Mnemonic training boosts long-lasting memories, supported by optimized brain processing and consolidation.
Abstract
Mnemonic techniques, such as the method of loci, can powerfully boost memory. We compared memory athletes ranked among the world’s top 50 in memory sports to mnemonics-naïve controls. In a second study, participants completed a 6-week memory training, working memory training, or no intervention. Behaviorally, memory training enhanced durable, longer-lasting memories. Functional magnetic resonance imaging during encoding and recognition revealed task-based activation decreases in lateral prefrontal, as well as in parahippocampal and retrosplenial cortices in both memory athletes and participants after memory training, partly associated with better performance after 4 months. This was complemented by hippocampal-neocortical coupling during consolidation, which was stronger the more durable memories participants formed. Our findings advance knowledge on how mnemonic training boosts durable memory formation through decreased task-based activation and increased consolidation thereafter. This is in line with conceptual accounts of neural efficiency and highlights a complex interplay of neural processes critical for extraordinary memory.
INTRODUCTION
Mnemonic techniques are powerful tools to enhance memory performance. One of the most common techniques is the so-called “method of loci,” which was developed in ancient Greece and draws upon mental navigation along well-known spatial routes (1). To-be-remembered material is mentally placed at salient landmarks on an imagined path and can subsequently be recalled by retracing the route, “picking up” the previously “dropped” information. The successful application of this method typically requires training and can then lead to exceptional memory performance, as can be seen in individuals participating in events such as the World Memory Championships, who are able to memorize and accurately reproduce tremendous amounts of arbitrary information (such as word lists, digit series, and decks of cards) (2). In these competitions, however, performance is frequently assessed shortly after study, which makes it impossible to draw conclusions about durable, longer-lasting memories. It is thus unclear whether the method of loci actually helps to form durable rather than weak memories that would eventually fade with time. Previously, we have shown that initially-mnemonics-naïve participants were also able to dramatically boost their memory performance after training the method of loci for several weeks (3). Here, we substantially expanded on these findings and investigated several previously unacknowledged key aspects of the data: We focused on the effects of memory training on durable, longer-lasting memory formation; leveraged neural data during active memory processing; and addressed consolidation-related processes during post-task rest.
When applying the method of loci during memory encoding (4–6) and retrieval (7), better memory performance appears dovetailed by increased activation within the hippocampus, as well as parahippocampal and retrosplenial cortices. These regions are typically involved in spatial processing (8, 9), including scene construction (10, 11), (mental) navigation (12–14), and episodic memory (15–17). In addition, previous work revealed increased neural processing within the lateral prefrontal cortex when using the technique during encoding (6), in line with the suggested role of this region in (durable) memory formation (16, 18) and in the cognitive control of memory processes via top-down projections (19). Most studies thus far investigated participants who were instructed in the method of loci shortly before the memory tasks were completed [but see (4)]. Here, we performed two separate studies that allowed a detailed characterization of mnemonic expertise, as well as tracking the buildup of experience over time. First, we assessed memory athletes with extraordinary training in using the method of loci, as shown by their ranking among the world’s top 50 in memory sports, and compared them to mnemonics-naïve controls. Second, we recruited mnemonics-naïve participants who underwent either an extensive method of loci training regime that spanned several weeks, a working memory training, or no intervention. In both studies, we focused on the neural correlates during encoding and retrieval and aimed at elucidating their contributions to durable memory formation.
Apart from task-based activation changes, we recently demonstrated the critical role of training-related reorganization among visuospatial brain networks during rest, before engaging in any memory-related activity (3). We found that changes in functional connectivity were associated with increased memory performance in initially mnemonics-naïve participants after method of loci training, becoming similar to those identified in memory athletes. While these training-related alterations were observed during baseline, possibly setting the grounds for optimal memory processing thereafter, it is currently unclear whether memory training also affects connectivity following learning. Such post-task connectivity is thought to reflect consolidation during which memory content becomes stabilized within a wider neocortical network (20). This entails hippocampal-neocortical interactions during awake rest (21, 22) or sleep (23), potentially indexing the reactivation, or “replay,” of neuronal ensembles that were engaged during the preceding experience (24). In the current work, we investigated hippocampal-neocortical connectivity after learning and its association with durable memory formation after training the method of loci.
Across two separate studies, we tested (i) memory athletes (compared to matched but mnemonics-naïve controls; “athlete study”) and (ii) mnemonics-naïve participants who completed an intense method of loci training across 6 weeks (compared to participants who underwent working memory training or no intervention; “training study”). For all participants, functional magnetic resonance imaging (fMRI) was performed during word list encoding and temporal order recognition, as well as during resting-state periods before and after the tasks. We specifically chose these tasks as they are often used during memory championships. In addition, we reasoned that the particular strength of the method of loci lies in the learning and the recall of ordered sequences due to mental navigation through the imagined “memory palace,” directly tapping into episodic memory. To assess the effects of training the method of loci, participants of the training study were reinvited to complete another fMRI session after 6 weeks. Memory performance was assessed during free recall tests immediately and 1 day after each session (Fig. 1, A to C). We hypothesized increased memory durability in initially mnemonics-naïve participants after memory training, compared to both control groups. This should be paralleled by training-related neural changes in visuospatial brain regions during both tasks and consolidation-related hippocampal-neocortical coupling during rest. Additionally, and in parallel to our previous study, we predicted that activation and connectivity profiles in mnemonics-naïve participants after training (compared to the respective control groups) would be similar to when comparing memory athletes with matched controls.
Fig. 1. Study design, procedures, and results from the free recall tests.
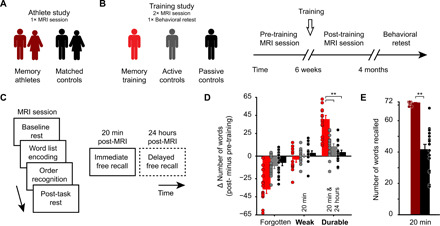
(A) In the athlete study, we tested memory athletes (n = 17) and compared them to matched controls (n = 16) during a single MRI session. (B) Participants of the training study were pseudo-randomized into three groups after an initial MRI session (pre-training): the memory training group (n = 17), active controls (n = 16), and passive controls (n = 17). Participants returned to the laboratory for a second MRI session (post-training) and took part in a behavioral retest after 4 months. (C) General structure of MRI sessions: baseline resting-state period (8 min), word list encoding and temporal order recognition tasks (10 min each), post-task resting-state period (8 min), immediate free recall test (5 + 5 min and 20 min post-MRI), and delayed free recall test after 24 hours (5 + 5 min; only completed by participants of the training study, dashed frame). (D) Training study: Change in the number of forgotten/weak/durable words from pre- to post-training sessions. Note that only weak and durable memories were included in the analysis (marked in bold). **P < 0.0001. (E) Athlete study: Free recall performance (20 min). **P = 0.0005. (D and E) Error bars reflect the SEM. See also Table 2 for an overview of free recall performance across the groups.
RESULTS
Study design and participant samples
We tested 17 participants who were experts in using the method of loci and were ranked among the world’s top 50 in memory sports (hereafter referred to as “memory athletes”) and compared them to a control group closely matched for age, sex, handedness, and intelligence (see Table 1 for a sample description, and see the “Participants of athlete and training studies” section). Within this so-called athlete study, memory performance and brain function were assessed during a single MRI session (Fig. 1A; memory athletes, n = 17; matched controls, n = 16).
Table 1. Descriptive sample details.
Sample size, number of males, left-handers, and smokers are given as absolute numbers; fluid reasoning and memory abilities are given as mean intelligence quotient (IQ) scores ± SD.
| Athlete study | Training study | ||||
| Memory athletes | Matched controls | Memory training group | Active controls | Passive controls | |
| n | 17 | 16 | 17 | 16 | 17 |
| Males | 9 | 9 | 17 | 16 | 17 |
| Age (years), means ± SD | 24.6 ± 4.3 | 25.4 ± 3.9 | 23.7 ± 2.7 | 24.3 ± 2.5 | 24.4 ± 3.8 |
| Age (years), range | 19–32 | 20–35 | 20–29 | 20–29 | 18–30 |
| Fluid reasoning | 128.1 ± 9.6 | 128.4 ± 10.8 | 117.4 ± 12.7 | 116.4 ± 14.6 | 118.2 ± 13.2 |
| Memory abilities | Not tested | 104.6 ± 27.8 | 103.3 ± 13.3 | 100.8 ± 21.9 | 101.8 ± 16.2 |
| Left-handers | 3 | 3 | 0 | 0 | 0 |
| Smokers | 1 | 1 | 0 | 0 | 0 |
In a second study (i.e., the training study; n = 50), mnemonics-naïve participants completed a method of loci training over 6 weeks (40 × 30 min). The memory training group (n = 17) was compared to an active (n = 16) and a passive control group (n = 17) that underwent working memory training (40 × 30 min) or no intervention across the 6-week interval, respectively (see the “Study procedures and tasks” section). Memory performance and brain function were assessed before and after training. To test whether method of loci training affected memory performance over a longer term, participants of the training group also completed a behavioral retest after 4 months (Fig. 1B).
Memory training enhances durable memory formation in initially mnemonics-naïve participants
Starting out, we put our focus on data from the training study and hypothesized that if the method of loci truly boosted durable rather than weak memories, we should see increased free recall performance at the delayed compared to the immediate free recall test. We thus analyzed the change in memory durability from before to after training (see Table 2 for the overall free recall performance). During both sessions, participants studied word lists and were asked to retrieve material during a free recall test 20 min after MR scanning (immediate free recall), as well as 24 hours later (delayed free recall; Fig. 1C). While some words were never recalled (i.e., forgotten material), weak memories were defined as those that could only be remembered at the immediate free recall but were forgotten afterwards. Durable memories were the ones also remembered after the delay, thus tackling stable, longer-term memory (18).
Table 2. Free recall and recognition performance across groups of the training study.
Free recall performance during the immediate and delayed free recall tests, performance during the retest after 4 months, as well as d-prime scores during temporal order recognition. Values represent the average number of freely recalled words/d-prime ± SD.
| Memory training group (n = 17) | Active controls (n = 16) | Passive controls (n = 17) | |
| Free recall performance | |||
| Pre-training session | 25.2 ± 16.9 | 30.7 ± 14.6 | 28.9 ± 15.4 |
| Immediate free recall after 20 min | |||
| Pre-training session | 16.1 ± 14.2 | 19.4 ± 12.5 | 18.5 ± 15.4 |
| Delayed free recall after 24 hours | |||
| Post-training session | 62.2 ± 10.9 | 41.7 ± 16.3 | 36.4 ± 19.4 |
| Immediate free recall after 20 min | |||
| Post-training session | 56.2 ± 16.2 | 30.5 ± 17.8 | 21.4 ± 19 |
| Delayed free recall after 24 hours | |||
| Retest after 4 months | 50.3 ± 16.5 (n = 16) | 30.4 ± 9.9 (n = 14) | 27.4 ± 9.8 (n = 15) |
| Change in free recall performance | 22.7 ± 18.8 (n = 16) | −0.7 ± 9.9(n = 14) | −1.5 ± 11.2 (n = 15) |
| (4 months > pre-training, immediate test) | |||
| Temporal order recognition | |||
| Pre-training session, d-prime | 1.3 ± 1.3 | 1.6 ± 0.5 | 1.6 ± 1.1 |
| Post-training session, d-prime | 2.5 ± 0.6 | 2.1 ± 1.3 | 2.3 ± 0.9 |
Results revealed a significant increase in durable memories in the memory training group from before to after training, compared to both active and passive control groups, while the change in the amount of weak memories from pre- to post-training did not significantly differ between the three groups [Fig. 1D; for the following analyses, we focused on weak and durable memories and solely illustrated the amount of forgotten material in the figure: mixed-model analysis of variance (ANOVA); memory training group, n = 17; active controls, n = 16; passive controls, n = 17; group × memory type interaction, F(2,94) = 30.85, P < 0.0001; pairwise comparisons, durable: memory training group > active control group, t(94) = 7.4, P < 0.0001; memory training group > passive control group, t(94) = 8.84, P < 0.0001; main effect of group, F(2,94) = 15.25, P < 0.0001; main effect of memory type, P > 0.05]. Additional analyses revealed that the change in memory durability was specifically related to memory training and was not due to potential performance differences already present pre-training (results S1). Thus, training the method of loci increased durable memories in initially mnemonics-naïve participants.
Different from the training study, the athlete study comprised a single experimental session; performance was tested immediately after the tasks and only once (20 min post-MRI; thus, the analysis of memory durability was not possible). As also shown previously [(3); but note that current analyses involved a subsample of participants], free recall performance within the athlete study was generally high (presumably due to the well-matched control group) but was significantly higher in memory athletes compared to matched controls (Wilcoxon signed-rank test; one matched pair excluded from analysis; memory athletes, n = 16, median = 72; matched controls, n = 16, median = 43; V = 136, P = 0.0005; Fig. 1E).
Method of loci decreases activation in lateral prefrontal cortex during word list encoding in athletes and initially mnemonics-naïve participants after training
Next, we turned to the fMRI data and investigated changes in brain activation from pre- to post-training while participants studied previously unstudied words (word list encoding task; Figs. 1C and 2A), in analogy to tasks often used during memory championships and tapping into episodic memory. During this task, memory athletes and the memory training group (post-training) were asked to use the method of loci during encoding (thus, they were asked to mentally navigate through their memory palace and to “place” the studied words at the specific loci). We hypothesized engagement of regions typically involved in visuospatial processing and successful memory encoding, including the hippocampus and adjacent medial temporal lobe (MTL) structures, retrosplenial cortex, and lateral prefrontal regions (4–6, 16). Because of the at-ceiling performance of memory athletes and the memory training group after training, we started out by testing activation changes during encoding compared to an implicit baseline, with individual memory durability scores added as a covariate (see also the “MRI data processing: Task data” section).
Fig. 2. Activation changes during word list encoding.
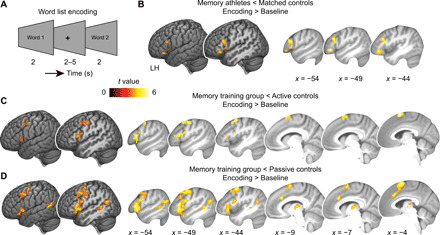
(A) Word list encoding task: Participants studied previously unstudied words during each MRI session. (B) Athlete study: Brain activation during encoding (encoding > baseline) is decreased in memory athletes compared to matched controls. (C and D) Training study: Brain activation is significantly decreased in the memory training group after training when compared to (C) active or (D) passive controls (group × session interactions; see table S1 for main effects and table S2 for a comparison between active and passive controls). Results are shown at P < 0.05 family-wise error (FWE)–corrected at cluster level (cluster-defining threshold P < 0.001). LH, left hemisphere.
First, we focused on data from the athlete study and compared memory athletes to matched controls. Unexpectedly, we found robust activation decreases within the left lateral prefrontal cortex (MNI coordinates of the two global maxima: x = −48, y = 32, z = −8, Z value = 4.55, 226 voxels; and x = −46, y = 20, z = 16, Z value = 4.38, 370 voxels) during word list encoding [independent-samples t test, contrast encoding > baseline, matched controls > memory athletes, covariate number of words freely recalled, statistical threshold for this and all subsequent analyses: P < 0.05, family-wise error (FWE)–corrected at cluster level using a cluster-defining threshold of P < 0.001; critical cluster size = 125 voxels; memory athletes, n = 17; matched controls, n = 16; Fig. 2B]. Contrary to what we had expected, there were no significant activation changes within the MTL or retrosplenial cortex.
Second, we leveraged data from the training study and found, notably similar to above, decreased activation within the left lateral prefrontal cortex in the memory training group after training, compared to both the active and the passive control groups (interaction effects, two separate full factorial designs, contrast encoding > baseline, covariate memory durability score; memory training group, n = 17; active controls, n = 16; passive controls, n = 17; Fig. 2, C and D; table S1, also for main effects of group and session). When comparing the memory training with the passive control group, activation decreases further included the thalamus and the left angular gyrus (Fig. 2D and table S1; see table S2 for a comparison between active and passive control groups). We also repeated the analyses without the performance covariates included, which led to highly similar results (results S2). Therefore, findings from both studies consistently revealed decreased activation within left lateral prefrontal regions when applying the method of loci during word list encoding.
To elucidate whether results were actually caused by a decrease in activation in the memory training group over time rather than by group differences already present pre-training, we performed additional region-of-interest (ROI) analyses and extracted average activation values from significant interaction clusters, together confirming the results above (results S3). Moreover, there were no significant differences in activation levels between the memory athletes and the memory training group (post-training) during word list encoding [independent-samples t test, contrast encoding > baseline, covariate number of words recalled during the immediate free recall test; memory athletes, n = 17; memory group (post-training), n = 17], indicating similar activation profiles of athletes and initially mnemonics-naïve participants after training the method of loci.
Lastly, we performed additional subsequent memory analyses (results S4; please note that this was only based on a subset of participants). If decreased activation was related to durable memory formation while applying the method of loci, we expected to find stronger decreases for durable compared to weak or forgotten encoding. While results confirmed our finding of generally decreased activation in the memory training group (post-training) compared to both control groups, results did not appear specific for durable memory formation. Thus, activation decreases were related to general memory processing during encoding in the different groups but were not significantly related to durable memory formation.
Expertise in the method of loci can positively affect recognition performance while slowing down response times
Following word list encoding, participants completed the temporal order recognition task where word triplets were presented in either the same or a different order as studied previously (Figs. 1C and 3A). We specifically developed this task as an MR-compatible measure of recall and reasoned that since memory athletes and participants of the memory training group (post-training) were asked to use the method of loci during temporal order recognition (i.e., they were asked to mentally move through their memory palace, serially retrieving word-loci associations), these participants should excel at judging the word order.
Fig. 3. D-prime and activation changes during temporal order recognition.
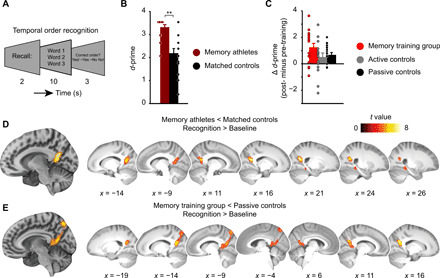
(A) After word list encoding, word triplets were presented in the same or a different order as studied previously and participants were asked to judge the order. (B) Athlete study: Recognition performance (d-prime) for memory athletes and matched controls. **P < 0.001. (C) Training study: Change in d-prime (from pre- to post-training sessions) across the groups (main effect of group, P = 0.133). Error bars (B and C) reflect the SEM. See also Table 2 for an overview of recognition performance across the groups. (D) Athlete study: Brain activation during temporal order recognition (recognition > baseline) is decreased in memory athletes compared to matched controls (see Results for MNI coordinates). (E) Training study: Brain activation is significantly decreased in the memory training group after training when compared to passive controls (group × session interaction; see table S3). Results are shown at P < 0.05 FWE-corrected at cluster level (cluster-defining threshold P < 0.001).
Memory athletes indeed showed significantly higher recognition performance (indexed through d-prime) compared to matched controls (Wilcoxon signed-rank test; one matched pair excluded from analysis; memory athletes, n = 16, median = 3.54; matched controls, n = 16, median = 2.38; V = 131.5, P = 0.001; Fig. 3B; see results S5 for response times). However, despite numerically increased d-prime scores in the memory training group after training, there was no significant difference in recognition performance between the three groups (change in d-prime; Fig. 3C; one-way ANOVA; memory training group, n = 17; active controls, n = 16; passive controls, n = 17; main effect of group, P = 0.133, pairwise comparisons: memory training > active controls: P = 0.135, effect size d = 0.592; memory training > passive controls: P = 0.294, d = 0.569; active > passive controls: P = 0.898, d = −0.161; see results S6 for a general increase in d-prime from pre- and post-training). In addition, participants of the memory training group showed slower response times after training (results S5). Thus, expertise in the method of loci positively affected recognition performance in memory athletes. While the effect of memory training on recognition performance in initially mnemonics-naïve participants appeared to be positive as well (but note that results were not significant), findings were accompanied by generally slower responses.
Method of loci decreases activation in posterior parahippocampal and retrosplenial cortices during temporal order recognition in athletes and initially mnemonics-naïve participants after training
We next turned to the neuroimaging data acquired during temporal order recognition. Since memory athletes and participants of the memory training group (post-training) were asked to use the method of loci during order recognition, we expected increased engagement of brain regions typically associated with visuospatial processing and successful memory retrieval, such as the hippocampus, parahippocampal, and retrosplenial cortices (4, 7–9, 12, 15, 17). As correct and incorrect trials were unevenly distributed between groups, we compared recognition trials against the implicit, active baseline (syllable counting), with individual d-prime scores added as a covariate (see also the “MRI data processing: Task data” section).
Similar to the profile of activation decreases during the preceding word list encoding task (see above), results indicated reduced activation within the right posterior parahippocampal and bilateral retrosplenial cortices (x = 30, y = −40, z = −12, Z value = 4.54, 142 voxels), as well as in bilateral superior parietal gyrus (left: x = −16, y = −58, z = 23, Z value = 5.31, 501 voxels; right: x = 20, y = −60, z = 22, Z value = 6.01, 435 voxels) in memory athletes compared to matched controls (independent-samples t test, contrast recognition > baseline, covariate d-prime, P < 0.05, FWE-corrected at cluster level using a cluster-defining threshold of P < 0.001, critical cluster size = 123 voxels; memory athletes, n = 17; matched controls, n = 16; Fig. 3D). This was dovetailed by decreased activation within the posterior parahippocampal and bilateral retrosplenial cortices and in the precuneus in the memory training group after training, when compared to passive controls (interaction effect, full factorial design, contrast recognition > baseline, covariate d-prime; memory training group, n = 17; passive controls, n = 17; Fig. 3E; see table S3 for main effects of group and session). There was no significant group × session interaction when comparing the memory training group with active controls, but activation in the precuneus (x = −4, y = −78, z = 50, Z value = 3.81, 143 voxels) and bilateral superior parietal gyrus (left: x = −12, y = −56, z = 16, Z value = 3.72, 148 voxels; right: x = 22, y = −60, z = 22, Z value = 4.55, 217 voxels) generally decreased over time (main effect of session, full factorial design, contrast recognition > baseline, covariate d-prime, P < 0.05, FWE-corrected at cluster level using a cluster-defining threshold of P < 0.001, critical cluster size = 134 voxels; memory training group, n = 17; active controls, n = 16). Furthermore, active and passive control groups did not differ significantly (full factorial design, contrast recognition > baseline, covariate d-prime; active controls, n = 16; passive controls, n = 17). We repeated the analyses without the performance covariates included, which did not change our results (results S2). To summarize, findings from both studies consistently revealed decreased activation within the posterior parahippocampal and retrosplenial cortices when applying the method of loci during temporal order recognition.
Additional ROI analyses confirmed that these results were actually related to memory training and not an effect of potential group differences already present pre-training (results S7). Finally, as also for data during word list encoding (see above), there were no significant differences in activation levels between the memory athletes and the memory training group (post-training) during temporal order recognition [independent-samples t test, contrast recognition > baseline, covariate d-prime; memory athletes, n = 17; memory group (posttraining), n = 17], indicating similar activation profiles in athletes and initially mnemonics-naïve participants after training the method of loci.
Training-related activation decreases during temporal order recognition are associated with better free recall performance after 4 months
Next, we asked whether the whole-brain activation changes from pre- to post-training sessions during the memory tasks (word list encoding and temporal order recognition) were associated with increased free recall performance beyond the 24-hour delay. During the retest after 4 months, participants of the training study were once more invited to the behavioral laboratory where they completed the word list encoding task followed by a free recall test (the same word list as during the pre-training session was used; see the “Retest after 4 months” section).
As also reported previously [(3); but note that current analyses include a subsample of participants], the memory training group showed significantly increased free recall performance after 4 months (4-month retest minus pre-training test 20 min post-MRI), as compared to both active and passive control groups [change in the number of words freely recalled, means ± SEM: memory training group, 22.67 ± 4.87; active controls, −0.71 ± 2.65; passive controls, −1.5 ± 2.79; five subjects were not available for the retest, analysis thus included 45 participants; memory training group, n = 15; active controls, n = 14; passive controls, n = 16; one-way ANOVA; main effect of group, F(2,42) = 14.67, P < 0.0001; pairwise comparisons: memory training > active controls, t(42) = 4.53, P = 0.0001; memory training > passive controls, t(42) = 4.84, P = 0.0001; active controls > passive controls, P = 0.987]. Hence, the memory training group was able to use the method of loci successfully (as indicated through increased free recall performance compared to both control groups), even after several months.
We then went on to test the cross-participant relationship between whole-brain activation decreases from pre- to post-training and the change in free recall performance (4-month retest minus pre-training20 min). To this end, we created individual difference maps (pre- minus post-training) based on the first-level contrasts (encoding > baseline, recognition > baseline), reflecting decreased activation over time. These difference maps were then submitted to two separate linear regression analyses with the change in free recall performance added as a covariate of interest.
During temporal order recognition, activation decreases across sessions were positively associated with free recall performance after 4 months. This included decreased activation from pre- to post-training within a widespread set of regions comprising the hippocampus, the posterior parahippocampal region, the left fusiform gyrus, retrosplenial cortex, precuneus, left angular gyrus, thalamus, bilateral striatum, medial prefrontal and orbitofrontal cortex, and the precentral gyrus (Fig. 4 and table S4). Thus, stronger activation decreases in these regions during temporal order recognition were coupled to increased free recall performance at the 4-month retest across all participants of the training study. In contrast, and in line with our results of general but not memory-specific activation decreases during encoding (see above and see also results S4), activation decreases during word list encoding appeared unrelated to memory performance after 4 months.
Fig. 4. Activation changes during temporal order recognition and association with memory performance at the 4-month retest.
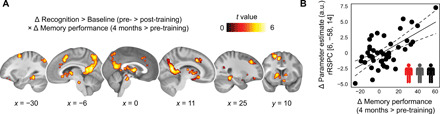
(A) Training study: Decreases in brain activation (recognition > baseline) from before to after training (pre- > post-training) that positively scaled with the change in free recall performance (referred to “memory performance” in the figure) from the pre-training session (20 min post-MRI) to the retest after 4 months (covariate of interest). Results are shown at P < 0.05 FWE-corrected at cluster level (cluster-defining threshold P < 0.001; see also table S4). (B) The scatterplot shows the relationship between the change in parameter estimates [arbitrary units (a.u.)] from the pre- to post-training sessions, extracted from the global maximum (right retrosplenial cortex, rRSPC; 8-mm sphere around MNI peak coordinate, x = 6, y = −58, z = 14), and the change in memory performance (4-month retest minus pre-training20 min). Given the clear inferential circularity, we would like to highlight that this plot serves visualization purposes only, solely illustrating the direction of association between the brain-behavior relationship.
Increased hippocampal-neocortical coupling during post-task rest is related to memory consolidation in athletes and initially mnemonics-naïve participants after training
So far, we documented increased memory performance (memory durability and recognition performance), along with decreased brain activation during memory-related processing (temporal order recognition) in memory athletes and (partly) in participants of the memory training group after training. In addition, we hypothesized that durable memory formation should be associated with increased consolidation processes during rest after learning, involving hippocampal-neocortical circuits (20–22), which should be related to durable memory formation. To test this, we took the anatomical boundaries of the bilateral hippocampus as a seed (Fig. 5A), calculated its whole-brain connectivity during each resting-state period and session, and tested whether connectivity varied as a function of memory performance (i.e., free recall performance in the athlete study and memory durability in the training study; see also the “MRI data processing: Resting-state periods” section).
Fig. 5. Hippocampal connectivity at rest and association with memory durability.
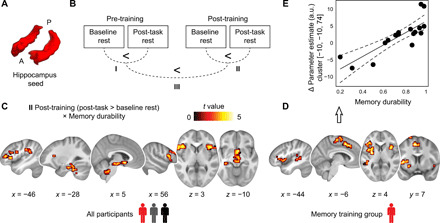
(A) Bilateral anatomical hippocampus seed used for whole-brain connectivity analysis. A, anterior, P, posterior. (B) Training study: Schematic of the analysis steps performed. We tested hippocampal connectivity increases (post-task > baseline rest) during the pre- (I) and post-training sessions (II) and investigated the increase in consolidation-related coupling from pre- to post-training sessions (III; [post-task > baseline rest]post > [post-task > baseline rest]pre). Analysis of data from the athlete study involved a single MRI session (post-task > baseline), which is not depicted here. (C) Training study: Hippocampal-neocortical connectivity increases from baseline to post-task rest during the post-training session positively scaled with the proportion of durable memories formed (i.e., memory durability) across all participants (see also table S5). (D) Follow-up analyses revealed that these effects were specifically driven by connectivity changes in the memory training group but were not present in passive or active controls (see results S9 and table S6). Given the clear inferential circularity, we would like to highlight that the scatterplot (E) serves visualization purposes only, solely illustrating the direction of association between the brain-behavior relationship. All results are shown at P < 0.05 FWE-corrected at cluster level (cluster-defining threshold P < 0.001).
First, we focused on data from the athlete study and investigated consolidation-related hippocampal connectivity increases from before to after the tasks. Across participants (including both memory athletes and matched controls, n = 33), we found coupling between the hippocampus and a bilateral cerebellar region (x = 32, y = −70, z = −52, Z value = 4.6, 416 voxels) that positively scaled with subsequent free recall performance [linear regression, contrast difference map (post-task > baseline rest), number of words freely recalled 20 min post-MRI added as a covariate of interest; P < 0.05, FWE-corrected at cluster level using a cluster-defining threshold of P < 0.001, cluster size = 62 voxels]. Thus, hippocampal connectivity with the cerebellum was stronger during rest the more words participants recalled. More specifically, these results appeared to be driven by stronger hippocampal-cerebellar coupling in memory athletes compared to matched controls (results S8).
Second, we turned toward data from the training study (including the memory training group, active and passive controls, n = 49). To draw precise conclusions about connectivity changes related to extensive memory training, we investigated hippocampal-neocortical coupling before and after training, as well as changes from before to after the tasks, and their association with durable memory formation. Correspondingly, and in line with the remaining analysis strategy of the paper, this involved three analysis steps: connectivity (I) during the pre-training session (post-task > baseline rest), (II) during the post-training session (post-task > baseline rest), and (III) changes from pre- to post-training sessions ([post-task > baseline rest]post > [post-task > baseline rest]pre; see also Fig. 5B).
Results from the post-training session (II; Fig. 5, B and C) revealed stronger connectivity from baseline to post-task rest between the hippocampus and the bilateral lateral prefrontal cortex, left angular gyrus, the left hippocampus and parahippocampal cortex, bilateral insula and right caudate nucleus, as well as the brainstem and cerebellum that positively scaled with memory durability across participants [linear regression, contrast difference map (post-task > baseline rest), memory durability (post-training) added as a covariate of interest; see Fig. 5C]. There was no negative association between hippocampal-neocortical connectivity and memory durability (but see table S5 for general connectivity increases from baseline to post-task rest). Therefore, hippocampal coupling with a widespread set of neocortical regions was stronger after training the more durable memories participants formed. Follow-up analyses confirmed that this effect was driven by connectivity changes in the memory training group and was not present in the active or passive controls (Fig. 5D; see results S9).
To be able to directly compare the results between the athlete and training studies, we repeated the above analysis but instead tested the association of hippocampal-neocortical coupling with the raw numbers of words freely recalled per participant (thus, both analyses involved the same covariate). This led to virtually identical results, confirming once more that hippocampal-neocortical coupling (post-training) was positively associated with free recall performance at the delayed but not at the immediate test across participants of the memory training group (results S10).
We did not find any significant, hippocampal-neocortical connectivity increases related to memory durability during the pretraining session (I; Fig. 5B) or from pre- to post-training sessions (III; Fig. 5B; but general connectivity increases across sessions in the right lingual gyrus, two global maxima: x = 14, y = −46, z = 0, Z value = 4.49, 102 voxels; and x = 4, y = −80, z = −7, Z value = 4.02, 49 voxels; P < 0.05, FWE-corrected at cluster level using a cluster-defining threshold of P < 0.001, critical cluster size = 37 voxels), and none of the results were associated to the change in memory performance from pre-training to after 4 months.
To summarize, stronger hippocampal-cerebellar connectivity during rest after memory processing was associated with increased memory performance across participants of the athlete study. In the training study, hippocampal connectivity with the lateral prefrontal cortex, MTL, and striatum was increased the more durable memories participants formed (post-training). Additional analyses confirmed that these effects were specifically driven by connectivity changes in memory athletes and the memory training group after training but were not present in any of the control groups.
Stronger activation decreases during temporal order recognition are associated with increased hippocampal-neocortical coupling during post-task rest
As a last step, we explored whether the training-induced activation decreases during temporal order recognition (which appeared stronger, the better participants performed during the 4-month retest; Fig. 4) were related to hippocampal-neocortical connectivity increases during the post-task rest (which appeared stronger, the more durable memories participants formed; Fig. 5). This analysis involved three steps: First, we created a whole-brain binary mask centered on the significant activation effects obtained during temporal order recognition (Fig. 6A; based on a sample of n = 45) and extracted the raw change in activation per participant (i.e., using the contrast pre- minus posttraining, recognition > baseline). Second, we created a whole-brain binary mask centered on the significant connectivity effects obtained during post-task resting-state period after training (Fig. 6A; based on a sample of n = 49) and extracted the raw change in hippocampal connectivity per participant (i.e., using the contrast post- minus pre-task, post-training). Third, we selected the subsample of participants from which both activation and resting-state data were available (n = 44) and performed a correlation analysis.
Fig. 6. Relation between task-based activation decreases and hippocampal connectivity at rest.
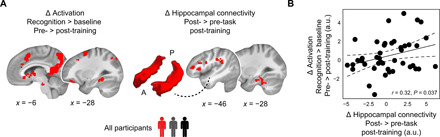
(A) (Left) We created a whole-brain binary mask centered on the significant activation effects obtained during temporal order recognition (Fig. 4; based on a sample of n = 45) and extracted the raw change in activation per participant (i.e., using the contrast pre- minus post-training, recognition > baseline). (Right) We created a whole-brain binary mask centered on the significant connectivity effects obtained during post-task resting state after training (Fig. 5; based on a sample of n = 49) and extracted the raw change in hippocampal connectivity per participant (i.e., using the contrast post- minus pre-task resting state, during the post-training session). The bilateral hippocampal seed is schematically indicated (A, anterior; P, posterior). (B) Correlational analysis across all participants of the training study. Larger activation decreases (i.e., more positive values pre-training) were coupled to larger increases in hippocampal connectivity after training.
Notably, we found a significantly positive association between activation decreases and connectivity increases across participants (rPearson = 0.32, P = 0.037; Fig. 6B). In other words, larger decreases in activation during the temporal order recognition task from pre- to post-training (and, thus, more positive activation values pre-training) were coupled with larger increases in hippocampal-neocortical coupling during the post-task rest after training. This highlights a direct association between task-based activation decreases and consolidation-related processes across participants of the training study.
DISCUSSION
In this study, we investigated memory training using the method of loci and its impact on memory durability and neural coding. To obtain a detailed characterization of long-term training effects and existing expertise with mnemonic techniques, we performed two separate experiments that involved memory athletes as well as mnemonics-naïve participants who underwent an extensive memory training over 4 weeks. We present several key findings that substantially expand our previous work (3) in the following ways: We show that the method of loci serves to boost durable, longer-lasting memories, leading to exceptional memory performance in athletes and initially mnemonics-naïve participants after training. Applying this mnemonic technique is related to decreased task-based activation, potentially due to strategy use and in line with theoretical accounts of neural efficiency. These task-based activation decreases are stronger, the better participants perform after 4 months, suggesting stable long-term effects of mnemonic training. After learning, memory training triggers hippocampal-neocortical connectivity, which is stronger the more durable memories participants formed. Lastly, we found that stronger activation decreases during temporal order recognition were directly linked to increased consolidation-related processes during rest.
Central to our question was the potential effect of mnemonic training on durable memory formation. We found that initially- mnemonics-naïve participants improved memory durability after training, compared to both active and passive control groups (Fig. 1D). These results were mirrored by the exceptional, close-to-ceiling performance in memory athletes compared to matched controls (Fig. 1E). Effectively using the method of loci requires mental navigation along well-known spatial routes and the anchoring of to-be-remembered information to salient locations on the path (1). The method thus combines several key aspects that are thought to affect memory. First, the method of loci relies on visuospatial processing that engages the hippocampus, parahippocampal, and retrosplenial cortices (4–7). These brain regions are typically associated with spatial processing and (mental) navigation (8–14), as well as (episodic) memory (15–17). A link between space and memory therefore appears natural, and spatial representations have been discussed to organize conceptual knowledge and to allow flexible behavior (12, 25). Second, the reliance on well-known spatial routes bears resemblance to the utilization of schema-like knowledge structures that are established during prior experiences. Schemas are assumed to provide a scaffolding that promotes memory encoding and consolidation (26). Instinctively, the stable formation of spatial routes for mental navigation takes time and should thus benefit from extensive method of loci training. While previous studies provided participants with an introduction into the mnemonic technique 1 day prior (5) or shortly before study (7), we recruited participants who underwent a training-regime that spanned several weeks [see also (3)]. Hence, our training allowed participants to build up stable spatial routes that could incorporate novel information more readily, drastically enhancing durable memories and sustainably increasing performance even after 4 months. Related to this, mnemonic techniques were discussed to speed up memory stabilization using schema-like structures, promoting the direct transfer from working memory into long-term storage [as proposed by the “long-term working memory” hypothesis, (27)]. Third, mentally placing arbitrary to-be-remembered information at salient locations along the imagined path likely produces relatively bizarre associations, thereby triggering neural mechanisms related to novelty (26). This, in turn, cranks up dopaminergic and noradrenergic release from the brainstem and ventral striatum toward the hippocampus (28), which is thought to promote memory persistence by triggering synaptic (29) and systems consolidation (20). Overall, we suggest that the method of loci favorably combines the abovementioned aspects (visuospatial processing, prior knowledge, and novelty) to boost durable memories, leading to exceptional memory performance in athletes and initially mnemonics-naïve participants after training.
We found consistent activation decreases in lateral prefrontal regions when memory athletes and participants of the memory training group (post-training) studied verbal material (Fig. 2, B to D). The lateral prefrontal cortex is involved in memory encoding while applying the method of loci (6) and supports durable memory formation (16, 18) as well as the selection and flexible organization of memories via top-down control (19). Our effects, however, appeared not specifically related to durable memory formation. Instead, results might indicate a diminished requirement for cognitive control due to extensive method of loci training and might be grounded upon the use of different cognitive strategies between the groups. An important difference to previous studies [for example, see (4)] is that we focused on changes in brain activation from before to after training. Previous work (4) assessed brain activation within a single session, therefore not capturing training-induced changes. Differences in results might thus stem from divergent approaches when contrasting brain activation, and we speculate that Maguire et al. (4) might have obtained similar effects if they would have compared their results to a pre-training baseline. In addition, we contrasted encoding-related activation to the implicit baseline (due to the close-to-ceiling performance of memory athletes and the memory training group after training). Although participants were instructed not to rehearse material between trials and blocks of the word list encoding task (and although we have no reason to doubt their compliance), we cannot preclude the possibility that participants rehearsed (some of) the material during this downtime, as we did not use any postexperimental questionnaires. This could, at least in part, explain the effects observed (i.e., decreased activation during the trial compared to rest). However, what speaks against this potential explanation is the fact that we found similar results also during temporal order recognition, during which trials were contrasted against an active baseline that involved a cognitive task (syllable counting). More specifically, we found decreased activation within the posterior parahippocampal and retrosplenial cortices during temporal order recognition in memory athletes and participants of the memory training group after training (Fig. 3, D and E). Although these results are in line with previous reports with regard to their spatial layout (4–7), we revealed diametrically opposite effects. In other words, we report robust activation decreases despite the fact that successful memory encoding (16, 18) and retrieval (15, 17) typically engage increased activation in a set of prefrontal, medial temporal, and visuospatial brain regions. Importantly, the training-related decreases were directly associated with better memory performance at the 4-month retest across participants (Fig. 4), and stronger activation decrease was coupled to increases in hippocampal-neocortical connectivity during rest after learning (Fig. 6).
Our results are in line with the so-called “neural efficiency hypothesis” (30), which proposes that highly skilled or intelligent individuals display lower (thus, more efficient) brain activation during cognitive tasks for reaching the same behavioral performance (31, 32). For instance, participants with higher verbal or visuospatial skills were found to show lower levels of brain activation when using the respective strategies during cognitive tasks (31). Such efficient neural coding might require extensive training (30, 33). Our 6-week training regime might thus resemble the buildup of expertise and could explain the differential findings compared to previous studies. The concept of neural efficiency has, however, been criticized in that (lateral prefrontal) activation effects could stem from differential strategy use between groups (34). Indeed, the memory training group (post-training) was asked to use the method of loci during memory encoding and temporal order recognition; their strategy thus differed from participants in both control groups. Heinzel et al. (33) demonstrated activation decreases after working memory training and their relationship with performance increases in related tasks. We speculate that the working memory group might have shown similar activation decreases when being tested with a working memory task during fMRI. However, we would not expect such an interpretation to also account for the formation of durable memories, as the working memory group showed no significant behavioral memory improvement from before to after training (Fig. 1D). Therefore, our crucial point here is that memory training served to improve durable memory formation through acquiring a previously unknown strategy, which altered task-based activation levels but also post-task connectivity linked to consolidation-related processes. Another criticism suggests that trained participants might spend less time on the task when performance is high (34). Here, we found that the memory training group (post-training) showed slower response times during temporal order recognition. We speculate that this was potentially related to increased memory search when mentally retracing previously studied information along the imagined path, an effect that was especially pronounced during incorrect trials where participants were presumably unable to recall some of the locus-word associations, spending more time trying to retrieve them. Response time differences between the groups thus appear unlikely to have influenced activation decreases since the memory training group actually spent more time-on-task. In addition, our results were directly related to memory performance at the 4-month retest, as well as to hippocampal connectivity increases during post-task rest, speaking for the relevant association of task-based activation decreases, behavioral improvements, and effects potentially related to memory consolidation. At this point, it is important to mention that we refrain from any direct conclusions regarding the specific neural or molecular mechanisms supporting efficiency, leveraging this account rather on the descriptive level and as a guiding theoretical framework.
Durable memory formation relies on consolidation during rest that is thought to stabilize memory content. This entails communication between hippocampal-neocortical networks (20–22), potentially reflecting replay of neuronal ensembles that were engaged during the preceding experience (24). Across participants of the training study, we found increased hippocampal connectivity during rest after training with the lateral prefrontal cortex, left angular gyrus, parahippocampal regions, and the caudate nucleus that was higher the more durable memories were formed (Fig. 5C). Follow-up analyses revealed that these effects were specific to the memory training group after training but were not present in any of the control groups (Fig. 5D). Connectivity effects during post-task rest were generally less widespread in the athlete compared to the training sample and were centered on increased hippocampal-cerebellar connectivity at higher memory performance. The cerebellum was associated with hippocampal-dependent navigation (35) and might thus contribute to the consolidation of previously studied material. Because of their long-standing experience with the mnemonic technique, memory athletes (compared to participants of the training study) might have formed even stronger memories already during the tasks, thereby alleviating the need for additional consolidation during rest. Together, our findings of hippocampal interactions after learning show an association with (durable) free recall performance, potentially linked to processes underlying memory consolidation.
We found that method of loci training positively affected free recall performance even after 4 months. One open limitation is that we used the same word list during the retest as also during the initial pre-training session (due to the fact that we decided to add the retest after the lists had already been constructed). However, participants of the training study were assigned to the different groups only after the first session was completed (i.e., after the delayed test, pre-training). Any material from the first session that might have been remembered also at the 4-month retest was thus independent of training. We acknowledge that recall during the pre-training session might have served to strengthen the memories for those successfully recalled words by means of the testing effect (36, 37), but this should have affected all groups of the training study to a similar extent.
Different from method of loci training, working memory training did not improve performance on the memory tasks used. This is in line with previous work highlighting that working memory training does not readily generalize to other tasks in different domains (38, 39), lacking so-called “far transfer” effects [i.e., effects that generalize to untrained tasks dissimilar from the training; (40, 41)]. In addition, working memory training has been associated with short- rather than long-term effects (38, 42), although results appear sometimes inconsistent [(43, 44), which reported small but long-lasting improvements in reasoning/intelligence but also small and only short-term effects for long-term memory]. Overall, previous work demonstrated weak effects of working memory training on other cognitive abilities, and evidence for stable long-term effects on, for example, memory performance is so far missing.
Together, we found that memory training enhanced durable memories. In both memory athletes and initially mnemonics-naïve participants after memory training, we found decreased brain activation in lateral prefrontal, as well as in posterior parahippocampal and retrosplenial cortices during encoding and recognition, respectively. These activation decreases were partly associated with better memory performance at a 4-month follow-up, indicating that participants were able to successfully use the method even after several months. Effects were paralleled by increased hippocampal-neocortical connectivity during rest that was higher the more durable memories participants formed. Lastly, task-based decreases during recognition were larger the stronger hippocampal connectivity was during rest after learning. We suggest that the method of loci favorably combines key aspects affecting memory, such as visuospatial processing, prior knowledge, and novelty. This serves to boost durable memories, leading to exceptional memory performance in athletes and initially mnemonics-naïve participants after training. On a neural level, applying this mnemonic technique appears linked to decreased task-based activation and to increased consolidation-related processes thereafter. In line with conceptual accounts of neural efficiency, this highlights a complex interplay between brain activation and connectivity critical for extraordinary memory.
MATERIALS AND METHODS
Participants of athlete and training studies
We tested 23 memory athletes (age, 28 ± 8.6 years; nine females) that were ranked among the top 50 of the world’s memory sports (www.world-memory-statistics.com). These participants were compared to an equally sized control sample that was matched for age, sex, handedness, smoking status, and intelligence quotient (IQ), recruited among gifted students of academic foundations and members of the high-IQ society Mensa (see also Table 1). Six participants of the matched control group were selected from the training study based on their cognitive abilities within the screening session (see below), evenly sampled from the three groups. These participants completed a standardized memory test (45) to avoid including “natural” superior memorizers (none of the participants reached this criterion), as well as a test for fluid reasoning (46). Experience with any kind of systematic memory training was an exclusion criterion. Together, all participants were part of the so-called athlete study. Of the 23 memory athletes, 17 completed a word list encoding and temporal order recognition task inside the MR scanner; current analyses were thus restricted to a subsample of participants [memory athletes, n = 17 (age, 25 ± 4 years; eight females); matched controls, n = 16 (age, 25 ± 4 years; seven females); see also the “MRI data processing: Task data” and “MRI data processing: Resting-state periods” sections for a detailed description of exclusions].
Next, we recruited 51 male participants (age, 24 ± 3 years; all students at the University of Munich) to test the behavioral and neural effects of mnemonic training in a mnemonics-naïve participant sample (i.e., the so-called training study). We included only male participants since memory appears affected by the menstrual cycle (47, 48) and since the longitudinal design of our study would have not allowed us to systematically control for this factor. On the basis of cognitive performance determined during an initial screening session (45, 46), participants were pseudo-randomly assigned to three groups to ensure similar cognitive baseline levels between the groups so that potential changes in memory performance were attributable to the specific training procedure (see also Table 1). As above, experience with any kind of systematic memory training was an exclusion criterion. All participants were offered to receive the non-assigned training condition for free after study completion if they wished to do so. A first group of participants underwent a 6-week training in the method of loci between the two test sessions (memory training group). These participants were directly compared to a sample who underwent an n-back working memory training between the sessions (active controls) and to a group who did not undergo any intervention (passive controls). Current analyses included 50 participants [memory training group, n = 17 (age: 24 ± 3 years); active controls, n = 16 (age: 24 ± 3 years); passive controls, n = 17 (age: 24 ± 4 years); see also the “MRI data processing: Task data” and “MRI data processing: Resting-state periods” sections for a detailed description of exclusions]. All participants provided written informed consent before participation, and the study was reviewed and approved by the ethics committee of the Medical Faculty of the University of Munich (Munich, Germany).
Study procedures and tasks
Participants of the memory athlete study completed a single MRI session (Fig. 1A). Participants of the training study took part in two MRI sessions that were placed 6 weeks apart, as well as in a behavioral session after 4 months (Fig. 1B). After the first MRI session (i.e., pre-training session), participants were pseudo-randomly grouped into one of three training groups and completed a training in the method of loci (memory training group), a working memory training (active controls), or no intervention (passive controls). Six weeks following the pre-training session, participants were invited to the second MRI session (i.e., post-training session) and were asked to complete a behavioral retest 4 months thereafter.
Method of loci training
Participants of the memory training group were familiarized with the method of loci at the Max Planck Institute of Psychiatry, where they were introduced to the method, were taught their first route within and outside the institute, applied their first route in an initial memory task under supervision, were familiarized with the online platform that was used to complete and monitor the home-based training (https://memocamp.com), were instructed on how to build new routes, and were provided with a training plan for the upcoming week. To ensure equal training of all routes and to reduce interference of word lists memorized on preceding days, training plans gave specific instructions on which set of locations to use. After this, participants completed 30 min of training each day for 40 days at home.
During the training, participants built and memorized another three loci routes (thus, a total of four trained routes), with which they trained to memorize random word lists. The task difficulty (i.e., the number of words that needed to be memorized) dynamically changed according to their individual performance. At the start of each daily training, five words were presented during a first run. The number of presented words increased in subsequent runs by +5 as soon as participants successfully recalled all words in a given run. Speed of training success was defined as the average number of runs needed per level increase until 40 words were successfully recalled (thus, eight runs). This final level was reached by most participants of the memory training group (16 of 17) but can hardly be achieved by mnemonics-naïve participants. Log files of the training sessions were checked daily to monitor compliance. In case a participant missed a training session or trained not long enough, he was contacted on the following morning and instructed to expand the next training session to make up for the missed training time. Participants came into the laboratory for an interview (within small groups of two to three participants) regarding potential training problems once every week where they were trained under direct supervision and received the training plan for the following week.
Working memory training
Participants of the active control group were familiarized with the dual n-back task where participants had to monitor and update a series of both visually presented locations and auditorily presented letters (3). Participants completed 30 min of training each day for 40 days. The training was completed using a home-based working memory training program, and training results were monitored daily to check compliance. In case a participant missed a training session or trained not long enough, he was contacted on the following morning and instructed to expand the next training session to make up for the missed training time. Participants came into the laboratory once a week for an interview (within small groups of two to three participants) regarding potential training problems and for a training under direct supervision. Participants were instructed to perform as well as possible, but to refrain from any systematic long-term memory training.
Passive controls
The passive controls did not receive any training between the two sessions and received no specific instructions beyond refraining from systematic memory training while being enrolled in the study.
General structure of MRI sessions
Each MRI session (athlete and training study) started out with the acquisition of a structural brain image, a baseline resting-state period, followed by the word list encoding and temporal order recognition tasks, as well as a post-task resting-state period (Fig. 1C). Participants then performed a free recall test in the behavioral laboratory 20 min after exiting the MR scanner (i.e., immediate free recall), and another free recall test 24 hours later via phone interview (i.e., delayed free recall). Participants of the memory athlete study only performed the immediate but no delayed free recall test.
Resting-state periods
A first 8-min resting-state period was acquired at the start of each MRI session (i.e., baseline rest; Fig. 1C). To assess intrinsic connectivity changes related to memory consolidation, another resting-state period (8 min) was placed after the temporal order recognition task (i.e., post-task rest). Thus, participants of the memory athletes and training studies completed two and four resting-state periods, respectively. All participants were instructed to think of nothing in particular and to not rehearse the studied word lists after the tasks.
Word list encoding task
We introduced this task (as well as the temporal order recognition task below) since we reasoned that the particular strength of the method of loci lies in the learning (and in the recall) of ordered sequences (due to the mental navigation through the imagined memory palace). A list of 72 concrete nouns was presented within each session. Thus, material was presented in two separate lists that were counterbalanced for word length and frequency and were presented in random order, and the order of lists was balanced across participants.
After an initial instruction (5 s), words were presented individually (3 s), separated by a jittered interval ranging between 2 and 5 s (mean = 3.5 s) during which a fixation cross was presented (Fig. 2A). Another fixation period (30 s) was inserted after every sixth word. Memory athletes and the memory training group (post-training) were asked to use the method of loci during word list encoding. In other words, participants were asked to mentally move through their memory palace, placing the different words at specific loci. Participants of the control groups received no specific instructions. In addition, all participants were instructed to not rehearse the studied material during the fixation periods (30 s) but rather to think of nothing in particular.
Temporal order recognition task
We developed this task to form an MR-compatible measure of recall performance. Participants viewed 24 triplets of words based on material from the previously encoded word list. This included all words from the previously encoding word lists. Triplets were formed from adjacent word presentations during the previous word list encoding task and were then shuffled. Hence, each word triplet consisted of three words that were previously presented in direct (0-distance) and close (1-distance) proximity.
A brief cue indicated the start of the next trial (2 s) after which a triplet was presented (10 s) and participants had to indicate whether the word order was the same as presented before (3 s; answer options “same, sure,” “same, maybe,” “different, maybe,” and “different, sure”; Fig. 3A). Triplet presentations were separated by an active control condition during which participants were asked whether triplets that consisted of new words were shown in ascending or descending order according to their number of syllables. Recognition trials alternated with control trials in ABAB fashion. The timing of the control trials was identical to the recognition trials (brief cue indicating the start of the next trial, 2 s, after which a word triplet was presented, 10 s, and participants had to provide an answer, 3 s). Memory athletes and the memory training group (post-training) were asked to use the method of loci during temporal order recognition. In other words, participants were asked to mentally move through their memory palace, retrieving individual words to subsequently judge whether they were presented in correct order. Participants of the control groups received no specific instructions.
Free recall tests
The free recall test was our main outcome measure of interest, as it is most comparable with tasks used at memory championships. Following MR scanning (approximately 20 min later), participants were asked to freely recall (i.e., to write down) the 72 words studied during the preceding word list encoding task (i.e., immediate free recall test). After 5 min, participants were asked whether they would need more time, and the free recall test was terminated after an additional 5 min. Another free recall test (5 + 5 min) was performed via telephone 24 hours later (i.e., delayed free recall test). Performance was determined by the number of words correctly recalled, ignoring word order or spelling mistakes. The delayed free recall test was announced to all participants, as we intended to keep pre- and post-training sessions identical. Participants of the athlete study completed only the immediate but not the delayed free recall.
Retest after 4 months
During the retest 4 months after the post-training session, participants of the memory training study completed the word list encoding task once more, followed by a delay filled with a reasoning task (15 min), and a free recall task (since free recall was our main outcome of interest). All tasks were completed in the behavioral laboratory, and the task material was the same as during the initial pre-training session. We did not use a novel word list as we decided to add the 4-month retest session after the lists had already been constructed. However, participants of the training study were assigned to the different groups only after the first session was completed (i.e., after the delayed test, pre-training). We reasoned that any material from the first session that might have been remembered also at the 4-month retest should thus be independent of training. Participants of the memory training group (post-training) were asked to use the method of loci during word list encoding. Five participants (two memory training group, two active controls, and one passive control) were not available for the retest after 4 months.
Behavioral measures: Memory durability
Memory durability was determined for participants of the memory training study by assessing performance at the immediate (20 min) and delayed (24 hours) free recall test, for both the pre- and post-training session separately. This resulted in three types of responses [see also (18)]: words that were (i) already forgotten during the immediate free recall test (“forgotten”), (ii) recalled during the immediate but forgotten during the delayed free recall test (“weak”), or (iii) recalled at both free recall tests (“durable”). Words that were not recalled at the immediate test but recalled at the delayed test were grouped together with words that were forgotten [number of words, means ± SEM; (pre-/post-training) memory training group, 1.29 ± 0.65/0.59 ± 0.21; active controls, 0.94 ± 0.48/1.29 ± 0.57; passive controls, 1.35 ± 0.49/0.35 ± 0.19].
We aimed at identifying activation and connectivity profiles that were associated with durable memory formation and, thus, calculated a behavioral “memory durability score” for each participant. We divided the number of durable by the total number of recalled words (durable ∩ weak; i.e., the proportion of durable memories), thereby normalizing individual memory durability scores for general memory performance. We did this separately for the pre- and post-training session and included these values as a covariate in group-level analyses (see below). We did not determine memory durability for the athlete study, as these participants only completed the immediate but not the delayed free recall test.
Behavioral measures: Recognition performance (d-prime)
Recognition performance was quantified using d-prime scores. To accommodate hit rates of 1 and false alarm rates of 0 in memory athletes and the memory training group (post-training), we adjusted the individual hit and false alarm rates (z scored) of all participants by adding 0.5 to the raw counts of individual hit and false alarm rates (49). D-prime was calculated as the difference between these adjusted hit and false alarm rates [z(hits) − z(false alarms)], collapsing across the different confidence levels (“sure,” “maybe”), as memory athletes and participants of the memory training group (post-training) had very few “maybe” responses [number of “maybe” triplets, means ± SEM; athlete study, memory athletes: 0.47 ± 0.1, matched controls: 4 ± 0.43; training study (pre-/post-training), memory training group: 6.1 ± 0.61/1.53 ± 0.54, active controls: 4.47 ± 0.85/2.36 ± 0.61, passive controls: 3.35 ± 0.74/2.65 ± 0.85]. There were very few missed responses that were collapsed together with incorrect triplets [number of missed triplets, means ± SEM; athlete study, memory athletes: 0.18 ± 0.1, matched controls: 0.29 ± 0.14; training study (pre−/post-training), memory training group: 0.59 ± 0.19/0.65 ± 0.17, active controls: 0.47 ± 0.17/0.29 ± 0.14, passive controls: 0.35 ± 0.12/0.35 ± 0.15].
Statistical analysis of behavioral measures
Analysis of all behavioral data was carried out using R (www.r-project.org). The general free recall performance of participants in both studies was reported previously (3). Here, we used a set of independent-samples t tests and ANOVA models to analyze previously unacknowledged data regarding memory durability and temporal order recognition performance (i.e., number of triplets correctly recognized, d-prime, and response times). Significant interaction effects were followed up with pairwise comparisons using the R package emmeans (https://cran.r-project.org/web/packages/emmeans/index.html) and were corrected for multiple comparisons (Tukey’s post hoc test). α was set to 0.05 throughout (two-tailed). Any exploratory analyses are explicitly described as such.
Imaging parameters
All imaging data were collected at the Max Planck Institute of Psychiatry (Munich, Germany), using a 3T scanner (GE Discovery MR750, General Electric, USA) equipped with a 12-channel head coil. We acquired 192 T2*-weighted blood oxygenation level–dependent (BOLD) images during each resting-state period, using the following echo-planar imaging (EPI) sequence: repetition time (TR), 2.5 s; echo time (TE), 30 ms; 34 axial slices; interleaved acquisition; field of view (FOV), 240 × 240 mm; 64 × 64 matrix; slice thickness, 3 mm; 1-mm slice gap. During each task (i.e., word list encoding and temporal order recognition), we obtained 292 T2*-weighted BOLD images with the following EPI sequence: TR, 2.5 s; TE, 30 ms; flip angle, 90°; 42 ascending axial slices; FOV, 240 × 240 mm; 64 × 64 matrix; slice thickness, 2 mm. The structural image was acquired with the following parameters: TR, 7.1 s; TE, 2.2 ms; flip angle, 12°; in-plane FOV, 240 mm; 320 × 320 × 128 matrix; slice thickness, 1.3 mm.
MRI data processing: Task data
MRI data preprocessing and participant exclusions
The fMRI data were processed using Statistical Parametric Mapping (SPM, version 8) (www.fil.ion.ucl.ac.uk/spm/) in combination with Matlab (The Mathworks, Natick, MA, USA). The first four volumes were excluded to allow for T1 equilibration. The remaining volumes were realigned to the mean image of each session (athlete study) or across sessions (training study). The structural scan was co-registered to the mean functional scan and segmented into gray matter, white matter, and cerebrospinal fluid using the “New Segmentation” algorithm. All images (functional and structural) were spatially normalized to the Montreal Neurological Institute (MNI) EPI template using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra [DARTEL; (50)], and functional images were further smoothed with a three-dimensional Gaussian kernel [8-mm full width at half maximum (FWHM)].
Head motion [quantified as framewise displacement (FD); (51)] was similar across groups for word list encoding and temporal order recognition tasks during the pre- and post-training sessions (results S11). We excluded one participant because of technical problems with the MR images (training study, active controls) and one participant because of strong motion (FD = 103.39; athlete study, matched controls; motion affected only the word list encoding task but the participant was excluded from all analyses). This left 50 participants within the training study (memory training group, n = 17; active controls, n = 16; passive controls, n = 17) and 33 participants within the athlete study (memory athletes, n = 17; matched controls, n = 16).
fMRI data modeling and statistical analysis: Word list encoding task
Memory athletes and participants of the memory training group (post-training) showed free recall performance close to ceiling level. Using a common subsequent memory analysis would have led to an uneven distribution of trials across participants (i.e., most memory athletes might remember all words and forget none, whereas this might be different for participants of the control groups, but see results S4 for additional analysis). Thus, we opted for an alternative approach and tested activation changes during word list encoding compared to an implicit baseline, with individual memory durability scores (see above) added as a covariate during statistical inference.
The BOLD response for all trials during the word list encoding task was modeled as a single task regressor, time-locked to the onset of each trial. Instructions were binned within a separate regressor of no interest (i.e., including two task-based regressors). All events were estimated as a boxcar function with a duration of 3 s (encoding) or 5 s (instructions) and were convolved with the SPM default canonical hemodynamic response function. To account for noise due to head movement, we included the six realignment parameters, their first derivatives, and the squared first derivatives into the design matrix. A high-pass filter with a cutoff at 128 s was applied. For participants of the memory training study, both sessions (i.e., pre- and post-training) were combined into one first-level model. The task regressors were then contrasted against the implicit baseline.
We then tested activation changes between the groups and over time on a second level, applying pairwise comparisons between the three groups. Specifically, we used three separate random-effects, mixed ANOVAs with group (i.e., memory training group versus active controls, memory training group versus passive controls, and active versus passive controls) as a between-subjects factor and session (pre- and post-training) as a within-subjects factor. We thus used several 2 × 2 designs for the different group comparisons. Individual memory durability scores were added as a covariate (see above). Conditions were compared using post hoc t tests. Differential activation between memory athletes and matched controls was investigated with an independent-samples t test, and the number of words freely recalled was added as a covariate (and see results S2 for additional analyses excluding performance covariates).
fMRI data modeling and statistical analysis: Temporal order recognition task
Following the rationale above, we tested activation changes during temporal order recognition compared to an active control condition (i.e., syllable counting) rather than contrasting correct and incorrect trials. Recognition trials were modeled as a single task regressor, time-locked to the onset of each trial (i.e., the presentation of the triplet) and with the duration set until a button press occurred (thus, the duration was equal to the response time). Instructions were binned within a separate regressor of no interest (duration, 2 s). As above, the first-level model thus included two task-based regressors. The remaining modeling steps and the statistical inference were performed identical to the word list encoding task (see above). Individual recognition performance (i.e., d-prime scores) was added as a covariate during group-level analyses for both studies (and see results S2 for additional analyses excluding performance covariates).
MRI data processing: Resting-state periods
MRI data preprocessing and participant exclusions
Data from resting-state periods were processed using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL, version 5.0.1; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). As a first step, the structural scan was processed (using fsl_anat), reoriented to the MNI standard space (using fslreorient2std), bias-field–corrected [FMRIB’s Automated Segmentation Tool (FAST)], and brain-extracted [Brain Extraction Tool (BET)]. The functional images were preprocessed using the FMRI Expert Analysis Tool (FEAT). We excluded the first four volumes to account for T1 equilibration, performed motion correction [Motion Correction using FMRIB’s Linear Image Registration Tool (MCFLIRT)], spatial smoothing with a Gaussian kernel (5-mm FWHM), and aligned images to the bias-corrected, brain-extracted structural image [FMRIB’s Linear Image Registration Tool (FLIRT)] using boundary-based registration. The structural image was aligned with the MNI 152 EPI template using nonlinear registration [FMRIB’s Non-linear Image Registration Tool (FNIRT)]. After manual inspection of the registered images, we used independent component analysis (ICA) to automatically detect and remove subject-specific, motion-related artifacts [ICA-based strategy for Automatic Removal Of Motion Artifacts, ICA-AROMA, version 0.3-beta; https://github.com/maartenmennes/ICA-AROMA; (52)].
Head motion (FD; see above) was similar across groups for all resting-state periods (baseline, post-task rest) during the pre- and post-training sessions (results S11). We excluded two participants because of technical problems with the MR images (both training study and active controls) and one participant because of strong motion during the resting-state period (see above; athlete study, matched controls). This left 49 participants within the training study (memory training group, n = 17; active controls, n = 15; passive controls, n = 17) and 33 participants within the athlete study (memory athletes, n = 17; matched controls, n = 16).
Seed-based hippocampal connectivity and statistical analysis
To test for hippocampal whole-brain connectivity related to consolidation, we placed a bilateral seed within the anatomical boundaries of the hippocampus [taken from the Automated Anatomical Labeling atlas, AAL; (53)] and calculated connectivity by regressing the average hippocampal time course against all other voxel time courses in the brain, resulting in connectivity maps for each resting-state period (baseline and post-task rest for both pre-/post-training sessions). We then created difference maps (post-task minus baseline rest) that yielded connectivity increases within each session and submitted them to group-level analyses.
The athlete study comprised a single MRI session, and connectivity increases (i.e., difference maps, post-task minus baseline) were analyzed using a linear regression with free recall performance (i.e., the number of words freely recalled 20 min post-MRI scanning) added as a covariate of interest. To follow our analysis rationale when assessing the task-based data, resting-state data from the training study (i.e., two MRI sessions) were analyzed in three steps: We investigated hippocampal coupling (i) during the pre-training session (post-task minus baseline rest), (ii) during the post-training session (post-task minus baseline rest), and (iii) changes from pre- to post-training sessions ([post-task minus baseline rest]post minus [post-task minus baseline rest]pre) using three separate linear regression models with memory durability added as a covariate of interest [i.e., (I and II) the session-specific proportion of durable memories formed or (III) the change in memory durability from pre- to post-training sessions].
Statistical thresholds for fMRI analyses and anatomical labeling
Throughout the manuscript, and unless stated otherwise, significance for all MRI analyses was assessed using cluster inference with a cluster-defining threshold of P < 0.001 and a cluster probability of P < 0.05 FWE-corrected for multiple comparisons. The corrected cluster size (i.e., the spatial extent of a cluster that is required in order to be labeled as significant) was calculated using the SPM extension “CorrClusTh.m” and the Newton-Raphson search method (script provided by T. Nichols, University of Warwick, UK, and M. Wilke, University of Tübingen, Germany; www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/spm/). Anatomical nomenclature for all tables was obtained from the Laboratory for Neuro Imaging (LONI) Brain Atlas (LBPA40; www.loni.usc.edu/atlases/).
Acknowledgments
We thank the five anonymous reviewers for taking the time to provide valuable and constructive feedback. Furthermore, we thank the International Association of Memory (IAM; www.iam-memory.org) for support in recruiting memory athletes. Funding: This article is based on work from a Veni project supported by the Netherlands Organisation for Scientific Research; the European Platform for Life Sciences, Mind Sciences, and the Humanities supported by the Volkswagen Foundation; and COST Action CA18106 supported by COST (European Cooperation in Science and Technology). Author contributions: B.N.K., D.R., K.O., S.K., M.C., and M.D. designed the study. B.N.K., P.S., S.W., M.C., and M.D. collected the data. I.C.W. analyzed the data and wrote the first version of the manuscript. I.C.W., C.L., and M.D. edited the manuscript. A.S., G.F., C.L., M.C., and M.D. supervised the study. I.C.W., B.N.K., P.S., S.W., D.R., K.O., S.K., A.S., G.F., C.L., M.C., and M.D. discussed and interpreted results and commented on the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. In addition, all anonymized data and analysis code are available upon request in accordance with the requirements of the institute, the funding body, and the institutional ethics board. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/10/eabc7606/DC1
REFERENCES AND NOTES
- 1.F. Yates, The Art of Memory (Routledge & Kegan, 1966). [Google Scholar]
- 2.J. Foer, Moonwalking with Einstein (Penguin Books, 2011). [Google Scholar]
- 3.Dresler M., Shirer W. R., Konrad B. N., Müller N. C. J., Wagner I. C., Fernández G., Czisch M., Greicius M. D., Mnemonic training reshapes brain networks to support superior memory. Neuron 93, 1227–1235.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maguire E. A., Valentine E. R., Wilding J. M., Kapur N., Routes to remembering: The brains behind superior memory. Nat. Neurosci. 6, 90–95 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Fellner M.-C., Volberg G., Wimber M., Goldhacker M., Greenlee M. W., Hanslmayr S., Spatial Mnemonic encoding: Theta power decreases and medial temporal lobe BOLD increases co-occur during the usage of the method of loci. eNeuro 3, ENEURO.0184-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyberg L., Sandblom J., Jones S., Neely A. S., Petersson K. M., Ingvar M., Backman L., Neural correlates of training-related memory improvement in adulthood and aging. Proc. Natl. Acad. Sci. U.S.A. 100, 13728–13733 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo Y., Suzuki M., Mugikura S., Abe N., Takahashi S., Iijima T., Fujii T., Changes in brain activation associated with use of a memory strategy: A functional MRI study. Neuroimage 24, 1154–1163 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Vann S. D., Aggleton J. P., Maguire E. A., What does the retrosplenial cortex do? Nat. Rev. Neurosci. 10, 792–802 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Hartley T., Lever C., Burgess N., O’Keefe J., Space in the brain: How the hippocampal formation supports spatial cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20120510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson J., The human imagination: The cognitive neuroscience of visual mental imagery. Nat. Rev. Neurosci. 20, 624–634 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Hassabis D., Maguire E. A., The construction system of the brain. Philos. Trans. R. Soc. B Biol. Sci. 364, 1263–1271 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein R. A., Patai E. Z., Julian J. B., Spiers H. J., The cognitive map in humans: Spatial navigation and beyond. Nat. Neurosci. 20, 1504–1513 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein R. A., Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn. Sci. 12, 388–396 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiers H. J., Maguire E. A., A navigational guidance system in the human brain. Hippocampus 17, 618–626 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rugg M. D., Vilberg K. L., Brain networks underlying episodic memory retrieval. Curr. Opin. Neurobiol. 23, 255–260 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H., Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage 54, 2446–2461 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Kim H., Default network activation during episodic and semantic memory retrieval: A selective meta-analytic comparison. Neuropsychologia 80, 35–46 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Wagner I. C., van Buuren M., Bovy L., Fernández G., Parallel engagement of regions associated with encoding and later retrieval forms durable memories. J. Neurosci. 36, 7985–7995 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenfeld R. S., Ranganath C., Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13, 280–291 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Frankland P. W., Bontempi B., The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Tambini A., Ketz N., Davachi L., Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65, 280–290 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Kesteren M. T. R., Fernández G., Norris D. G., Hermans E. J., Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc. Natl. Acad. Sci. U.S.A. 107, 7550–7555 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diekelmann S., Born J., The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Carr M. F., Jadhav S. P., Frank L. M., Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nat. Neurosci. 14, 147–153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behrens T. E. J., Muller T. H., Whittington J. C. R., Mark S., Baram A. B., Stachenfeld K. L., Kurth-Nelson Z., What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Fernández G., Morris R. G. M., Memory, novelty and prior knowledge. Trends Neurosci. 41, 654–659 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Ericsson K. A., Kintsch W., Long-term working memory. Psychol. Rev. 102, 211–245 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Duszkiewicz A. J., McNamara C. G., Takeuchi T., Genzel L., Novelty and dopaminergic modulation of memory persistence: A tale of two systems. Trends Neurosci. 42, 102–114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redondo R. L., Morris R. G. M., Making memories last: The synaptic tagging and capture hypothesis. Nat. Rev. Neurosci. 12, 17–30 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Neubauer A. C., Fink A., Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 33, 1004–1023 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Reichle E. D., Carpenter P. A., Just M. A., The neural bases of strategy and skill in sentence–picture verification. Cogn. Psychol. 40, 261–295 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Kühn S., Schmiedek F., Noack H., Wenger E., Bodammer N. C., Lindenberger U., Lövden M., The dynamics of change in striatal activity following updating training. Hum. Brain Mapp. 34, 1530–1541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinzel S., Lorenz R. C., Pelz P., Heinz A., Walter H., Kathmann N., Rapp M. A., Stelzel C., Neural correlates of training and transfer effects in working memory in older adults. Neuroimage 134, 236–249 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Poldrack R. A., Is “efficiency” a useful concept in cognitive neuroscience? Dev. Cogn. Neurosci. 11, 12–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iglói K., Doeller C. F., Paradis A.-L., Benchenane K., Berthoz A., Burgess N., Rondi-Reig L., Interaction between hippocampus and cerebellum crus I in sequence-based but not place-based navigation. Cereb. Cortex 25, 4146–4154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karpicke J. D., Roediger H. L. III, The critical importance of retrieval for learning. Science 319, 966–968 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Roediger H. L. III, Butler A. C., The critical role of retrieval practice in long-term retention. Trends Cogn. Sci. 15, 20–27 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Melby-Lervåg M., Redick T. S., Hulme C., Working memory training does not improve performance on measures of intelligence or other measures of “far transfer”: Evidence from a meta-analytic review. Perspect. Psychol. Sci. 11, 512–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shipstead Z., Redick T. S., Engle R. W., Does working memory training generalize? Psychol. Belg. 50, 245–276 (2010). [Google Scholar]
- 40.Barnett S. M., Ceci S. J., When and where do we apply what we learn?: A taxonomy for far transfer. Psychol. Bull. 128, 612–637 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Sala G., Gobet F., Does far transfer exist? Negative evidence from chess, music, and working memory training. Curr. Dir. Psychol. Sci. 26, 515–520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melby-Lervåg M., Hulme C., Is working memory training effective? A meta-analytic review. Dev. Psychol. 49, 270–291 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Au J., Sheehan E., Tsai N., Duncan G. J., Buschkuehl M., Jaeggi S. M., Improving fluid intelligence with training on working memory: A meta-analysis. Psychon. Bull. Rev. 22, 366–377 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Weicker J., Villringer A., Thöne-Otto A., Can impaired working memory functioning be improved by training? A meta-analysis with a special focus on brain injured patients. Neuropsychology 30, 190–212 (2016). [DOI] [PubMed] [Google Scholar]
- 45.G. Bäumler, Lern-und Gedächtnistest: LGT-3 (1974).
- 46.R. Weiß, CFT 20-R: Grundintelligenztest skala 2-revision (2006).
- 47.Genzel L., Kiefer T., Renner L., Wehrle R., Kluge M., Grözinger M., Steiger A., Dresler M., Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology 37, 987–998 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Sattari N., McDevitt E. A., Panas D., Niknazar M., Ahmadi M., Naji M., Baker F. C., Mednick S. C., The effect of sex and menstrual phase on memory formation during a nap. Neurobiol. Learn. Mem. 145, 119–128 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Stanislaw H., Todorov N., Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 31, 137–149 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Ashburner J., A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Power J. D., Barnes K. A., Snyder A. Z., Schlaggar B. L., Petersen S. E., Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pruim R. H. R., Mennes M., van Rooij D., Llera A., Buitelaar J. K., Beckmann C. F., ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M., Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Shattuck D. W., Mirza M., Adisetiyo V., Hojatkashani C., Salamon G., Narr K. L., Poldrack R. A., Bilder R. M., Toga A. W., Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 39, 1064–1080 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/10/eabc7606/DC1


