Abstract
Neuropilins (NRP1 and NRP2) are multifunctional receptor proteins that are involved in nerve, blood vessel, and tumor development. NRP1 was first found to be expressed in neurons, but subsequent studies have demonstrated its surface expression in cells from the endothelium and lymph nodes. NRP1 has been demonstrated to be involved in the occurrence and development of a variety of cancers. NRP1 interacts with various cytokines, such as vascular endothelial growth factor family and its receptor and transforming growth factor β1 and its receptor, to affect tumor angiogenesis, tumor proliferation, and migration. In addition, NRP1+ regulatory T cells (Tregs) play an inhibitory role in tumor immunity. High numbers of NRP1+ Tregs were associated with cancer prognosis. Targeting NRP1 has shown promise, and antagonists against NRP1 have had therapeutic efficacy in preliminary clinical studies. NRP1 treatment modalities using nanomaterials, targeted drugs, oncolytic viruses, and radio-chemotherapy have gradually been developed. Hence, we reviewed the use of NRP1 in the context of tumorigenesis, progression, and treatment.
Keywords: Neuropilin-1, Anti-tumor, Immunotherapy, Tumor targeting
Introduction
Neuropilins (NRPs) is unique to vertebrates and is a highly conserved multifunctional type I single-pass transmembrane protein about 130,000 to 140,000 Da in size. It is involved in various physiological and pathological processes in the body.[1–5] NRPs include two subtypes, that is, NRP1 and NRP2. They regulate cell function by acting as co-receptors for multiple ligands. NRPs have been demonstrated to be involved in angiogenesis, cell migration, immune cell regulation, axon growth, and so on.[6–11] NRP1 is essential for the development of neurons and the cardiovasculature, while NRP2 plays a key role in neuronal patterns and lymphangiogenesis.[1,12–14]
Increasing evidence has demonstrated that high NRP1 expression is closely associated with tumor occurrence, progression, invasion, metastasis, and prognosis.[15–19] NRP1 can not only form complexes directly with vascular endothelial growth factor A (VEGFA) and vascular endothelial growth factor receptor 2 (VEGFR2) to enhance angiogenesis, but also promote RhoA activation after binding with VEGFA to directly affect the growth and metastasis of tumor cells and promote tumor development. In addition, NRP1 can also accelerate tumor progression by stabilizing the function of regulatory T cells (Tregs) and preventing tumor-associated macrophages (TAM) from entering the normoxic tumor area.[19] NRP1 has become a key therapeutic target for tumor therapy. Antagonists that target NRP1 have shown promise in several studies.[20]
Structure, Expression, and Function of NRP1 Protein
The structure of NRP1 protein
NRP1 was discovered in 1987 and was originally named A5. It was discovered as an antigen of a monoclonal antibody that was bound to neuronal cell surface proteins in the Xenopus nervous system.[6] The NRP1 gene is 112 kb in length and is located on the human chromosome 10q12. It contains 17 exons and 16 introns.[21] NRP1 has an intracellular, transmembrane, and extracellular domain. Its intracellular domain is relatively small, lacks an inherent kinase domain, and does not participate in signal transduction. Its extracellular domain consists of five subdomains, that is, a1, a2, b1, b2, and c, with each subdomain associated with different molecular and/or cellular interactions [Figure 1].
Figure 1.
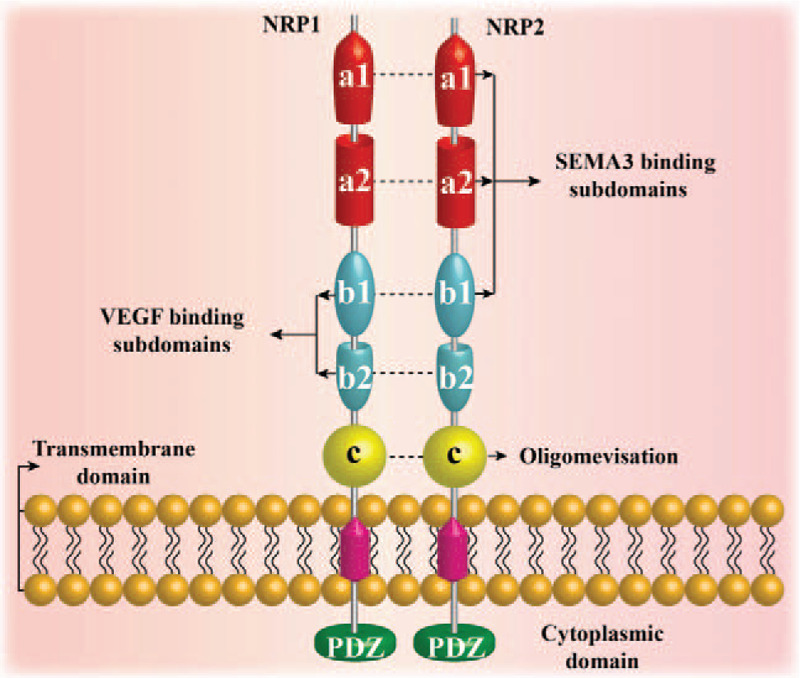
Schematic of the neuropilin (NRP) molecular structure. NRP1 and NRP2 are unique transmembrane glycoproteins in vertebrates. In humans, NRP1 is located on chromosome 10, and NRP2 is located on chromosome 2. They have about 44% sequence homology at the amino acid level. The overall structure of the two NRPs is similar, including a large N-terminal extracellular domain, a short transmembrane domain, and a small cytoplasmic domain. The extracellular domain is divided into three domains: the complement protein binding homology domain (CUB domain or a1a2 domain), coagulation factor V/VIII homology domain (b1b2 domain), and the MAM domain (c domain). The a1a2 b1 domain binds to SEMA3, the b1b2 domain binds to vascular endothelial growth factor (VEGF), and the c domain is considered to play a role in NRP1 oligomerization. The C-terminus of NRP contains a three amino acid (Ser-Glu-Ala) sequence called SEA, which binds to the kinase through the PDZ domain.
The expression of NRP1 protein
NRP1 was originally found to be expressed in neurons, but later, was observed to be also expressed on the surface of several types of cells. High expression levels of NRP1 have been observed in osteoblasts, nerve cells, immune cells, adipocytes, glomerular stromal cells, endothelial cells, and hepatic stellate cells, and so on[22–25] [Figure 2]. Almost all tumor cells express NRP1 or NRP2 or both. These include certain leukemias, malignant melanomas, malignant gliomas, osteosarcomas (OSs), lung cancer, gastric cancer, and so on. Expression of both NRP1 and NRP2 has been associated with poor prognosis.[26,27]
Figure 2.
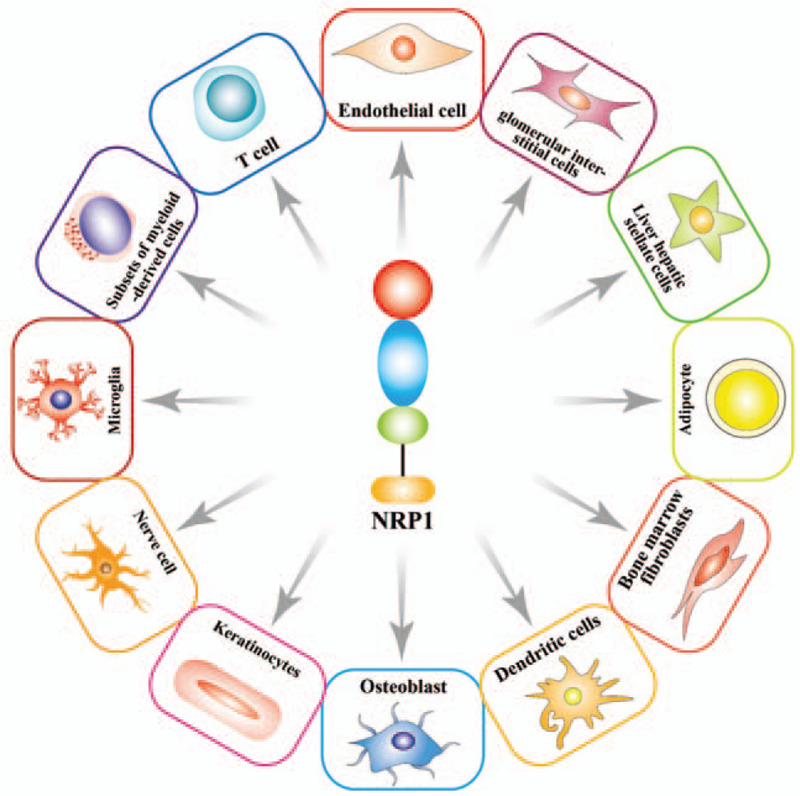
Cell types expressing neuropilin-1 (NRP1). NRP1 is expressed in several cell types, including endothelial cells, T lymphocytes, myeloid cell subsets, microglia, nerve cells, keratinocytes, osteoblasts, dendritic cells, bone marrow fibroblasts, fat cells, hepatic stellate cells, and glomerular interstitial cells.
The function of NRP1 protein
NRP1 was initially identified as a co-receptor for class 3 semaphorins (Sema3A). It forms a dimer with plexin A3 and is involved in axon guidance and nervous system development.[28] Later studies have found that NRP1 could form cis-acting complexes with the vascular endothelial growth factor (VEGF) family and its receptor (VEGFR) on the same cell to promote tumor angiogenesis.[29,30] Recent studies have shown that NRP1 could interact with glycosylation-dependent galectin-1 to activate transforming growth factor β1 (TGF-β1) and its receptors to accelerate liver fibrosis. In addition, NRP1 could promote cell migration induced by hepatocyte growth factor (HGF) or platelet-derived growth factor (PDGF) by phosphorylating p130Cas. Furthermore, NRP1 activates fibroblast growth factors (FGFs) and their receptors by interacting with heparin-binding proteins. NRP1 interacts with a variety of activated tyrosine kinase receptors and integrins to enhance tumor growth, survival, and invasion. NRP1 has been shown to play a regulatory role in the immune system. Overexpression of NRP1 on the surface of dendritic cells (DC) and Tregs has been demonstrated to play a role in promoting tumor development.[31–33]
NRP1 Functions in a Variety of Immune Cells
NRP1 is widely expressed in lymphoid and myeloid cells. In vitro and in vivo studies have demonstrated its important role in the immune response, cell proliferation, chemotaxis, and cytokine production in DC.[34–36] The occurrence and development of tumors have been linked to immune cell function.
The role of NRP1 in Tregs
T cells, an important type of immune cell in the body, are involved in all aspects of tumor progression. A subset of T-cells, Tregs, are involved in inhibiting anti-tumor immunity. Tregs that infiltrate tumors inhibit the anti-tumor effects of CD4+ and CD8+ T cells through multiple pathways. This results in immune escape and tumor progression, that is, anti-cancer immunity of the micro-environment (TME).[37–40] In recent years, NRP1 has been demonstrated to play a role in the stability and function of Tregs. NRP1 interacts with the ligand Semaphorin-4a (Sema4a) expressed on Tregs to enhance the function and survival of Tregs in tumors. This in turn restricts the anti-tumor immune response.[41–44] In mouse models, knockout of the NRP1 gene acting on Tregs could reduce tumor growth. This highlights the importance of NRP1 in suppressing anti-tumor immunity.
NRP1 has also been shown to act on DC. Sema4A secreted by DCs bind to NRP1 on Tregs and recruit PTEN to inhibit AKT phosphorylation. This in turn promotes the nuclear translocation of Foxo3a, which is important for the survival and stability of Tregs[45] [Figure 3]. Jung et al[20] demonstrated enhanced anti-tumor activity by inhibiting the function of Tregs in a mouse tumor model using NRP1 antagonists. In addition, Overacre-Delgoffe et al[46] demonstrated that a high percentage of NRP1+ Tregs in patients with melanoma and squamous cell carcinoma of the head and neck were associated with poor prognosis.
Figure 3.
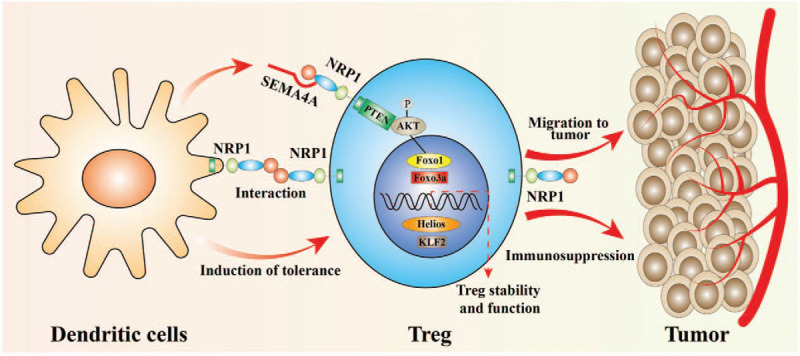
Schematic depicting the role of neuropilin-1 (NRP1) in dendritic cells (DC) and regulatory T cells (Tregs). NRP1 is mainly associated with the inhibitory function of Tregs. NRP1 is important for the formation of immune synapses between dendritic cells (DC) and T cells. Sema4A secreted by DC binds to NRP1 and recruits phosphatase and tensin homolog deleted on chromosome ten (PTEN) to inhibit protein kinase B (AKT) phosphorylation, thereby promoting the nuclear translocation of Forkhead box O3 (Foxo3a). This is important for the survival and stability of Tregs. NRP1 also plays an important role in the migration of Tregs into the tumor microenvironment in response to tumor cell-derived vascular endothelial growth factor.
Wang et al[47] found that NRP1 signaling-mediated accumulation of Tregs in tumors may play a key role in aggravating ischemic brain damage in tumor-bearing mice. When anti-NRP1 was combined with anti-PD-1 immunotherapy, it could enhance CD8+ T cell proliferation, cytotoxicity, and tumor control.[48] Hence, targeting NRP1 in combination with immunotherapy may be a promising approach.
The role of NRP1 in TAM
In addition to Tregs, TAM also play a role in promoting tumor progression. TAMs are macrophages in the tumor stroma. They participate in the process of tumorigenesis, growth, infiltration, and spread, and has been associated with tumor angiogenesis and lymphangiogenesis.[49–54] Deletions in the NRP1 gene in macrophages facilitate the entry of TAMs into the area of normoxic tumors. This reduces the pro-angiogenic and immunosuppressive functions of TAMs and inhibits the growth and metastasis of tumors.[44,55,56] Conversely, when TAM are recruited to avascular areas, tumor progression could be maintained.[55] These results were supported by the study conducted by Miyauchi et al[57] Hence, modulation of NRP1 in peripheral macrophages or microglia could make them more anti-tumorigenic, reduce neovascularization, and modulate glioma adaptive immune response.
Correlation Between NRP1 Expression and Tumor-initiating Cells (TIC)
Recent studies have demonstrated the relationship between NRP1 expression and TIC. TIC have the capacity for self-renewal and are responsible for the initiation and maintenance of a tumor.[19,58–61] TICs have been extensively investigated for their function.[62]
Recent studies have demonstrated that endothelial progenitor cells could be identified by their expression levels of NRP1. NRP1 is essential for the proliferation and cell migration of adult mesenchymal stem cells.[63–66] In addition, NRP1 maintains a tumor-initiating phenotype in gliomas and skin cancer cells.[67] Jimenez-Hernandez LE et al and others have also demonstrated that cells expressing NRP1 exhibit similar characteristics as TIC with high clonal ability. This suggests that NRP1+ lung cancer cells have tumor-initiating properties.[19] These findings provide new insights for cancer treatment and potential biomarkers for the study of TIC.
NRP1 Promotes Tumor Angiogenesis
Angiogenesis is essential during tumorigenesis and malignancy. Angiogenesis is a complex mechanism that induces new capillary formation from pre-existing vessels. The signaling pathways include the involvement of NRP1 and VEGF and their interactions with receptor VEGFRs.[68–71] Studies have confirmed that knocking out NRP1 in mice can affect the development of nervous and cardiovascular systems.
VEGFA is the predominant VEGF and is one of the main stimuli to induce angiogenesis. Within the VEGFA family, VEGF165 has a major role in neovascularization. The carboxy terminus of the gene encodes exons 7 and 8 and binds with the b1/b2 domain of NRP1.[72–75]
The formation of cis NRP1-VEGFA-VEGFR2 complexes within cells plays a crucial role in enhancing angiogenesis.[76–78] However, trans-NRP1-VEGFA-VEGFR2 complexes across cells play an inhibitory role in angiogenesis.[78] Pan et al[79] using a mouse xenograft tumor model, determined that antibodies that blocked VEGFA binding to NRP1 enhanced the anti-tumor effect of anti-VEGFA antibodies. Interestingly, in acute myeloid leukemia (AML), SEMA3A may partially reverse AML progression by inducing VEGFA overexpression. However, it is generally believed that SEMA3A binding to NRP1 plays a role in neurological development.[80] In addition, the VEGFR2/NRP1 complex plays a role in the early signaling of liver regeneration.[81]
In addition to interacting with VEGF, NRP1 also interacts with other pro-angiogenic cytokines, including FGF and HGF.[23,82–86] NRP1 binds to and promotes PDGF-β, as well as, TGF-β1 signaling pathway, thereby contributing to the activation and recruitment of perivascular cells.[22,87] Genetic studies have provided strong evidence that NRP1 is required for vascular morphogenesis. NRP1 deficiency leads to vascular reconstruction and branching defects. NRP1 expression has been shown to increase tumorigenicity in several tumor models such as murine hepatocellular carcinoma, human colon cancer, and non-small cell lung cancer. This may be by promoting VEGF-mediated angiogenesis.[42,88–91]
NRP1 Promotes Tumor Proliferation and Migration
Tumor infiltration and migration are important processes in tumor development and are the main reasons for poor prognosis. NRP1 promotes tumor cell growth, migration, invasion, and survival by interacting with several growth factors and their cognate signaling receptors.[92–96] Binding of VEGFA to NRP1 promotes RhoA activation and then, activated RhoA contributes to the degradation of p27kip1, which in turn, promotes tumor cell proliferation. This has been demonstrated in skin cancer, prostate cancer, and glioblastoma[60,97–99] [Figure 4]. In addition, PDGF and its receptor (PDGFR) are angiogenic factors closely associated with tumorigenesis and progression, and their overexpression is a common feature in different cancers.[100–102] Binding of NRP1 to PDGF and PDGFR promotes the phosphorylation of PDGF and consequently stimulates tumor growth.[22,103] Abelson tyrosine kinase (ABL), a non-receptor tyrosine kinase, itself promotes cell adhesion and migration, while NRP1 can promote endothelial cell migration through the NRP1-ABL1 pathway. This has been demonstrated in non-small cell lung and breast cancers.[24,104–106] In addition, NRP1 could affect the expression of the Bcl-2 protein family and block the mitogen-activated protein kinase signaling pathway. Inhibition of NRP1 has been shown to significantly inhibit the proliferation of glioma cells.[107] NRP1 is highly expressed in the metastatic MDA-MB-231 and MDA-MB-435 breast cancer cell lines, but not in the non-metastatic MDA-MB-453 breast cancer cell lines.[108] NRP1 not only directly promotes tumor growth and migration but also modulates the tumor microenvironment by interacting with integrins and remodeling the extracellular matrix to influence tumor growth.[4]
Figure 4.
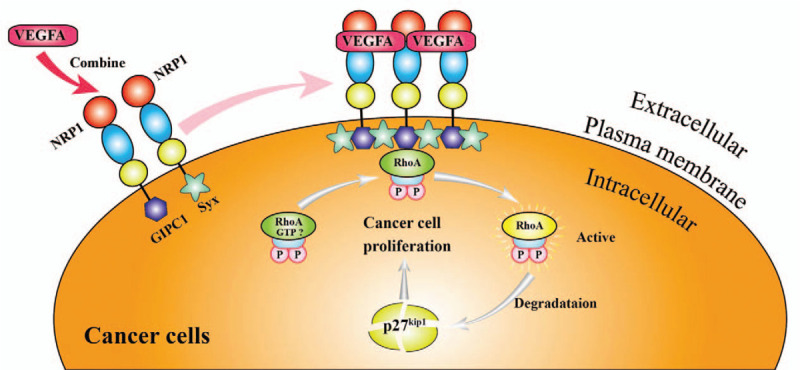
VEGFA induces RhoA protein activation through NRP1 to promote tumor cell proliferation. When VEGFA binds to NRP1, it promotes the interaction between NRP1 and GIPC1 (a scaffold protein) and enhances the assembly of the molecular complex of GIPC1 and Syx, resulting in GTP binding of RhoA. The active form is increased and activated RhoA contributes to the degradation of p27kip1. This promotes tumor cell proliferation. GIPC1 has anti-apoptotic effects in human breast cancer and colorectal cancer cells. Syx is involved in endothelial cell migration and endothelial cell connection integrity, barrier function, and vascular leakage. GTP: Guanosine triphosphate; NRP1: Neuropilin-1; RhoA: ras homolog family member A; Syx: Synectin-binding guanine exchange factor; VEGFA: Vascular endothelial growth factor A.
NRP1 in Cancer Treatment
Based on the function of NRP1 and its interactions with proteins involved in tumorigenesis, targeting NRP1 could have potent anti-tumor activity for several cancers. In recent years, NRP1 has been extensively studied, and the main therapeutic focus has been summarized in the following areas [Table 1].
Table 1.
Anti-tumor therapy targeting NRP1.
| Items | Targeted association | Drugs or agents | Cancer models and cell lines | References |
| Block pathway | Block tumor angiogenesis | Bevacizumab EG00229 Nb-HS45 | Glioma, squamous cell carcinoma, and so on | [109–113,114–116,117] |
| NRP1-Tregs | Release anti-tumor immune response | Fc(AAG)-TPP11 | [20] | |
| Reduce expression | Decrease the expression of NRP1 | miR-130a, miR-130b miR-9–5p, miR-628 miR-1247 miR-9 5. NDGA | Epithelial ovarian cancer, Gastric cancer, Osteosarcoma, ALL, Adenocarcinoma, and so on | [125,15,126,127,128,4] |
| Competitive inhibitors of NRP1 | Inhibition of NRP1 binding to its downstream targets | Combination of Sema3A protein and VEGFA inhibitor The SEMA3A point mutant | AML, Pancreatic cancer, and so on | [80,5] |
| NRP1 alternative splicing variants | Competitive NRP1 combination | s12NRP1, s11NRP1, sIIINRP1, sIVNRP1 NRP1-Δ7 | Breast cancer, Prostate cancer, and so on | [130–132,17] |
| Multi-drug combination therapy | Enhance treatment effect | Nrp1 coupled multifunctional drug nanocarrier NRP1 complex iRGD+5-FU | Glioblastoma, Gastric cancer, and so on | [137,138,139] |
EG00229: (S)-2-(3-(benzo[c][1,2,5] thiadiazole-4-sulfonamido)thiophene-2-carboxamido)-5- ((diaminomethylene)amino)pentanoic acid; Nb-HS45: Nanobody HS45; Fc(AAG)-TPP11: NRP1 antagonist; miR: MicroRNAs; NDGA: Nordihydroguaiaretic acid; ALL: Acute lymphoblastic leukemia; AML: Acute myeloid leukemia; s12NRP1, s11NRP1, sIIINRP1, sIVNRP1, NRP1-Δ7: Soluble forms of NRP1; iRGD: Tumor homing peptide; 5-FU: 5-Fluorouracil.
Blocking the NRP1 pathway interaction to block tumor angiogenesis
NRP1 primarily promotes tumor angiogenesis by forming NRP1/VEGF/VEGFR2 complexes with the VEGF family and its receptors.
The anti-VEGFA antibody, bevacizumab, has been clinically used to treat patients.[109–113] To date, the most characteristic inhibitor of NRP1 is EG00229. It interacts with the extracellular b1b2 domain of NRP1 and has been identified as a specific inhibitor of NRP1 interaction with VEGFA. It has significant tumor-suppressive effects in gliomas and squamous cell carcinomas.[114–116] Rizzolio S et al were also successful in generating an NRP1-specific nanoantibody HS45 that showed high levels of affinity to human NRP1.[117]
Inhibiting NRP1 in Tregs to increase anti-tumor immune response
NRP1 is barely detectable in human peripheral Tregs, however, it is expressed in tumor Tregs. NRP1+ Tregs have been shown to significantly suppress anti-tumor immune responses.[20,115,118,119] The reduction of NRP1+ Tregs in cancer has been strongly associated with chemotherapy success.[22] Jung et al[20] synthesized an NRP1 antagonist, Fc(AAG)-TPP11, that selectively inhibits the function and survival of NRP1+ Tregs to enhance anti-tumor activity in TME. They validated their findings in a mouse model with no apparent toxicity.
Improving tumor efficacy by inhibiting NRP1 expression
There are several types of NRP1 inhibitors, and microRNAs, as one of them, can regulate gene expression at the post-transcriptional level by forming RNA-induced silencing complexes. This leads to translational repression or degradation of target genes. It has been shown that microRNAs targeting NRP1 could be used for the treatment of cancers.[120–124] NRP1 was a target of miR-130a and miR-130b and was the first to report that NRP1 was associated with multidrug resistance in ovarian epithelial carcinoma.[125] In gastric cancer cells, miR-9-5p and miR-628 bind to NRP1 and inhibit NRP1 expression to inhibit the proliferation and invasion of gastric cancer cells, while at the same time, increasing the sensitivity of gastric cancer cells to chemotherapeutic agents.[15,126] In OS, NRP1 was identified as a direct target of miR-1247 and has been shown to inhibit the viability and metastasis of OS cells.[127] In acute lymphoblastic leukemia (ALL), it was demonstrated for the first time that NRP1 was a direct downstream target of miR-9 in ALL. These suggested that the development of novel therapeutic interventions targeting the miR-9/NRP1 signaling pathway could be a therapeutic option for ALL patients.[128] In the adenocarcinoma A549 cell line, miR-9 was found to directly target NRP1, and was found to enhance radio-sensitivity in A549 cells.[129]
Certain drugs can also affect NRP1 expression. Nordihydroguaiaretic acid (NDGA) is a natural product that down-regulates NRP1 expression. NDGA could inhibit NRP1 expression and attenuate cell motility and adhesion of cancer cells to the ECM, in addition to attenuating tumor metastasis in a nude mouse model.[4]
Competitive inhibitors of NRP1 binding proteins
Inhibition of NRP1 binding to its downstream targets will inevitably lead to an attenuation of NRP1 oncogenic signaling. SEMA3A could partially reverse the binding of VEGFA to the NRP1 receptor. Combining the SEMA3A protein with a VEGFA inhibitor may be beneficial for the treatment of AML.[80] Similarly, Gioelli et al[5] designed and generated a safe, non-intestinal-delivery, non-NRP1-dependent subtype of the SEMA3A point mutant. This SEMA3A point mutant could bind with nanomolar affinity to PLXNA4 compared to the wild-type SEMA3A.
SEMA3A is a direct binding co-receptor for NRP1, which in turn, is associated with PLXN receptor signaling. However, PLXN receptor signaling is critical for cancer vasculature. SEMA3A point mutants can competitively bind with PLXNA4 and prevent NRP1 from binding to PLXNA4. This accelerates vascular normalization, reduces tissue hypoxia, and increases perfusion to inhibit tumor growth. The effectiveness of the SEMA3A point mutants for the treatment of cancer has been successfully demonstrated in a mouse model of pancreatic cancer.[5]
Application of recombinant sNRP-1 in tumor treatment
In addition to the anti-tumor therapy directly targeting NRP1, the emergence of NRP1 alternative splicing variants (sNRP1) is also a new direction of tumor treatment. At present, s12NRP1, s11NRP1, sIIINRP1, and sIVNRP1 are the most studied NRP1 variants. The proteins encoded by s12NRP1 and s11NRP1 mRNA contain a1a2 and b1b2 domains and some b/c junctions.[130,131] They are known as VEGF165 antagonists. s12NRP1 can inhibit the binding of VEGF165 to NRP1-expressing cells and inhibit the tyrosine phosphorylation of VEGFR-2 induced by VEGF165. In the rat model of prostate cancer, overexpression of s12NRP1 results in a high percentage of apoptotic cells, intratumoral hemorrhage, and few blood vessels. Both sIIINRP1 and sIVNRP1 contain a1a2 and b1b2 domains, but no c domain or the rest NRP1 sequence. It has been found that these two recombinant proteins sIIINRP1 and sIVNRP1 can inhibit the migration of MDA-MB-231 breast cancer cells mediated by NRP1.[132] Recently, Hendricks et al[17] characterized a novel splicing variant NRP1-Δ7, which lost seven amino acids on two residues downstream of O-glycosylation site compared with NRP1. The proliferation, migration, and anchorage-independent growth of cells with increased NRP1-Δ7 expression decrease significantly in vitro, and NRP1-Δ7 inhibits the growth and angiogenesis of prostate tumor in vivo.
Multi-drug combination therapy targeting NRP1
Regarding cancer, a single drug often fails to achieve the desired therapeutic effect. Hence, a multi-drug combination therapy is generally used in clinical practice.[133–136] Teijeiro-Valino et al[137] coupled a multifunctional drug nanocarrier consisting of hyaluronic acid nanocapsules to NRP1. This significantly improved drug delivery capacity and demonstrated good efficacy. Benachour et al[138] generated polysiloxane nanoparticles chelated to NRP1 targeting peptides and 1,4,7,10 tetraazacyclododecane-N,N’,N,N’-tetraacetic acid (DOTA) derivatives. This was therapeutically efficacious in eliminating intracranial U87 glioblastomas in a rat model Zhang et al[139] demonstrated that a novel tumor homing peptide, iRGD, increased tumor penetration of chemotherapeutic agents and that the NRP1 protein was the key mediator of iRGD. Hence, combining iRGD with 5-fluorouracil, the standard first-line chemotherapeutic agent for locally advanced or metastatic gastric cancer, maybe a novel and effective approach to improving tumor prognosis.
Conclusions and Future Directions
NRP1 plays a key role in the occurrence and development of tumors. It is involved in angiogenesis, cancer migration, and tumor immunity. Some of the NRP1 signaling pathways have been mentioned earlier in this report. Targeting these pathways may be efficacious in treating a variety of cancers. However, additional studies need to be performed to decipher the molecular mechanism of NRP1 as it relates to cancer progression and metastasis. For effective cancer therapy, inhibitors of NRP1 function have to be combined with other treatment modalities, including immunotherapy, radiotherapy, and chemotherapy to achieve a complete response in patients with cancers.
Acknowledgements
The authors thank our laboratory members and collaborators for useful discussions.
Conflicts of interest
None.
Footnotes
How to cite this article: Liu SD, Zhong LP, He J, Zhao YX. Targeting neuropilin-1 interactions is a promising anti-tumor strategy. Chin Med J 2021;134:508–517. doi: 10.1097/CM9.0000000000001200
References
- 1.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res 2006; 312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Prud’homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012; 3:921–939. doi: 10.18632/oncotarget.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raimondi C, Ruhrberg C. Neuropilin signalling in vessels, neurons and tumours. Semin Cell Dev Biol 2013; 24:172–178. doi: 10.1016/j.semcdb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Fan S, Pan X, Xiaokaiti Y, Duan J, Shi Y, et al. Nordihydroguaiaretic acid impairs prostate cancer cell migration and tumor metastasis by suppressing neuropilin 1. Oncotarget 2016; 7:86225–86238. doi: 10.18632/oncotarget.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gioelli N, Maione F, Camillo C, Ghitti M, Valdembri D, Morello N, et al. A rationally designed NRP1-independent superagonist SEMA3A mutant is an effective anticancer agent. Sci Transl Med 2018; 10:eaah4807.doi: 10.1126/scitranslmed.aah4807. [DOI] [PubMed] [Google Scholar]
- 6.Wild JR, Staton CA, Chapple K, Corfe BM. Neuropilins: expression and roles in the epithelium. Int J Exp Pathol 2012; 93:81–103. doi: 10.1111/j.1365-2613.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuckran CA, Liu C, Bruno TC, Workman CJ, Vignali DA. Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy. J Immunother Cancer 2020; 8:e000967.doi: 10.1136/jitc-2020-000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang G, Chen L, Sun K, Khan AA, Yan J, Liu H, et al. Neuropilin-1 (NRP-1)/GIPC1 pathway mediates glioma progression. Tumour Biol 2016; 37:13777–13788. doi: 10.1007/s13277-016-5138-3. [DOI] [PubMed] [Google Scholar]
- 9.Niland S, Eble JA. Neuropilins in the context of tumor vasculature. Int J Mol Sci 2019; 20:639.doi: 10.3390/ijms20030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niland S, Eble JA. Neuropilin: handyman and power broker in the tumor microenvironment. Adv Exp Med Biol 2020; 1223:31–67. doi: 10.1007/978-3-030-35582-1_3. [DOI] [PubMed] [Google Scholar]
- 11.Schramek H, Sarkozi R, Lauterberg C, Kronbichler A, Pirklbauer M, Albrecht R, et al. Neuropilin-1 and neuropilin-2 are differentially expressed in human proteinuric nephropathies and cytokine-stimulated proximal tubular cells. Lab Invest 2009; 89:1304–1316. doi: 10.1038/labinvest.2009.96. [DOI] [PubMed] [Google Scholar]
- 12.Sulpice E, Plouet J, Berge M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 2008; 111:2036–2045. doi: 10.1182/blood-2007-04-084269. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Huang Y, Zhang J, Xing B, Xuan W, Wang H, et al. NRP-2 in tumor lymphangiogenesis and lymphatic metastasis. Cancer Lett 2018; 418:176–184. doi: 10.1016/j.canlet.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Li WP, Zhao H, Zhang X, Liang X, Liu Y, Zhang W, et al. Study on the white matter neuronal integrity in amnestic mild cognitive impairment based on automating fiber-tract quantification (in Chinese). Natl Med J China 2020; 100:172–177. doi: 10.3760/cma.j.issn.0376-2491.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Hang C, Yan HS, Gong C, Gao H, Mao QH, Zhu JX. MicroRNA-9 inhibits gastric cancer cell proliferation and migration by targeting neuropilin-1. Exp Ther Med 2019; 18:2524–2530. doi: 10.3892/etm.2019.7841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zhou R, Curry JM, Roy LD, Grover P, Haider J, Moore LJ, et al. A novel association of neuropilin-1 and MUC1 in pancreatic ductal adenocarcinoma: role in induction of VEGF signaling and angiogenesis. Oncogene 2016; 35:5608–5618. doi: 10.1038/onc.2015.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendricks C, Dubail J, Brohee L, Delforge Y, Colige A, Deroanne C. A novel physiological glycosaminoglycan-deficient splice variant of neuropilin-1 is anti-tumorigenic in vitro and in vivo. PLoS One 2016; 11:e0165153.doi: 10.1371/journal.pone.0165153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Shareef H, Hiraoka SI, Tanaka N, Shogen Y, Lee AD, Bakhshishayan S, et al. Use of NRP1, a novel biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to predict lymph node metastasis in squamous cell carcinoma of the tongue. Oncol Rep 2016; 36:2444–2454. doi: 10.3892/or.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez-Hernandez LE, Vazquez-Santillan K, Castro-Oropeza R, Martinez-Ruiz G, Munoz-Galindo L, Gonzalez-Torres C, et al. NRP1-positive lung cancer cells possess tumor-initiating properties. Oncol Rep 2018; 39:349–357. doi: 10.3892/or.2017.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung K, Kim JA, Kim YJ, Lee HW, Kim CH, Haam S, et al. A neuropilin-1 antagonist exerts antitumor immunity by inhibiting the suppressive function of intratumoral regulatory T cells. Cancer Immunol Res 2020; 8:46–56. doi: 10.1158/2326-6066.CIR-19-0143. [DOI] [PubMed] [Google Scholar]
- 21.Lampropoulou A, Ruhrberg C. Neuropilin regulation of angiogenesis. Biochem Soc Trans 2014; 42:1623–1628. doi: 10.1042/BST20140244. [DOI] [PubMed] [Google Scholar]
- 22.Hu C, Jiang X. Role of NRP-1 in VEGF-VEGFR2-independent tumorigenesis. Target Oncol 2016; 11:501–505. doi: 10.1007/s11523-016-0422-0. [DOI] [PubMed] [Google Scholar]
- 23.Hellec C, Diawara M, Carpentier M, Denys A, Allain F. The pro-tumoral activity of heparan sulfate 3-O-sulfotransferase 3B (HS3ST3B) in breast cancer MDA-MB-231 cells is dependent on the expression of neuropilin-1. Molecules 2018; 23:2718.doi: 10.3390/molecules23102718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raimondi C, Fantin A, Lampropoulou A, Denti L, Chikh A, Ruhrberg C. Imatinib inhibits VEGF-independent angiogenesis by targeting neuropilin 1-dependent ABL1 activation in endothelial cells. J Exp Med 2014; 211:1167–1183. doi: 10.1084/jem.20132330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhary B, Khaled YS, Ammori BJ, Elkord E. Neuropilin 1: function and therapeutic potential in cancer. Cancer Immunol Immunother 2014; 63:81–99. doi: 10.1007/s00262-013-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou K, Zhang YY, Cen Y, Zhu GN, Zhang YG. Effect of RKIP on proliferation and migration of malignant melanoma cells and potential mechanism (in Chinese). Natl Med J China 2019; 99:616–621. doi: 10.3760/cma.j.issn.0376-2491.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Bobinski M, Okla K, Kotarski J, Szumilo J, Polak G, Sobstyl M, et al. Neuropilin 1 in uterine leiomyosarcoma. Clinical and pathological analysis. Ginekol Pol 2018; 89:7–12. doi: 10.5603/GP.a2018.0002. [DOI] [PubMed] [Google Scholar]
- 28.Oplawski M, Dziobek K, Grabarek B, Zmarzly N, Dabrus D, Januszyk P, et al. Expression of NRP-1 and NRP-2 in endometrial cancer. Curr Pharm Biotechnol 2019; 20:254–260. doi: 10.2174/1389201020666190219121602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarabipour S, Mac Gabhann F. VEGF-A121a binding to neuropilins - a concept revisited. Cell Adh Migr 2018; 12:204–214. doi: 10.1080/19336918.2017.1372878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 2016; 17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 31.Gotot J, Dhana E, Yagita H, Kaiser R, Ludwig-Portugall I, Kurts C. Antigen-specific Helios(–), Neuropilin-1(-) Tregs induce apoptosis of autoreactive B cells via PD-L1. Immunol Cell Biol 2018; 96:852–862. doi: 10.1111/imcb.12053. [DOI] [PubMed] [Google Scholar]
- 32.Yang ZG, Wen RT, Qi K, Li J, Zheng GX, Wang YF, et al. The neuropilin-1 ligand, Sema3A, acts as a tumor suppressor in the pathogenesis of acute leukemia. Anat Rec (Hoboken) 2019; 302:1127–1135. doi: 10.1002/ar.24016. [DOI] [PubMed] [Google Scholar]
- 33.Gao YL, Yu MM, Shou ST, Yao Y, Liu YC, Wang LJ, et al. Tuftsin prevents the negative immunoregulation of neuropilin-1highCD4+CD25+regulatory T cells and improves survival rate in septic mice. Oncotarget 2016; 7:81791–81805. doi: 10.18632/oncotarget.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med 2012; 209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dejda A, Mawambo G, Daudelin JF, Miloudi K, Akla N, Patel C, et al. Neuropilin-1-expressing microglia are associated with nascent retinal vasculature yet dispensable for developmental angiogenesis. Invest Ophthalmol Vis Sci 2016; 57:1530–1536. doi: 10.1167/iovs.15-18598. [DOI] [PubMed] [Google Scholar]
- 36.Mo Z, Yu F, Han S, Yang S, Wu L, Li P, et al. New peptide MY1340 revert the inhibition effect of VEGF on dendritic cells differentiation and maturation via blocking VEGF-NRP-1 axis and inhibit tumor growth in vivo. Int Immunopharmacol 2018; 60:132–140. doi: 10.1016/j.intimp.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Wu P, Sun HP, Wan LJ, Zhou CY, Wang T, Liu HX, et al. Cell morphological analysis of hepatosplenic T-cell lymphoma gamma-delta type (in Chinese). Natl Med J China 2020; 100:1805–1811. doi: 10.3760/cma.j.cn112137-20200221-00382. [DOI] [PubMed] [Google Scholar]
- 38.Yu S, Wang Y, Hou J, Li W, Wang X, Xiang L, et al. Tumor-infiltrating immune cells in hepatocellular carcinoma: Tregs is correlated with poor overall survival. PLoS One 2020; 15:e0231003.doi: 10.1371/journal.pone.0231003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauch DA, Conlon KC, Janakiram M, Brammer JE, Harding JC, Ye BH, et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood 2019; 134:1406–1414. doi: 10.1182/blood.2019002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu G, Li Z, Wang S. Tumor-infiltrating FoxP3(+) Tregs predict favorable outcome in colorectal cancer patients: a meta-analysis. Oncotarget 2017; 8:75361–75371. doi: 10.18632/oncotarget.17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C, Somasundaram A, Manne S, Gocher AM, Szymczak-Workman AL, Vignali KM, et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat Immunol 2020; 21:1010–1021. doi: 10.1038/s41590-020-0733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Zhang Y, Wu J, Li L, Chen N, Ni P, et al. Neuropilin 1 (NRP1) is a novel tumor marker in hepatocellular carcinoma. Clin Chim Acta 2018; 485:158–165. doi: 10.1016/j.cca.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 43.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013; 501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol 2004; 34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 45.Chang L, Wang Z, Li S, Chen X, Li X, Zhao J, et al. Type 2 inflammation suppression by T-regulatory cells attenuates the eosinophil recruitment in mucosa of chronic sinusitis. Clin Sci (Lond) 2020; 134:123–138. doi: 10.1042/CS20190388. [DOI] [PubMed] [Google Scholar]
- 46.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-gamma drives Treg fragility to promote anti-tumor immunity. Cell 2017; 169:1130–1141.e11. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Zhou Y, Yin J, Gan Y, Wang X, Wen D, et al. Cancer exacerbates ischemic brain injury Via Nrp1 (neuropilin 1)-mediated accumulation of regulatory T cells within the tumor. Stroke 2018; 49:2733–2742. doi: 10.1161/STROKEAHA.118.021948. [DOI] [PubMed] [Google Scholar]
- 48.Leclerc M, Voilin E, Gros G, Corgnac S, de Montpreville V, Validire P, et al. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat Commun 2019; 10:3345.doi: 10.1038/s41467-019-11280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bercovici N, Guerin MV, Trautmann A, Donnadieu E. The remarkable plasticity of macrophages: a chance to fight cancer. Front Immunol 2019; 10:1563.doi: 10.3389/fimmu.2019.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol 2010; 32:153–158. [PubMed] [Google Scholar]
- 51.Ji XY, Shi J, Dai XX, Sheng YJ, Xue YP, Liu JC, et al. Relevant molecular characteristics analysis on malignant transformation of interstitial cells induced by tumor stem cells in glioma microenvironment (in Chinese). Natl Med J China 2018; 98:3339–3344. doi: 10.3760/cma.j.issn.0376-2491.2018.41.010. [DOI] [PubMed] [Google Scholar]
- 52.Werneck-Gomes H, Campolina-Silva GH, Maria BT, Barata MC, Mahecha GAB, Hess RA, et al. Tumor-associated macrophages (TAM) are recruited to the aging prostate epithelial lesions and become intermingled with basal cells. Andrology 2020; 8:1375–1386. doi: 10.1111/andr.12783. [DOI] [PubMed] [Google Scholar]
- 53.Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer 2019; 136:136–144. doi: 10.1016/j.lungcan.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 54.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev 2017; 114:206–221. doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 2013; 24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 57.Miyauchi JT, Caponegro MD, Chen D, Choi MK, Li M, Tsirka SE. Deletion of neuropilin 1 from microglia or bone marrow-derived macrophages slows glioma progression. Cancer Res 2018; 78:685–694. doi: 10.1158/0008-5472.CAN-17-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duhagon MA, Hurt EM, Sotelo-Silveira JR, Zhang X, Farrar WL. Genomic profiling of tumor initiating prostatospheres. BMC Genomics 2010; 11:324.doi: 10.1186/1471-2164-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mercurio AM. VEGF/neuropilin signaling in cancer stem cells. Int J Mol Sci 2019; 20:490.doi: 10.3390/ijms20030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grun D, Adhikary G, Eckert RL. NRP-1 interacts with GIPC1 and SYX to activate p38 MAPK signaling and cancer stem cell survival. Mol Carcinog 2019; 58:488–499. doi: 10.1002/mc.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu W, Wu T, Dong X, Zeng YA. Neuropilin-1 is upregulated by Wnt/beta-catenin signaling and is important for mammary stem cells. Sci Rep 2017; 7:10941.doi: 10.1038/s41598-017-11287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Najafi M, Mortezaee K, Ahadi R. Cancer stem cell (a)symmetry & plasticity: tumorigenesis and therapy relevance. Life Sci 2019; 231:116520.doi: 10.1016/j.lfs.2019.05.076. [DOI] [PubMed] [Google Scholar]
- 63.Cimato T, Beers J, Ding S, Ma M, McCoy JP, Boehm M, et al. Neuropilin-1 identifies endothelial precursors in human and murine embryonic stem cells before CD34 expression. Circulation 2009; 119:2170–2178. doi: 10.1161/CIRCULATIONAHA.109.849596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fantin A, Vieira JM, Plein A, Denti L, Fruttiger M, Pollard JW, et al. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood 2013; 121:2352–2362. doi: 10.1182/blood-2012-05-424713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gualandris A, Noghero A, Geuna M, Arese M, Valdembri D, Serini G, et al. Microenvironment drives the endothelial or neural fate of differentiating embryonic stem cells coexpressing neuropilin-1 and Flk-1. FASEB J 2009; 23:68–78. doi: 10.1096/fj.08-112847. [DOI] [PubMed] [Google Scholar]
- 66.Chu W, Song X, Yang X, Ma L, Zhu J, He M, et al. Neuropilin-1 promotes epithelial-to-mesenchymal transition by stimulating nuclear factor-kappa B and is associated with poor prognosis in human oral squamous cell carcinoma. PLoS One 2014; 9:e101931.doi: 10.1371/journal.pone.0101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med 2012; 209:507–520. doi: 10.1084/jem.20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hori Y, Ito K, Hamamichi S, Ozawa Y, Matsui J, Umeda IO, et al. Functional characterization of VEGF- and FGF-induced tumor blood vessel models in human cancer xenografts. Anticancer Res 2017; 37:6629–6638. doi: 10.21873/anticanres.12120. [DOI] [PubMed] [Google Scholar]
- 69.Su J, Li ZQ, Cui S, Ji LH, Chai KX, Geng H, et al. The expressions of VEGF and VEGFR signaling pathway in the bone marrow mononuclear cells with chronic mountain sickness (in Chinese). Natl Med J China 2018; 98:1088–1092. doi: 10.3760/cma.j.issn.0376-2491.2018.14.008. [DOI] [PubMed] [Google Scholar]
- 70.Sitohy B, Nagy JA, Jaminet SC, Dvorak HF. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res 2011; 71:7021–7028. doi: 10.1158/0008-5472.CAN-11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farzaneh Behelgardi M, Zahri S, Gholami Shahvir Z, Mashayekhi F, Mirzanejad L, Asghari SM. Targeting signaling pathways of VEGFR1 and VEGFR2 as a potential target in the treatment of breast cancer. Mol Biol Rep 2020; 47:2061–2071. doi: 10.1007/s11033-020-05306-9. [DOI] [PubMed] [Google Scholar]
- 72.Auriau J, Roujeau C, Belaid Choucair Z, Oishi A, Derviaux C, Roux T, et al. Gain of affinity for VEGF165 binding within the VEGFR2/NRP1 cellular complex detected by an HTRF-based binding assay. Biochem Pharmacol 2018; 158:45–59. doi: 10.1016/j.bcp.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Chen CK, Yu WH, Cheng TY, Chen MW, Su CY, Yang YC, et al. Inhibition of VEGF165/VEGFR2-dependent signaling by LECT2 suppresses hepatocellular carcinoma angiogenesis. Sci Rep 2016; 6:31398.doi: 10.1038/srep31398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsai JL, Lee YM, Pan CY, Lee AY. The novel VEGF121-VEGF165 fusion attenuates angiogenesis and drug resistance via targeting VEGFR2-HIF-1alpha-VEGF165/Lon signaling through PI3K-AKT-mTOR pathway. Curr Cancer Drug Targets 2016; 16:275–286. doi: 10.2174/156800961603160206125352. [DOI] [PubMed] [Google Scholar]
- 75.Fantin A, Lampropoulou A, Senatore V, Brash JT, Prahst C, Lange CA, et al. VEGF165-induced vascular permeability requires NRP1 for ABL-mediated SRC family kinase activation. J Exp Med 2017; 214:1049–1064. doi: 10.1084/jem.20160311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pei J, Yu L, Xia X, Xia S, Lin X, Jin J, et al. Association of vascular endothelial growth factor gene polymorphisms with Crohn's disease among Chinese patients (in Chinese). Natl Med J China 2018; 35:582–586. doi: 10.3760/cma.j.issn.1003-9406.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 77.Morin E, Sjoberg E, Tjomsland V, Testini C, Lindskog C, Franklin O, et al. VEGF receptor-2/neuropilin 1 trans-complex formation between endothelial and tumor cells is an independent predictor of pancreatic cancer survival. J Pathol 2018; 246:311–322. doi: 10.1002/path.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarabipour S, Mac Gabhann F. Tumor and endothelial cells collaborate via transcellular receptor complexes. J Pathol 2019; 247:155–157. doi: 10.1002/path.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 2007; 11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 80.Palodetto B, da Silva Santos Duarte A, Rodrigues Lopes M, Adolfo Corrocher F, Marconi Roversi F, Soares Niemann F, et al. SEMA3A partially reverses VEGF effects through binding to neuropilin-1. Stem Cell Res 2017; 22:70–78. doi: 10.1016/j.scr.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Elpek GO. Neuropilins liver. World J Gastroenterol 2015; 21:7065–7073. doi: 10.3748/wjg.v21.i23.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gacche RN, Meshram RJ. Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth. Prog Biophys Mol Biol 2013; 113:333–354. doi: 10.1016/j.pbiomolbio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 83.McGowan SE, Lansakara TI, McCoy DM, Zhu L, Tivanski AV. Platelet-derived growth factor-alpha and neuropilin-1 mediate lung fibroblast response to rigid collagen fibers. Am J Respir Cell Mol Biol 2020; 62:454–465. doi: 10.1165/rcmb.2019-0173OC. [DOI] [PubMed] [Google Scholar]
- 84.Holmes DI, Zachary IC. Vascular endothelial growth factor regulates stanniocalcin-1 expression via neuropilin-1-dependent regulation of KDR and synergism with fibroblast growth factor-2. Cell Signal 2008; 20:569–579. doi: 10.1016/j.cellsig.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 85.West DC, Rees CG, Duchesne L, Patey SJ, Terry CJ, Turnbull JE, et al. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J Biol Chem 2005; 280:13457–13464. doi: 10.1074/jbc.M410924200. [DOI] [PubMed] [Google Scholar]
- 86.Liu W, Parikh AA, Stoeltzing O, Fan F, McCarty MF, Wey J, et al. Upregulation of neuropilin-1 by basic fibroblast growth factor enhances vascular smooth muscle cell migration in response to VEGF. Cytokine 2005; 32:206–212. doi: 10.1016/j.cyto.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist 2015; 20:660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Liu P, Jiang Y, Dou X, Yan J, Ma C, et al. High expression of neuropilin-1 associates with unfavorable clinicopathological features in hepatocellular carcinoma. Pathol Oncol Res 2016; 22:367–375. doi: 10.1007/s12253-015-0003-z. [DOI] [PubMed] [Google Scholar]
- 89.Tomida C, Yamagishi N, Nagano H, Uchida T, Ohno A, Hirasaka K, et al. VEGF pathway-targeting drugs induce evasive adaptation by activation of neuropilin-1/cMet in colon cancer cells. Int J Oncol 2018; 52:1350–1362. doi: 10.3892/ijo.2018.4291. [DOI] [PubMed] [Google Scholar]
- 90.Barr MP, Gray SG, Gately K, Hams E, Fallon PG, Davies AM, et al. Correction to: vascular endothelial growth factor is an autocrine growth factor, signaling through neuropilin-1 in non-small cell lung cancer. Mol Cancer 2020; 19:16.doi: 10.1186/s12943-020-1142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding Z, Zhu J, Zeng Y, Du W, Zhang Y, Tang H, et al. The regulation of neuropilin 1 expression by miR-338-3p promotes non-small cell lung cancer via changes in EGFR signaling. Mol Carcinog 2019; 58:1019–1032. doi: 10.1002/mc.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishizuka Y, Koshinaga T, Hirano T, Nagasaki-Maeoka E, Watanabe Y, Hoshi R, et al. NRP1 knockdown promotes the migration and invasion of human neuroblastoma-derived SKNAS cells via the activation of beta1 integrin expression. Int J Oncol 2018; 53:159–166. doi: 10.3892/ijo.2018.4397. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Luo JT, Liu YM, Wei WB. miRNA-145/miRNA-205 inhibits proliferation and invasion of uveal melanoma cells by targeting NPR1/CDC42. Int J Ophthalmol 2020; 13:718–724. doi: 10.18240/ijo.2020.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiso M, Tanaka S, Saji S, Toi M, Sato F. Long isoform of VEGF stimulates cell migration of breast cancer by filopodia formation via NRP1/ARHGAP17/Cdc42 regulatory network. Int J Cancer 2018; 143:2905–2918. doi: 10.1002/ijc.31645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Y, Yang L, Li Y. Neuropilin-1 (NRP-1) upregulated by IL-6/STAT3 signaling contributes to invasion in pancreatic neuroendocrine neoplasms. Hum Pathol 2018; 81:192–200. doi: 10.1016/j.humpath.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 96.Ding Y, Zhou J, Wang S, Li Y, Mi Y, Gao S, et al. Anti-neuropilin-1 monoclonal antibody suppresses the migration and invasion of human gastric cancer cells via Akt dephosphorylation. Exp Ther Med 2018; 16:537–546. doi: 10.3892/etm.2018.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El Baba N, Farran M, Khalil EA, Jaafar L, Fakhoury I, El-Sibai M. The role of Rho GTPases in VEGF signaling in cancer cells. Anal Cell Pathol (Amst) 2020; 2020:2097214.doi: 10.1155/2020/2097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshida A, Shimizu A, Asano H, Kadonosono T, Kondoh SK, Geretti E, et al. VEGF-A/NRP1 stimulates GIPC1 and Syx complex formation to promote RhoA activation and proliferation in skin cancer cells. Biol Open 2015; 4:1063–1076. doi: 10.1242/bio.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dachsel JC, Ngok SP, Lewis-Tuffin LJ, Kourtidis A, Geyer R, Johnston L, et al. The Rho guanine nucleotide exchange factor Syx regulates the balance of dia and ROCK activities to promote polarized-cancer-cell migration. Mol Cell Biol 2013; 33:4909–4918. doi: 10.1128/MCB.00565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Appiah-Kubi K, Wang Y, Qian H, Wu M, Yao X, Wu Y, et al. Platelet-derived growth factor receptor/platelet-derived growth factor (PDGFR/PDGF) system is a prognostic and treatment response biomarker with multifarious therapeutic targets in cancers. Tumour Biol 2016; 37:10053–10066. doi: 10.1007/s13277-016-5069-z. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Qiu H, Hu W, Li S, Yu J. Over-expression of platelet-derived growth factor-D promotes tumor growth and invasion in endometrial cancer. Int J Mol Sci 2014; 15:4780–4794. doi: 10.3390/ijms15034780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang W, Fridman Y, Bonfil RD, Ustach CV, Conley-LaComb MK, Wiesner C, et al. A novel function for platelet-derived growth factor D: induction of osteoclastic differentiation for intraosseous tumor growth. Oncogene 2012; 31:4527–4535. doi: 10.1038/onc.2011.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruffini F, Levati L, Graziani G, Caporali S, Atzori MG, D’Atri S, et al. Platelet-derived growth factor-C promotes human melanoma aggressiveness through activation of neuropilin-1. Oncotarget 2017; 8:66833–66848. doi: 10.18632/oncotarget.18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steinestel K, Bruderlein S, Lennerz JK, Steinestel J, Kraft K, Propper C, et al. Expression and Y435-phosphorylation of Abelson interactor 1 (Abi1) promotes tumour cell adhesion, extracellular matrix degradation and invasion by colorectal carcinoma cells. Mol Cancer 2014; 13:145.doi: 10.1186/1476-4598-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cleary RA, Wang R, Waqar O, Singer HA, Tang DD. Role of c-Abl tyrosine kinase in smooth muscle cell migration. Am J Physiol Cell Physiol 2014; 306:C753–761. doi: 10.1152/ajpcell.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao H, Chen MS, Lo YH, Waltz SE, Wang J, Ho PC, et al. The Ron receptor tyrosine kinase activates c-Abl to promote cell proliferation through tyrosine phosphorylation of PCNA in breast cancer. Oncogene 2014; 33:1429–1437. doi: 10.1038/onc.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X, Tang T, Lu X, Zhou H, Huang Y. RNA interference targeting NRP-1 inhibits human glioma cell proliferation and enhances cell apoptosis. Mol Med Rep 2011; 4:1261–1266. doi: 10.3892/mmr.2011.550. [DOI] [PubMed] [Google Scholar]
- 108.Lee E, Koskimaki JE, Pandey NB, Popel AS. Inhibition of lymphangiogenesis and angiogenesis in breast tumor xenografts and lymph nodes by a peptide derived from transmembrane protein 45A. Neoplasia 2013; 15:112–124. doi: 10.1593/neo.121638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang H, Chen C, Wang S, Li X, Fan T. Efficacy of bevacizumab combined with nedaplatin in the treatment of ovarian cancer and its effects on tumor markers and immunity of patients. J BUON 2020; 25:80–86. [PubMed] [Google Scholar]
- 110.Tamura R, Tanaka T, Morimoto Y, Kuranari Y, Yamamoto Y, Takei J, et al. Alterations of the tumor microenvironment in glioblastoma following radiation and temozolomide with or without bevacizumab. Ann Transl Med 2020; 8:297.doi: 10.21037/atm.2020.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.You XH, Wen C, Xia ZJ, Sun F, Li Y, Wang W, et al. Primary tumor sidedness predicts bevacizumab benefit in metastatic colorectal cancer patients. Front Oncol 2019; 9:723.doi: 10.3389/fonc.2019.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tosca EM, Rocchetti M, Pesenti E, Magni PA. Tumor-in-host DEB-based approach for modeling cachexia and bevacizumab resistance. Cancer Res 2020; 80:820–831. doi: 10.1158/0008-5472.CAN-19-0811. [DOI] [PubMed] [Google Scholar]
- 113.Sostelly A, Mercier F. Tumor size and overall survival in patients with platinum-resistant ovarian cancer treated with chemotherapy and bevacizumab. Clin Med Insights Oncol 2019; 13:1179554919852071.doi: 10.1177/1179554919852071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pal K, Madamsetty VS, Dutta SK, Wang E, Angom RS, Mukhopadhyay D. Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer. NPJ Precis Oncol 2019; 3:31.doi: 10.1038/s41698-019-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Powell J, Mota F, Steadman D, Soudy C, Miyauchi JT, Crosby S, et al. Small molecule neuropilin-1 antagonists combine antiangiogenic and antitumor activity with immune modulation through reduction of transforming growth factor beta (TGFbeta) production in regulatory T-cells. J Med Chem 2018; 61:4135–4154. doi: 10.1021/acs.jmedchem.8b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Z, Cheng C, Xiong H, Wang Y, Chen KK, Yang J, et al. NRP1 promotes cell migration and invasion and serves as a therapeutic target in nasopharyngeal carcinoma. Int J Clin Exp Pathol 2018; 11:2460–2469. [PMC free article] [PubMed] [Google Scholar]
- 117.Rizzolio S, Cagnoni G, Battistini C, Bonelli S, Isella C, Van Ginderachter JA, et al. Neuropilin-1 upregulation elicits adaptive resistance to oncogene-targeted therapies. J Clin Invest 2018; 128:3976–3990. doi: 10.1172/JCI99257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cari L, Nocentini G, Migliorati G, Riccardi C. Potential effect of tumor-specific Treg-targeted antibodies in the treatment of human cancers: a bioinformatics analysis. Oncoimmunology 2018; 7:e1387705.doi: 10.1080/2162402X.2017.1387705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hansen W. Neuropilin 1 guides regulatory T cells into VEGF-producing melanoma. Oncoimmunology 2013; 2:e23039.doi: 10.4161/onci.23039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wei Y, Guo S, Tang J, Wen J, Wang H, Hu X, et al. MicroRNA-19b-3p suppresses gastric cancer development by negatively regulating neuropilin-1. Cancer Cell Int 2020; 20:193.doi: 10.1186/s12935-020-01257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim H, Ko Y, Park H, Zhang H, Jeong Y, Kim Y, et al. MicroRNA-148a/b-3p regulates angiogenesis by targeting neuropilin-1 in endothelial cells. Exp Mol Med 2019; 51:1–11. doi: 10.1038/s12276-019-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yogi K, Sridhar E, Goel N, Jalali R, Goel A, Moiyadi A, et al. MiR-148a, a microRNA upregulated in the WNT subgroup tumors, inhibits invasion and tumorigenic potential of medulloblastoma cells by targeting Neuropilin 1. Oncoscience 2015; 2:334–348. doi: 10.18632/oncoscience.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P, et al. microRNA-320a inhibits tumor invasion by targeting neuropilin 1 and is associated with liver metastasis in colorectal cancer. Oncol Rep 2012; 27:685–694. doi: 10.3892/or.2011.1561. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P, et al. [Corrigendum.] MicroRNA-320a inhibits tumor invasion by targeting neuropilin 1 and is associated with liver metastasis in colorectal cancer. Oncol Rep 2015; 33:2093.doi: 10.3892/or.2015.3773. [DOI] [PubMed] [Google Scholar]
- 125.Chen C, Hu Y, Li L. NRP1 is targeted by miR-130a and miR-130b, and is associated with multidrug resistance in epithelial ovarian cancer based on integrated gene network analysis. Mol Med Rep 2016; 13:188–196. doi: 10.3892/mmr.2015.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pang W, Zhai M, Wang Y, Li Z. Long noncoding RNA SNHG16 silencing inhibits the aggressiveness of gastric cancer via upregulation of microRNA-628-3p and consequent decrease of NRP1. Cancer Manag Res 2019; 11:7263–7277. doi: 10.2147/CMAR.S211856. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Wei QF, Yao JS, Yang YT. MicroRNA-1247 inhibits the viability and metastasis of osteosarcoma cells via targeting NRP1 and mediating Wnt/beta-catenin pathway. Eur Rev Med Pharmacol Sci 2019; 23:7266–7274. doi: 10.26355/eurrev_201909_18831. [DOI] [PubMed] [Google Scholar]
- 128.Zang Y, Yu R, Bai Y, Chen X. MicroRNA-9 suppresses cancer proliferation and cell cycle progression in acute lymphoblastic leukemia with inverse association of neuropilin-1. J Cell Biochem 2018; 119:6604–6613. doi: 10.1002/jcb.26799. [DOI] [PubMed] [Google Scholar]
- 129.Xiong K, Shao LH, Zhang HQ, Jin L, Wei W, Dong Z, et al. MicroRNA-9 functions as a tumor suppressor and enhances radio-sensitivity in radio-resistant A549 cells by targeting neuropilin 1. Oncol Lett 2018; 15:2863–2870. doi: 10.3892/ol.2017.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gadermaier E, Tesarz M, Wallwitz J, Berg G, Himmler G. Characterization of a sandwich ELISA for quantification of total human soluble neuropilin-1. J Clin Lab Anal 2019; 33:e22944.doi: 10.1002/jcla.22944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romano E, Chora I, Manetti M, Mazzotta C, Rosa I, Bellando-Randone S, et al. Decreased expression of neuropilin-1 as a novel key factor contributing to peripheral microvasculopathy and defective angiogenesis in systemic sclerosis. Ann Rheum Dis 2016; 75:1541–1549. doi: 10.1136/annrheumdis-2015-207483. [DOI] [PubMed] [Google Scholar]
- 132.Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: in vivo expression and antitumor activity. Proc Natl Acad Sci U S A 2000; 97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S, Wang Z, Yu G, Zhou Z, Jacobson O, Liu Y, et al. Tumor-specific drug release and reactive oxygen species generation for cancer chemo/chemodynamic combination therapy. Adv Sci (Weinh) 2019; 6:1801986.doi: 10.1002/advs.201801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang X, Feng L, Dong Z, Xin X, Yang Z, Deng D, et al. Protein-drug conjugate programmed by pH-reversible linker for tumor hypoxia relief and enhanced cancer combination therapy. Int J Pharm 2020; 582:119321.doi: 10.1016/j.ijpharm.2020.119321. [DOI] [PubMed] [Google Scholar]
- 135.Seyedin SN, Hasibuzzaman MM, Pham V, Petronek MS, Callaghan C, Kalen AL, et al. Combination therapy with radiation and PARP inhibition enhances responsiveness to anti-PD-1 therapy in colorectal tumor models. Int J Radiat Oncol Biol Phys 2020; 108:81–92. doi: 10.1016/j.ijrobp.2020.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kunimasa K, Nakamura H, Nishino K, Nakatsuka SI, Kumagai T. Extrinsic upregulation of PD-L1 induced by pembrolizumab combination therapy in patients with NSCLC with low tumor PD-L1 expression. J Thorac Oncol 2019; 14:e231–e233. doi: 10.1016/j.jtho.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 137.Teijeiro-Valino C, Novoa-Carballal R, Borrajo E, Vidal A, Alonso-Nocelo M, de la Fuente Freire M, et al. A multifunctional drug nanocarrier for efficient anticancer therapy. J Control Release 2019; 294:154–164. doi: 10.1016/j.jconrel.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 138.Benachour H, Seve A, Bastogne T, Frochot C, Vanderesse R, Jasniewski J, et al. Multifunctional Peptide-conjugated hybrid silica nanoparticles for photodynamic therapy and MRI. Theranostics 2012; 2:889–904. doi: 10.7150/thno.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang L, Xing Y, Gao Q, Sun X, Zhang D, Cao G. Combination of NRP1-mediated iRGD with 5-fluorouracil suppresses proliferation, migration and invasion of gastric cancer cells. Biomed Pharmacother 2017; 93:1136–1143. doi: 10.1016/j.biopha.2017.06.103. [DOI] [PubMed] [Google Scholar]


