation was seemingly bolstered by the patient’s personality assessment, which had revealed traits of anger, impulsivity, and noncompliance. However, the revised EEG interpretation demonstrated that the patient performed poorly on memory measures only when he had been experiencing epileptiform activity. Having these EEG data allowed us to avoid a very misleading and potentially damaging conclusion. The patient was considered a generally good surgical candidate but never opted to undergo surgery, eventually experiencing improved seizure control with further medication management.
4. DISCUSSION:
4.1. Findings summary
In this study we demonstrated that: 1) 68% of epilepsy patients undergoing NP evaluation on their normal ASM regimen manifested concurrent EEG abnormalities 2) Epileptiform abnormalities (IEDs and/or sEDs) were seen in 38% of epilepsy patients undergoing NP evaluation, 3) Epileptiform (IEDs and sEDs) but not non-epileptiform (non-ED) discharges were associated with poorer performance on measures sensitive to global cognitive dysfunction (e.g., complex attention, processing speed, generative fluency), 4) IEDs, sEDs, and non-EDs occurred in patients regardless of functional status (i.e., ranging from patients employed and living independently to those who were developmentally delayed), and 5) PWE across all functional levels tended to perform significantly worse when compared to patients of their same functional level on these global measures of cognition when epileptiform activity was present during the evaluation.
These findings from the first large study examining concurrent EEG during clinical NP evaluation provide evidence that IEDs and sEDs are associated with poorer cognitive function regardless of baseline level of ability (i.e., here we refer to baseline level of ability based upon functional status not NP testing). As the relationship is correlational in the group level analysis, it does not prove that the poorer cognitive scores resulted from the direct effects of the IEDs and sEDs, but it does contribute to the growing evidence that epileptiform activity may affect cognition. That is, as subgroups were equivalent on variables including psychiatric status, medication treatment, and demographic and epilepsy-related factors, the presence of epileptiform activity during their cognitive testing session may implicate a direct effect on cognitive performance or may reflect some yet undetermined cause that is co-occurring with their presence. As the test results of many of these patients with epileptiform activity are below expectations for their educational and current vocational achievements, it suggests that the cognitive scores are not valid for them (i.e., such scores likely represent transient impairment, as many of their performances would not fit with their documented level of functional performance in current everyday life). Additionally, in the case of the two patients for whom we were able to show improved performance when epileptiform activity was not present during their current testing, this may be taken as direct evidence of the effects of epileptiform upon cognition. Both of these patients failed PVTs when IEDs were present, but not when they were absent on the same or subsequent evaluation dates.
Our efforts reflect the early stages of research attempting to examine the possible effects of epileptiform activity (interictal and subclinical ictal) on an NP test battery. We believe that our findings raise further questions that need to be explored with a larger sample using EEG data that is acquired simultaneously and at least a full day before testing. Ideally, the cognitive testing would be time-locked to the concurrent EEG recordings. Nevertheless, we believe our findings argue against the position that there is no relationship between the presence of IEDs and cognitive dysfunction,11,12,42 which has resulted from an argument that both phenomena are simply co-occurring in the most impaired patients (i.e., those with the worst underlying brain functioning/neural substrates experience poor cognition and abundant IED rates). Instead, our findings suggest that IEDs and sEDs are regularly present in PWE across the entire functional spectrum, and that IEDs/sEDs may be detrimental to global cognitive function. Across each functional level, when epileptiform activity was present, scores on these more global measures were 0.5 to 1.75 SD below those of equivalent, functional level counterparts who were not experiencing epileptiform activity.
4.2. Association of interictal epileptiform discharges with cognition
The present study strengthens the existing link between epileptiform activity and cognitive impairment, extending it to the presurgical arena, and demonstrating this link in the largest sample of clinical patients being examined to date while undergoing concurrent EEG recordings and while on standard ASM regimens. Most prior studies have either examined cognitive testing obtained on a different day than the EEG recordings or have examined concurrent EEG recordings in the individual patient or small case series level during a single test (e.g., a memory measure).4,8,28,43 Our work demonstrates that IEDs and sEDs are likely present quite frequently among PWE undergoing NP evaluation and that their presence is associated with worse global functioning. Non-epileptiform abnormalities such as background slowing have only rarely been linked with poor cognitive performance44 and we found no significant relationship in our data.
Frequency of IEDs has been previously reported as a significant parameter of cognitive performance. In prior studies of children36 and adults,37 an IED index of >10% was deemed sufficient to impair cognitive dysfunction. We were not able to replicate this plausible association, within the confines that our patients exhibited overall modest IED frequency given that they were mostly adults with focal epilepsy monitored in wakefulness on their standard ASM regimen. However, the occurrence of IEDs, even when restricted in scope (e.g., 1% of the total EEG recording) was associated with poorer performance on these global cognitive functions, which would likely have meaningful real-world impact on function. We also found that multifocal/generalized seizures were more associated with poor processing speed than were unifocal spikes. These patterns should be further evaluated in subsequent studies.
Laterality of epileptiform activity has been shown to be specific to the impaired cognitive task16,45 in small case-controlled series and on from anecdotal observations. We have limited our focus to more global measures of cognitive ability in the current study, as we will need substantially more patients to be able to demonstrate such focal patterns in the group setting across a range of pertinent cognitive tasks. Morphology of IEDs has also been previously investigated as a potential predictor of poor cognitive performance in some studies.9,15 However, we did not provide specifics on spike morphology and duration given the small size of our cohort.
4.3. Prevalence of IEDs
We were able to demonstrate that IEDs commonly occur in PWE even when such individuals are being treated with their standard ASM regimen. While temporal lobe spikes were most common, there was a wide range of both focal and multifocal/generalized events. Six patients actually experienced subclinical seizures during the testing, which reflect events that were not recognized by the patients or their examiners (only one of these six seizures were recognized during testing). This again contradicts the idea that an observer will readily recognize clinically meaningful seizure episodes,11 and highlights that healthcare professionals fail to recognize such occurrences.
4.4. Clinical implications
Epileptiform activity during NP evaluation can remain unsuspected by patients and providers alike and appears capable of affecting its interpretation in an appreciable manner. If it is conclusively proven to account for TCI, it may not only lead to erroneous conclusions about lateralization and localization of seizure onset, but could preclude patients from surgical candidacy by providing specious estimates of their cognitive reserve. Although our primary findings provided a cross-sectional description of the problem, we have previously demonstrated a case series of epilepsy patients with dramatic variation in their cognitive performance when tested during the presence or absence of subclinical or interictal epileptiform activity.28 We also added an additional case example in the current study. Based on our initial experience with concurrent EEG data during NP testing, we feel that having the knowledge of whether unrecognized subclinical seizures have occurred or whether there has been recent IEDs is beneficial when making sense of unexpectedly poor performances. We believe that concurrent EEG provides a greater opportunity to appreciate the possible negative effects of subclinical discharges on cognitive performance through electrographic guidance and feel that such EEG data should be taken into account when interpreting cognitive test results when available. After all, this is considered standard of care when performing other presurgical testing (e.g. intracarotid amytal injections, positron emission tomography, stimulation mapping), where delineation of epileptiform activity through EEG guidance is paramount in accurate interpretation of the findings. Even postoperatively, a cognitive deterioration may be masked by poor preoperative NP performance due to latent epileptiform activity. In this manner, it is also likely that currently used reliable change indices (RCIs),46 which were developed in samples of PWE without concurrent EEG tracings, are contaminated by IEDs. This would lead to an underestimation of the actual capacity of patients, spurious variance in performance estimates, and a tendency to overlook deficits following a procedure when they actually occur. PVT measures can also be affected by IEDs,28 casting flawed doubts to the patient’s effort and motivation during the evaluation, as could have been the case with both of the individual patients reported on in this manuscript (i.e., both failed PVT when IEDs were present but not when they were absent).
4.4. Strengths and limitations
This study indirectly amplifies prior animal and human experimental and clinical data on TCI6 and emphasizes in a systematic way the potential magnitude and impact of epileptiform activity in the clinical practice of neuropsychology. Concurrently blinded neurophysiological and neuropsychological data in addition to well-characterized clinical information, were collected through standardized protocols by experienced providers. Several parameters related to the biology and the treatment of the disease that may independently affect cognition in PWE were managed both statistically and due to the happenstance formation of subgroups not differing on these clinical variables.
On the other hand, the study population was recruited from a single, academic epilepsy center, albeit representative of the majority of patients who undergo presurgical evaluation. With the exception of two subject cases, data collection was cross-sectional allowing only for correlation and not causation inferences. Some IED parameters that were previously related with TCI (such as frequency and localization of IEDs) were not sufficiently evaluated in the current study given the overall low spike-wave index of our cohort, their predominantly temporal lobe localization, and the need for a larger sample size. In actuality, power analyses suggest a sample size of at least 800–1000 patients is likely required to begin exploring lateralized effects of IEDs due to the many combinations of possible IED types, cognitive functions, and critical brain regions. Additionally, we did not examine other factors such as duration or configuration of IED activity. Finally, albeit concurrent with the cognitive evaluation, the EEG monitoring was not time-locked to each test that was applied and therefore a plausible link but no conclusive proof of TCI could be suggested at the group level.
4.5. Future directions
It appears that longitudinal NP evaluations under EEG guidance have the potential to elucidate whether epileptiform activity is a mere epiphenomenon of a dysfunctional brain or can act as an independent predictor of poor cognitive performance during formal testing. The relationship of specific electrophysiological characteristics of IEDs (frequency, morphology, duration, location) with specific cognitive and behavioral tasks warrants further investigation, ideally utilizing invasive EEG recordings that the stereotactic era of presurgical evaluation with depth electrodes nowadays lavishly offers. If the potential interaction between IEDs and sEDs and specific NP tasks can be determined over time, it may be possible to design intervention studies to allow for more accurate neuropsychological evaluation. This could include medication trials to suppress this activity with caution for medication side effects. Finally, integrating cognitive performance with real-time neurophysiology and neuroimaging may one day allow for better insight in neural networks underpinning cognitive function.28
5. CONCLUSION:
These findings call in question the validity of conclusions drawn from presurgical neuropsychological evaluations where the possibility of TCI, whether due to IEDs or sEDs, has not been investigated with concurrent EEG monitoring. In accord with other investigators,5,9 we also emphasize the close association of epileptiform activity with cognitive performance and highlight to the epilepsy surgery community the need for its evaluation during cognitive testing. We tentatively recommend simultaneous EEG at the time of NP testing, particularly for pre-surgical patients.
Key Points Box:
68% of epilepsy patients undergoing neuropsychological evaluation on their normal drug regimen manifested concurrent EEG abnormalities.
Epileptiform abnormalities (IEDs and/or subclinical seizures) were seen in 38% of epilepsy patients undergoing NP evaluation.
Epileptiform, but not non-epileptiform, EEG abnormalities were associated with measures sensitive to global cognitive dysfunction (e.g., attention, processing speed).
Epilepsy patients across all functional levels performed worse at the group level on global cognitive measures when epileptiform activity was present.
Concurrent EEG should be explored as a means of distinguishing transient from chronic impairment during neuropsychological testing.
ACKNOWLEDGEMENTS:
We would like to thank Kelsey Hewitt with assistance in assimilating the data used in this manuscript, Gloria Novak for her assistance with lab coordination, Dr. Scott Millis for suggestions related to statistical methodology, and Dr. Nigel P. Pedersen for editorial suggestions.
These results were presented in part at the 2017 American Academy of Neurology Meeting in Boston, MA.
This manuscript is consistent with Epilepsy and Behavior’s guidelines for ethical publications. This study was approved by the Emory University Institutional Review Board (IRB: #00010651 and #00083323).
Dr. Karakis, Dr. Staikova and Casey Lynam have nothing to disclose.
Dr. Taraschenko receives salary support from the University of Nebraska College of Medicine Physician-Scientist Training Award.
Dr. Drane’s research efforts are currently funded by two grants from the NIH/NINDS, which partially supported his work on this manuscript (K02 NS070960 & R01NS088748). These grants provide salary support for Dr. Drane and his laboratory staff. He also receives grant funding from Medtronic, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strauss E, Hunter M, & Wada J. Risk factors for cognitive impairment in epilepsy. Neuropsychology. 1995;9(4):457–463. [Google Scholar]
- 2.Hermann B, Meador KJ, Gaillard WD, Cramer JA. Cognition across the lifespan: Antiepileptic drugs, epilepsy, or both? Epilepsy and Behavior. 2010;17:1–5. [DOI] [PubMed] [Google Scholar]
- 3.Aldenkamp A, Arends J. The relative influence of epileptic EEG discharges, short nonconvulsive seizures, and type of epilepsy on cognitive function. Epilepsia. 2004;45(1):54–63. [DOI] [PubMed] [Google Scholar]
- 4.Binnie CD. Significance and management of transitory cognitive impairment due to subclinical EEG discharges in children. Brain Dev. 1993;15(1):23–30. [DOI] [PubMed] [Google Scholar]
- 5.Kasteleijn-Nolst Trenite DG. Transient cognitive impairment during subclinical epileptiform electroencephalographic discharges. Semin Pediatr Neurol. 1995;2(4):246–253. [DOI] [PubMed] [Google Scholar]
- 6.Faught E, Karakis I, Drane DL. The Impact of Interictal Discharges on Performance. Curr Neurol Neurosci Rep. 2018;18(12):88. [DOI] [PubMed] [Google Scholar]
- 7.Aldenkamp AP, Arends J. Effects of epileptiform EEG discharges on cognitive function: Is the concept of "transient cognitive impairment" still valid? Epilepsy & Behavior. 2004;5(Supplement 1):25–34. [DOI] [PubMed] [Google Scholar]
- 8.Aarts JH, Binnie CD, SA M, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107(1):239–308. [DOI] [PubMed] [Google Scholar]
- 9.Binnie CD, Kasteleijn-Nolst Trenite DG, Smit AM, Wilkins AJ. Interactions of epileptiform EEG discharges and cognition. Epilepsy Res. 1987;1(4):239–245. [DOI] [PubMed] [Google Scholar]
- 10.Glennon JM, Weiss-Croft L, Harrison S, Cross JH, Boyd SG, Baldeweg T. Interictal epileptiform discharges have an independent association with cognive impairment in children with lesional epilepsy. . Epilepsia. 2016;57:1436–1442. [DOI] [PubMed] [Google Scholar]
- 11.Dodrill CB, Ojemann GA. Do recent seizures and recent changes in antiepileptic drugs impact performances on neuropsychological tests in subtle ways that might easily be missed? Epilepsia. 2007;48(10):1833–1841. [DOI] [PubMed] [Google Scholar]
- 12.Korff CM, Brunklaus A, Zuberi SM. Epileptic activity is a surrogate for an underlying etiology and stopping the activity has a limited impact on developmental outcome. Epilepsia. 2015;56(10):1477–1481. [DOI] [PubMed] [Google Scholar]
- 13.Schwab RS. A method of measuring consciousness in petit mal epilepsy Journal of Nervous and Mental Disease. 1939;89:690–691. [Google Scholar]
- 14.Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107 ( Pt 1):293–308. [DOI] [PubMed] [Google Scholar]
- 15.Kasteleijn-Nolst Trenite DG, Smit AM, Velis DN, Willemse J, van Emde Boas W. On-line detection of transient neuropsychological disturbances during EEG discharges in children with epilepsy. Dev Med Child Neurol. 1990;32(1):46–50. [DOI] [PubMed] [Google Scholar]
- 16.Shewmon DA, Erwin RJ. Transient impairment of visual perception induced by single interictal occipital spikes. J Clin Exp Neuropsychol. 1989;11(5):675–691. [DOI] [PubMed] [Google Scholar]
- 17.Hutt SJ, Newton J, Fairweather H. Choice reaction time and EEG activity in children with epilepsy. Neuropsychologia. 1977;15(2):257–267. [DOI] [PubMed] [Google Scholar]
- 18.De Negri M, Baglietto MG, Battaglia FM, Gaggero R, Pessagno A, Recanati L. Treatment of electrical status epilepticus by short diazepam (DZP) cycles after DZP rectal bolus test. Brain Dev. 1995;17(5):330–333. [DOI] [PubMed] [Google Scholar]
- 19.Grote CL, Van Slyke P, Hoeppner JA. Language outcome following multiple subpial transection for Landau-Kleffner syndrome. Brain. 1999;122 ( Pt 3):561–566. [DOI] [PubMed] [Google Scholar]
- 20.Groppel G, Dorfer C, Dressier A, et al. Immediate termination of electrical status epilepticus in sleep after hemispherotomy is associated with significant progress in language development. Dev Med Child Neurol. 2017;59(l):89–97. [DOI] [PubMed] [Google Scholar]
- 21.Filippini M, Boni A, Ginannotta M, Gobbi G. Neuropsychological development in children belonging to BECTS spectrum: Long-term effect of epileptiform activity Epilepsy and Behavior. 2013;28(3):504–511. [DOI] [PubMed] [Google Scholar]
- 22.Berroya AG, McIntyre J, Webster R, et al. Speech and language deterioration in benign rolandic epilepsy. J Child Neurol. 2004;19(l):53–58. [DOI] [PubMed] [Google Scholar]
- 23.Kasteleijn-Nolst Trenite DG, Bakker DJ, Binnie CD, Buerman A, Van Raaij M. Psychological effects of subclinical epileptiform EEG discharges. I. Scholastic skills. Epilepsy Res. 1988;2(2):111–116. [DOI] [PubMed] [Google Scholar]
- 24.Kasteleijn-Nolst Trenite DG, Riemersma JB, Binnie CD, Smit AM, Meinardi H. The influence of subclinical epileptiform EEG discharges on driving behaviour. Electroencephalogr Clin Neurophysiol. 1987;67(2):167–170. [DOI] [PubMed] [Google Scholar]
- 25.Nirkko AC, Bernasconi CA, vonAllmen A, Liechti C, Mathis J, Krestel HE. Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia. 2016;57(5):832–840. [DOI] [PubMed] [Google Scholar]
- 26.Kasteleijn-Nolst Trenite DG, Vermeiren R. The impact of subclinical epileptiform discharges on complex tasks and cognition: relevance for aircrew and air traffic controllers. Epilepsy Behav. 2005;6(l):31–34. [DOI] [PubMed] [Google Scholar]
- 27.Drane DL. Neuropsychological evaluation of the epilepsy surgical candidate. In: Barr WB, Morrison C, eds. Handbook on the neuropsychology or epilepsy. NY: Springer; 2015:87–121. [Google Scholar]
- 28.Drane DL, Ojemann JG, Kim MS, et al. Interictal epileptiform discharge effects on neuropsychological assessment and epilepsy surgical planning. Epilepsy Behav. 2016;56:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen NB, Alving J, Beniczky S. Effect of medication withdrawal on the interictal epileptiform EEG discharges in presurgical evaluation Seizure. 2010;19(3):137–139. [DOI] [PubMed] [Google Scholar]
- 30.Bennett TL, Ho MR. The neuropsychology of pediatric epilepsy and antiepileptic drugs. In: Reynolds CR, Fletcher-Janzen E, eds. Handbook of Clinical Child Neuropsychology. Boston, MA: Springer; 1997. [Google Scholar]
- 31.Sirven J I, Westmoreland BF. Adult EEG: Abnormal Non-epileptiform activity. In: Rubin Dl, Daube JR, eds. Clinical Neurophysiology. 4th ed. Oxford, England: Oxford University Press; 2016. [Google Scholar]
- 32.Aldenkamp AP, Arends J. The relative influence of elileptic EEG discharges, short nonconvulsive seizures, and type of epilepsy on cognitive function. Epilepsia. 2004;45(l):54–63. [DOI] [PubMed] [Google Scholar]
- 33.Drane DL, Meador KJ. Cognitive and behavioral effect of antiepileptic drugs Epilepsy and Behavior. 2002;3(5):49–53. [DOI] [PubMed] [Google Scholar]
- 34.Ojemann LM, Ojemann GA, Dodrill CB, Crawford CA, Holmes MD, Dudley DL. Language disturbances as side effects of topiramate and zonisamide therapy. Epilepsy and Behavior. 2001;2:579–584. [DOI] [PubMed] [Google Scholar]
- 35.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22(2):79–91. [DOI] [PubMed] [Google Scholar]
- 36.Ebus S, Arends J, Hendriksen J, et al. Cognitive effects of interictal epileptiform discharges in children. Eur J Paediatr Neurol. 2012;16(6):697–706. [DOI] [PubMed] [Google Scholar]
- 37.Lv Y, Wang Z, Cui L, Ma D, Meng H. Cognitive correlates of interictal epileptiform discharges in adult patients with epilepsy in China. Epilepsy Behav. 2013;29(1):205–210. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D Wechsler Adult Intelligence Scale - Fourth Edition. San Antonio, TX: Pearson; 2009. [Google Scholar]
- 39.Delis DC, Kaplan E, Kramer JH. Dells-Kaplan Executive Function System: Examiner's Manual. San Antonio, Texas: The Psychological Corporation; 2001. [Google Scholar]
- 40.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 41.Busch RM, Hogue O, Kattan MW, et al. Nomograms to predict naming decline after temporal lobe surgeryt in adults with epilepsy. Neurology. 2018;91:e2144–e2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodrill CB. Interictal cognitive aspects of epilepsy. Epilepsia. 1992;33(Suppl. 6):S7–10. [PubMed] [Google Scholar]
- 43.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? The Lancet Neurology. 2003;2(12):725–730. [DOI] [PubMed] [Google Scholar]
- 44.Aldenkamp AP, Arends J, Overweg-Plandsoen TC, et al. Acute cognitive effects of nonconvulsive difficult-to-detect epileptic seizures and epileptiform electroencephalographic discharges. J Child Neurol. 2001;16(2):119–123. [DOI] [PubMed] [Google Scholar]
- 45.Piccinelli P, Borgatti R, Aldini A, et al. Academic performance in children with rolandic epilepsy. Developmental Medicine and Child Neurology. 2008;50(5):353–356. [DOI] [PubMed] [Google Scholar]
- 46.Martin R, Sawrie S, Gilliam F, Mackey M, Faught E, Knowlton RK, R. Determining reliable cognitive change after epilepsy surgery: Development of reliable change indices and standardized regression-based change norms for the WMS-III and WAIS-III. Epilepsia. 2002;43(12):1551–1558. [DOI] [PubMed] [Google Scholar]


