Summary
Breast reconstruction is an option that should be considered for any patient facing a mastectomy. Autologous breast reconstruction provides the benefits of excellent longterm results, natural appearance, natural feel, and the best opportunity for sensory restoration. These factors lead many patients to choose autologous tissue over implant-based reconstruction. With improved anatomic and technical knowledge, the donor site morbidity previously associated with abdominally based autologous reconstruction has been significantly reduced. Today, the DIEP flap is the preferred autologous method allowing restoration of a “natural,” aesthetic breast with potential for sensation while simultaneously minimizing abdominal donor site morbidity. Alternative flaps and adjunctive procedures provide options when dealing with patients who present with challenging clinical scenarios because of an inadequate abdominal donor site. This paper reviews current methods employed by a high volume breast reconstruction practice to achieve these goals.
INTRODUCTION
Breast reconstruction is an integral component of comprehensive breast cancer care. Autologous reconstruction promotes establishment of enduring natural aesthetic and tactile results favored by patients over typical implant-based reconstruction outcomes.1–3 With improved anatomic and technical knowledge, donor site morbidity after autologous tissue reconstruction has been significantly reduced. For example, better patient selection and preservation of muscle and fascia have led to decreased abdominal donor site morbidity following Deep Inferior Epigastric Artery Perforator (DIEP) flap dissection.4,5 Reconstructive microsurgeons routinely use team approaches to shorten operative times, decrease complications, and improve outcomes.6 Enhanced recovery after surgery protocols have also significantly reduced hospital stays and have reduced postoperative narcotic usage.7 Additionally, patients who are not candidates for abdominally based reconstructions, but still prefer autologous tissue reconstruction, now have various other autologous tissue options. Innovation and improved imaging techniques have given plastic surgeons the ability to map out perforators and design potential flaps from every portion of the human body. Flaps from the thigh, posterior trunk, and gluteal region have all been evaluated and used as alternative flaps in breast reconstruction.8–13 In our experience, flaps from the posterior trunk and gluteal region are not ideal because of poor donor site aesthetics, multiple position changes required, small flap size, and difficult to shape adipose tissue. The aim of this study was to review current best approaches to autologous breast reconstruction, as seen in a high volume breast reconstruction practice.
DEEP INFERIOR EPIGASTRIC ARTERY PERFORATOR (DIEP) FLAP
Abdominally based microsurgical tissue methods are preferred in general due to superior aesthetic, long-lasting, and well-tolerated results, even in high-risk populations.4
Procedure
Harvest techniques for the DIEP flap are well described in other published studies.14,15 As a high volume practice, however, some of the salient points and pearls we have learned are described below.
The patient’s anatomic landmarks are marked in the standing position preoperatively. The midline, inframammary fold, and breast footprint are marked for reference points. Prior scars on the breast or abdomen should be noted. In the supine position, flap markings are then made with the entire trunk in full view. A lenticular pattern is created from above the umbilicus down to the natural skin crease above the mons pubis. This is frequently adjusted and tailored to fit the patient’s body habitus, skin/fat distribution, or perforator location based on preoperative imaging or Doppler examination. When abdominal scars are present, the flap design can be adjusted to exclude the scar (ie, shifted cephalad or caudal).
The cranial flap incision is made first. Degree of flap beveling in the cranial direction is determined by flap volume requirements and abdominal wall thickness. Once the anterior abdominal fascia is reached, discontinuous undermining of the upper abdominal apron is performed with maximal preservations of perforators. Suprafascial dissection is routinely continued centrally up to the xiphoid. The lower abdominal incision is made after the patient is flexed on the operating table to ensure not only adequate flap volume and appropriate wound closure tension, but also an aesthetic, low-lying final scar position. While creating the lower incision, care is taken to preserve the superficial inferior epigastric vein as a potential secondary venous outflow source. Once flap dimensions are defined, flap elevation proceeds from lateral to medial.
Although expedient and appropriate perforator selection is a crucial step in DIEP flap elevation, certain principles or maneuvers may facilitate a decision-making process. First and foremost, surgeons must also be familiar with the anatomic variations of the epigastric pedicle and its perforators. For the DIEP flap, a wide range of perforator size and intramuscular anatomy exists. Typically, the flap is based on 1 or 2 perforators. In our experience, an upper medial perforator (peri-umbilical) frequently includes a large draining vein, ranging from 2.0 to 2.5 mm in diameter. Although eccentrically positioned, inclusion of this perforator may promote adequate venous outflow. In instances of observed venous insufficiency through selected deep perforators, commonly referred to as “superficial venous dominance,” DIEP flap venous supercharging via superficial draining veins is necessary.16 The temporal relationship of development of venous congestion to flap dissection may suggest a possible etiology and effective corrective measures.17
If more than 1 perforator is selected, these should be ideally aligned vertically so as to minimize abdominal muscle division. In the absence of such favorable perforator location, the inclusion of a small cuff of rectus muscle to permit the inclusion of 2–3 small perforators will minimize the incidence of fat necrosis and will have little impact on abdominal wall strength and overall recovery.18,19
An excellent technique for determining clinically dominant perforators is intraoperative indocyanine green (ICG) fluorescence angiography. This is utilized once the flap has been isolated on several potential perforators. Perforators with questionable contribution or unfavorable position are temporarily occluded with atraumatic Acland clamps. ICG angiography is then used to verify flap perfusion on the remaining patent perforators. This system can also be used for precise determination of regional flap perfusion, enabling appropriate flap debridement before flap transfer or insetting decreasing the risk of peripheral fat necrosis.20
Maintaining abdominal integrity and functionality is of paramount importance as well. The infraumbilical segment of rectus muscle is innervated by T9-L1 motor nerves entering laterally and posteriorly.21 Every effort is made to preserve these motor nerve branches. However, a motor nerve located between perforators may require division to extricate a multiple-perforator flap from its bed. In this instance, the transected motor nerve is repaired via microneurorrhaphy after completion of the flap dissection whenever possible.21,22
Following flap transfer and vessel anastomosis, a sensory microneurorrhaphy is usually performed between the anterior (medial) cutaneous branch of the 3rd or 4th intercostal nerve in the chest and either the 10th, 11th, or 12th sensory intercostal nerve branch harvested with the contralateral DIEP flap. The lateral 4th intercostal nerve is occasionally used as a recipient nerve if preserved during the mastectomy. Neurotization of autologous flaps for restoration of sensory function represents a significant advancement in breast reconstruction. Given the sensory neuronal density of the infraumbilical abdominal wall, restoration of breast sensation may be readily achieved.23,24 We routinely evaluate patients for a neurotization procedure during a DIEP flap procedure, but in cases where nipple preservation is performed during the mastectomy, surgeon judgment is used to determine mastectomy skin flaps and nipple–areolar complex have sufficient thickness to have potential for nerve preservation in the mastectomy skin flaps. In these cases, no neurotization is performed to maximize potential for preserved nipple sensation. (See Video 1 [online], which displays sensory neurorrhaphy of DIEP flap breast reconstruction.)
Video 1. Sensory neurorrhaphy. Video 1 from “Post Mastectomy Breast Reconstruction with Autologous Tissue – Current Methods and Techniques”.
Regarding donor-site closure, progressive tension sutures allow advancement of the upper abdominal flap inferiorly. This not only decreases tension at the closure site but can also effectively lower the position of the final donor site scar and helps close dead space, decreasing potential for seromas. Abdominal donor site complications have been shown to decrease significantly with the use of progression tension sutures versus no progressive tension sutures (complication rate of 9% versus 38%, respectively).25
The final component of an aesthetic donor site closure in the umbilicoplasty. A low-lying umbilicus and a high-riding donor site scar is the worst combination, leading to a final result that can make patients feel they have been “bisected” when they look in the mirror. Ideally, the neo-umbilicus is inset well above the abdominal incision line. A narrow vertical ellipse is favored for the umbilical inset, but this should be tailored to match the remaining abdominal habitus. The umbilical stalk edges should be trimmed to create a small round circular pattern. Anchor sutures between the umbilical stalk and the anterior abdominal wall, along with defatting at the inset site are used to recreate hooding and maximize the cosmetic result of the umbilicoplasty. The final result should mimic normal umbilicus aesthetics of midline location, superior hooding, and shallow depth.26
ALTERNATIVE FLAP OPTIONS
In patients who are not candidates for abdominal-based breast reconstruction due to anatomic restrictions or patient preference, but require extensive soft tissue coverage such as in the setting of previous radiation, an alternative donor site is necessary.27,28 Ideally, donor sites should (1) contain enough subcutaneous fat to create an acceptable breast mound, (2) provide an acceptable size skin paddle (which can be used to resurface a chest wall) and have enough surface area to mold into a convex shape, (3) create minimal donor site functional morbidity, (4) have reliable perforator and feeding vessel anatomy, (5) be easily approached in a two-team setting, (6) have easy ability to hide scar burden at donor sites, (7) have the ability to create a sensate flap, (8) match skin quality/color to chest-wall or breast, and (9) have fat consistency that mimics normal breast tissue. When evaluating donor sites for a given patient, these factors must be considered (Fig. 1).
Fig. 1.
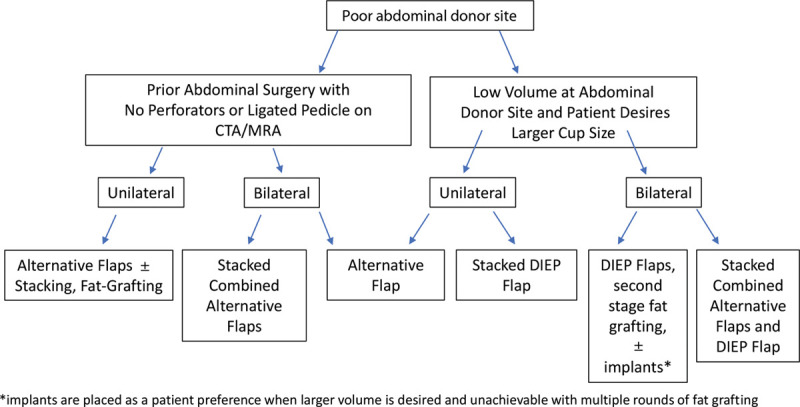
Algorithmic approach to patients who desire autologous tissue breast reconstruction. *Implants are placed as a patient preference when larger volume is desired and is unachievable with multiple rounds of fat grafting.
PROFUNDA ARTERY PERFORATOR FLAP
The profunda artery perforator (PAP) flap is designed along the upper inner thigh with the skin paddle oriented in a transverse direction and utilizes the “banana roll” area of fat distribution.29 Preoperative imaging is paramount in determining location of perforators and their course to source vessels. This is typically done with CT or MR angiography both with high-resolution cuts (Fig. 2).30 The flap design can be adjusted to capture perforators seen on imaging. (See Video 2 [online], which displays PAP flap dissection.)
Fig. 2.
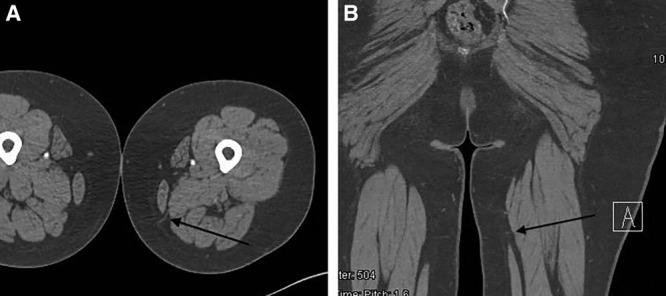
A, Axial cut of left lower extremity PAP flap, with arrow at perforator through adductor magnus. B, Coronal cut of left lower extremity PAP flap, with arrow at perforator through adductor magnus.
Video 2. PAP flap harvest. Video 2 from “Post Mastectomy Breast Reconstruction with Autologous Tissue – Current Methods and Techniques”.
The majority of perforators have an intramuscular course (69%) or with a minority classified as true septocutaneous perforators. Most of the septocutaneous perforators are located more caudally within the flap design. The flap can include 1–3 perforators. Flap dissection can be done in several positions: frog leg or lithotomy are common for approaching this dissection and is largely surgeon preference. (See Video 2 [online].) Insetting is done with the caudal edge of the flap placed at the inframammary fold. The lateral edges can be shaped and positioned to cone the reconstructed breast, adding projection to the breast mound.
Advantages of this flap are the relatively easy dissection and anatomic familiarity. The pedicle length and vessel caliber are adequate, but typically smaller and shorter compared with the DIEP flap pedicle. In addition, a 2-team approach can be used, which helps expedite the procedure and the donor site often yields an inconspicuous scar.
The principal drawback to this flap is the limited harvest volume obtained safely from most patients, averaging 425 g.31 Patient selection is paramount to getting optimal results. Patients should have adequate adiposity and skin laxity in the upper inner thigh region. Additionally, patients should be counseled about size limitations and the need for multiple rounds of fat grafting that may be required to achieve size/shape goals.
LATERAL THIGH PERFORATOR FLAP
When evaluating alternative donor sites, the lateral thigh typically provides sufficient subcutaneous tissue to reconstruct a breast, even in patients who have a low BMI.32 The tensor fasciae latae (TFL) myocutaneous free flap was first reported as a method of breast reconstruction in 1990.13 Most recently, Tuinder et al further classified pedicle anatomy and popularized the renamed “lateral thigh perforator (LTP) flap.”32,33 The LTP flap is a good option for women with a favorable soft tissue distribution over the upper lateral thigh (“saddle bag”) region. Several options exist regarding design of the flap over the lateral thigh (Fig. 3).
Fig. 3.
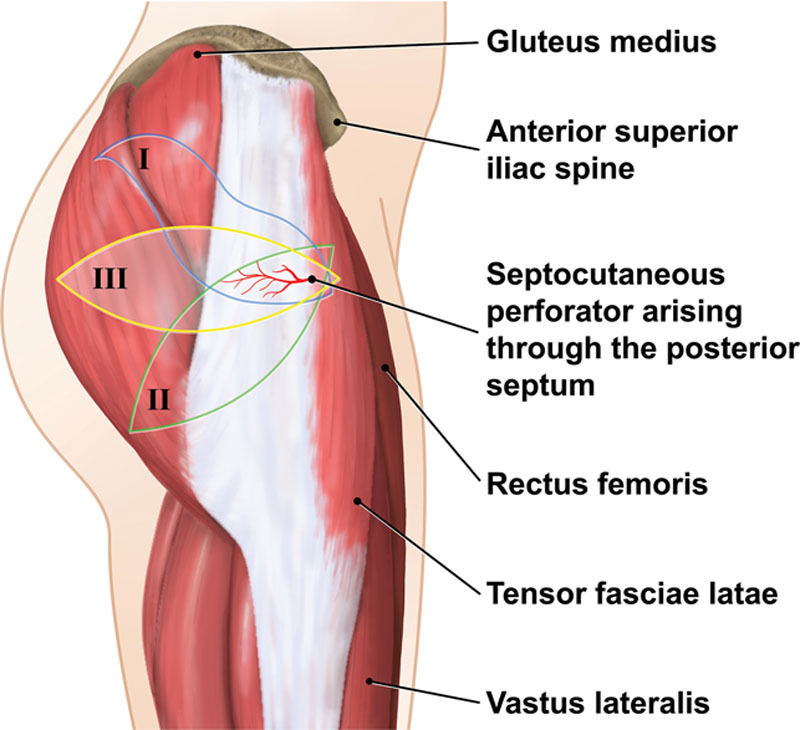
LTP design. There are 3 options for skin island design: a lateral ellipse (III), an S-shaped design extending superiorly over the lateral gluteal region (I), or inferiorly angled ellipse toward the gluteal crease (II). Flap design is guided by tissue distribution and patient preference in terms of final scar placement. A lateral gluteal design does not significantly impact the outer thigh contour and creates a scar which can be easily hidden by underwear.
Perforators to the TFL and overlying soft tissues arise from the ascending branch of the lateral circumflex femoral artery.34–36 Septocutaneous perforators are present in 97% of patients (1.8 perforators/thigh). Musculocutaneous perforators are present in 64% (0.9 perforators/thigh).35 The main source of perfusion to the LTP flap is a consistent septocutaneous perforator that travels within the posterior septum between the TFL and the gluteus medius muscles.34–36 The mean distance between anterior superior iliac spine and this perforator is 9.9 cm (±1.5 cm).35 Because the pedicle travels in a septocutaneous plane, flap dissection is technically straightforward and proceeds quickly. Additionally, some patients also have a large anterior septocutaneous perforator traveling in the septum between the TFL and rectus femoris / vastus lateralis muscles, but this is unfavorable because it is eccentrically located and smaller.32
Pre-operative imaging (CTA or MRI) is mandatory. Preoperative markings are drawn with the patient standing. A conservative pinch test should be used to mark the width of the flap (preferably limited to 7 cm), to minimize the risk of postoperative donor site dehiscence.
Flap dissection is performed with the patient in the supine position. Initially, dissection is limited to the medial 50% of the flap. This prevents inadvertent lateral migration of the flap and a potential traction injury to the pedicle during dissection. The lateral femoral cutaneous nerve is identified and preserved, unless a neurotized flap is planned, in which case a branch is included with the flap. A variable number of perforators will be seen piercing the thick, white posterior septum between the TFL and gluteus medius. The septum is opened longitudinally, and the largest caliber perforator is followed medially under the TFL. Frequently, 2 or more perforators will come together within the septum. The dissection is extended down to the anterior branch of the lateral circumflex femoral artery. The typical length of the pedicle is 6–8 cm. Once the bulk of the pedicle dissection is complete, the remaining flap dissection is completed over the lateral portion of the flap. Beveling should be avoided at all times to prevent a donor site deformity. In situations where both LTP flaps are required to have sufficient volume for one breast reconstruction, the flaps can be “stacked” on one side and the anastomosis can be performed in various ways.37
The ipsilateral LTP flap is often chosen for breast reconstruction. The internal mammary vessels are our preferred recipient vessels, and the LTP pedicle matches well in terms of caliber. In cases involving stacked LTP flaps, the contralateral flap is typically anastomosed to the retrograde internal mammary vessels.37
The posterior septum incision is closed using 2-0 PDS suture. The donor site is undermined at the supra-fascial plane cranially and caudally. A Byron Lockwood Underminer Dissector is used to extensively undermine and mobilize the soft tissues of the thigh. This degree of undermining greatly facilitates closure and ensures an aesthetic donor site contour. Progressive tension sutures (2-0 PDS) are used to close dead space and decrease the risk of seroma formation (Figs. 4 and 5).
Fig. 4.

Byron Lockwood Underminer Dissector (38 cm).
Fig. 5.
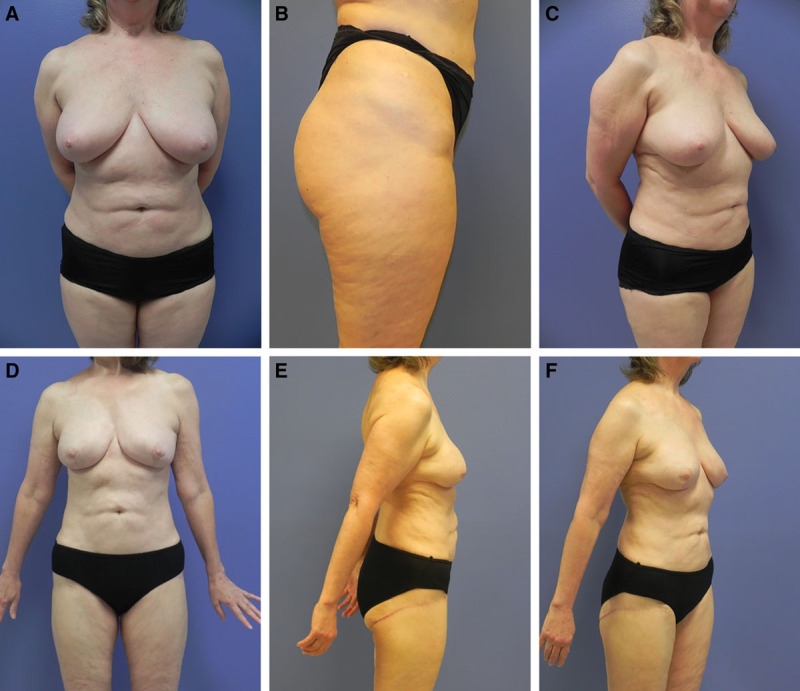
LTP Flap. This 69-year-old woman was diagnosed with left breast infiltrating ductal carcinoma and chose to undergo bilateral nipple-sparing mastectomies with immediate LTP flap reconstruction. Three months later, she underwent revision surgery consisting of bilateral thigh donor site recontouring with liposuction, and lipofilling of both breasts to address upper pole volume deficiency 110 mL fat graft to each breast: initial picture frontal view (A); initial picture lateral view (B); initial picture ¾ view (C); final frontal view (D); final lateral view (E); and final ¾ view (F).
GRACILIS MYOCUTANEOUS FREE FLAPS
The gracilis myocutaneous flap is primarily suited for patients with small to moderately sized breasts with tissue excess in the hip and thigh areas who are not candidates for abdominal-based reconstruction. The gracilis myocutaneous free flap for breast reconstruction is an essential alternative flap option in breast reconstruction.12,38 The elevation of the flap and anatomic descriptions are well known in the plastic surgery literature. The internal mammary vessels are preferentially used for anastomosis given the relatively short pedicle length as well as facilitate central flap positioning. Experience reveals that optimal results are achieved with adjunctive autologous fat grafting and shaped insetting of the flap. To optimize a natural breast contour, conical flap shaping can be achieved by approximating the superior limb borders, improving projection at the inferior pole.
Risks of lower extremity lymphedema or seroma formation are best avoided by limiting anterior limb dissection at the medial border of the great saphenous vein.39 Similarly, limiting the posterior limb boundary to not extend past midline of the posterior thigh minimizes the risk of distal flap skin necrosis and injury to the posterior femoral cutaneous nerve.40,41 Postoperatively, use of negative pressure dressings at the lower extremity donor site may reduce wound dehiscence, as reported by McKane.42
SMALL VOLUME AUTOLOGOUS TISSUE DONOR SITES
One frequent issue that arises in patients who desire autologous tissue reconstruction is the lack of volume from the donor site. This creates a challenge when trying to reach the patient’s desired goals for reconstruction. A scenario where this is particularly challenging is when a patient has very specific size goals, is larger breasted (more skin envelope to fill), and lack of donor site adiposity. In these instances, it is imperative for the surgeon to establish appropriate expectations with patients regarding likely outcomes relative to the patient’s desired goals. Having pictures of patients who have had similar scenarios and showing postoperative results is invaluable. The patient must be involved in the decision-making process. Shared decision-making between the patient and surgeon ensures that the patient has a good understanding of the reconstructive options and yields higher patient satisfaction.1,43 Once the surgeon and patient are comfortable with operative plan and agree that autologous tissue reconstruction is the best option for the patient, there are several techniques that can be employed to increase the volume of the reconstructed breast. Beveling at the perimeter of the flap dissection can maximize the volume obtained from any donor site. The edges of the flap can be trimmed as needed to healthy bright red bleeding to avoid fat necrosis and is necessary when aggressive beveling has been performed. Additionally, intraoperative use of SPY angiography to assess flap perfusion is extremely useful when designing larger flaps.20
For unilateral or bilateral breast reconstruction, stacked flaps or bi-pedicled DIEP flaps are often used to maximize volume in the reconstructed breast. This is a great option for patients who have minimal adiposity in their potential donor sites, but have large breasts. Vessels are often anastomosed to the antegrade and retrograde internal mammary vessels, but the thoracodorsal vessels can also be used for the more laterally placed flap (Fig. 6).44,45
Fig. 6.
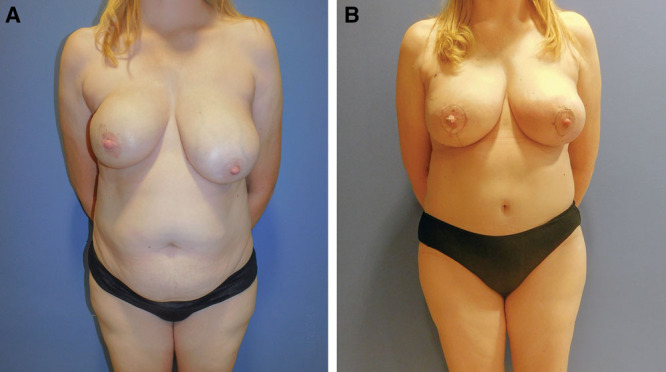
Bipedicled DIEP flap. A 44-year-old patient with right-sided breast cancer presented with large breasts (42 D cup) relative to abdominal donor site. Bipedicled DIEP flaps performed to provide adequate volume match to contralateral side. Nipple reconstruction and matching mastopexy performed to contralateral breast 6 months after initial surgery. Photographs taken 9 weeks after revision surgery. A, Initial frontal view. B, Final frontal view.
Fat grafting at a secondary stage is almost always done in these cases. As an adjunct, autologous fat grafting has proved effective in addressing areas of asymmetry, contour deformity, or volume deficits in patients following autologous flap breast reconstruction.46,47 Fat grafting has been shown to have minimal risk for complications and does not increase risk for local regional cancers.48,49 In our experience, 150 cm3 of fat graft is approximately ¾ to 1 cup size breast volume. The surgeon and patient must remember that 30%–50% of fat graft may not take, and multiple rounds of fat grafting may be required to reach size goals (Fig. 4).50
When fat grafting alone will not allow for patient goals to be met, implants placed under autologous tissue can provide abundant volume when needed.51 Small implants are usually selected and can be placed primarily at the time of autologous reconstruction or delayed during a revision surgery. In our experience, delayed placement is preferable because there are no concerns with pedicle compression/kinking and less fat necrosis related to implant compression of flap edges. Furthermore, because of the longer operative time associated with autologous tissue reconstruction, placement of implants in this setting may have a higher incidence of infection and/or capsular contracture. Long-term data are not available in the literature, but published studies in the short term reveal favorable results.52 For the best possible outcomes, extensive preoperative discussions with the patient and having a shared decision-making approach will optimize patient satisfaction1,43 (Figs. 7–8).
Fig. 7.
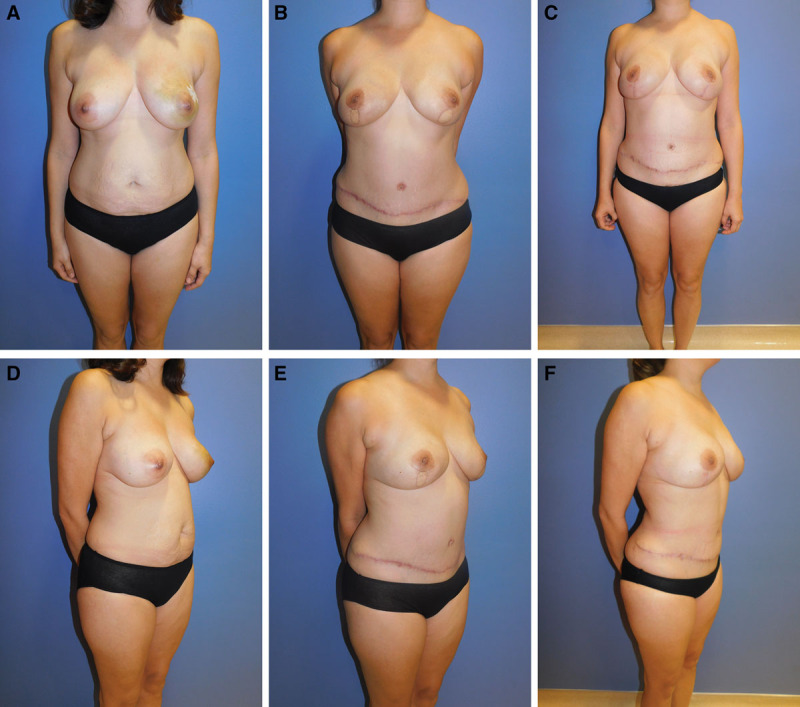
Examples of low BMI breast reconstruction patient . Patient 1: 38-year-old woman with left DCIS and BRCA 1 Gene mutation and BMI 24. She desired a similar volume match to her current breast size. She underwent bilateral nipple-sparing mastectomies and bilateral breast reconstruction with DIEP flaps. After her initial surgery, she underwent revision surgery 7 months later. Liposuction was performed to her flanks and abdomen to obtain fat graft and to shape her abdominal donor site. A 42-cm3 fat graft was placed to the right breast, and an 88-cm3 placed to her left breast. DIEP flap skin paddles were excised from both breasts. Final photographs taken 3 months after her revision surgery:
initial (A), frontal pre-revision (B), frontal final (C), ¾ view pre-operative (D), ¾ view pre-revision (E), and 3/4 view final (F).
Fig. 8.
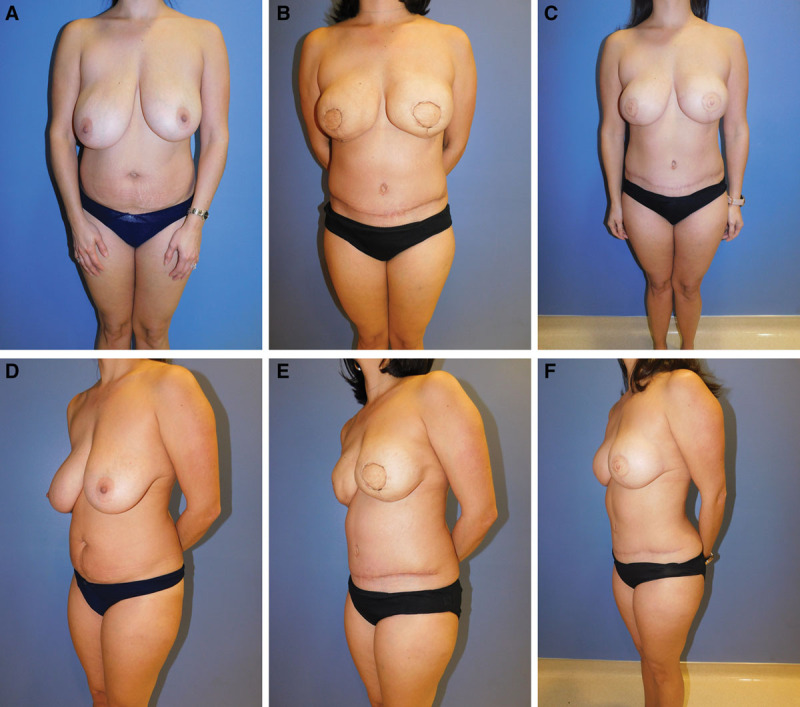
Examples of low BMI breast reconstruction patient. Patient 2: 37-year-old woman with left invasive cancer and right-side pseudo- stromal hyperplasia. Her preoperative bra and cup size was 34 G. She desired a cup size that was smaller and preferred to be in the D cup range. Her preoperative BMI was 23. She underwent bilateral skin-sparing mastectomies and immediate reconstruction with DIEP flaps. Her revision surgery was performed 4 months after her initial surgery. Fat graft to the right 135 mL, and to the left 70 mL revision surgery performed 4 months after her initial reconstruction. Nipple reconstruction was done at a later time. Final photographs taken 6 months after revision surgery: initial (A), frontal pre-revision (B), frontal final (C), 3/4 view preoperative (D), 3/4 view pre-revision (E), and ¾ view final (F).
CONCLUSIONS
Autologous-tissue–based breast reconstruction provides excellent results for breast cancer patients undergoing mastectomy. With the DIEP flap as the preferred method utilized, plastic surgeons are able to restore and create an aesthetic breast mound with potential for sensation in the reconstructed breast while simultaneously minimizing abdominal donor site morbidity. Alternative flaps and adjunctive procedures create options for patients who are not candidates for a DIEP flap or present with challenging clinical scenarios.
Footnotes
Published online 18 February 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Santosa KB, Qi J, Kim HM, et al. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2018;153:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coriddi M, Shenaq D, Kenworthy E, et al. Autologous breast reconstruction after failed implant-based reconstruction: evaluation of surgical and patient-reported outcomes and quality of life. Plast Reconstr Surg. 2019;143:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochoa O, Garza R, III, Pisano S, et al. Prospective longitudinal patient-reported satisfaction and health-related quality of life following DIEP flap breast reconstruction: relationship with body mass index. Plast Reconstr Surg. 2019;143:1589–1600. [DOI] [PubMed] [Google Scholar]
- 4.Ochoa O, Chrysopoulo M, Nastala C, et al. Abdominal wall stability and flap complications after deep inferior epigastric perforator flap breast reconstruction: does body mass index make a difference? Analysis of 418 patients and 639 flaps. Plast Reconstr Surg. 2012;130:21e–33e. [DOI] [PubMed] [Google Scholar]
- 5.Garvey PB, Buchel EW, Pockaj BA, et al. DIEP and pedicled TRAM flaps: a comparison of outcomes. Plast Reconstr Surg. 2006;117:1711–9; discussion 1720. [DOI] [PubMed] [Google Scholar]
- 6.Pisano SM, Ledoux PR, Nastala CL. Breast reconstruction in private practice. Semin Plast Surg. 2004;18:157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astanehe A, Temple-Oberle C, Nielsen M, et al. An enhanced recovery after surgery pathway for microvascular breast reconstruction is safe and effective. Plast Reconstr Surg Glob Open. 2018;6:e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Weerd L, Elvenes OP, Strandenes E, et al. Autologous breast reconstruction with a free lumbar artery perforator flap. Br J Plast Surg. 2003;56:180–183. [DOI] [PubMed] [Google Scholar]
- 9.Granzow JW, Levine JL, Chiu ES, et al. Breast reconstruction with gluteal artery perforator flaps. J Plast Reconstr Aesthet Surg. 2006;59:614–621. [DOI] [PubMed] [Google Scholar]
- 10.LoTempio MM, Allen RJ. Breast reconstruction with SGAP and IGAP flaps. Plast Reconstr Surg. 2010;126:393–401. [DOI] [PubMed] [Google Scholar]
- 11.Schoeller T, Wechselberger G. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg. 2004;57:481–482. [DOI] [PubMed] [Google Scholar]
- 12.Wechselberger G, Schoeller T. The transverse myocutaneous gracilis free flap: a valuable tissue source in autologous breast reconstruction. Plast Reconstr Surg. 2004;114:69–73. [DOI] [PubMed] [Google Scholar]
- 13.Elliott LF, Beegle PH, Hartrampf CR, Jr. The lateral transverse thigh free flap: an alternative for autogenous-tissue breast reconstruction. Plast Reconstr Surg. 1990;85:169–78; discussion 179. [PubMed] [Google Scholar]
- 14.Xue EY, Cen N, Reece E, et al. A Standardized approach to deep inferior epigastric perforator flap marking. Plast Reconstr Surg Glob Open. 2019;7:e2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damen TH, Morritt AN, Zhong T, et al. Improving outcomes in microsurgical breast reconstruction: lessons learnt from 406 consecutive DIEP/TRAM flaps performed by a single surgeon. J Plast Reconstr Aesthet Surg. 2013;66:1032–1038. [DOI] [PubMed] [Google Scholar]
- 16.Sbitany H, Mirzabeigi MN, Kovach SJ, et al. Strategies for recognizing and managing intraoperative venous congestion in abdominally based autologous breast reconstruction. Plast Reconstr Surg. 2012;129:809–815. [DOI] [PubMed] [Google Scholar]
- 17.Ochoa O, Pisano S, Chrysopoulo M, et al. Salvage of intraoperative deep inferior epigastric perforator flap venous congestion with augmentation of venous outflow: flap morbidity and review of the literature. Plast Reconstr Surg Glob Open. 2013;1:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:576–583. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117:737–46; discussion 747. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee A, Krishnan NM, Van Vliet MM, et al. A comparison of free autologous breast reconstruction with and without the use of laser-assisted indocyanine green angiography: a cost-effectiveness analysis. Plast Reconstr Surg. 2013;131:693e–701e. [DOI] [PubMed] [Google Scholar]
- 21.Rozen WM, Ashton MW, Murray ACA, et al. Avoiding denervation of rectus abdominis in DIEP flap harvest: the importance of medial row perforators. Plast Reconstr Surg. 2008;122:710–716. [DOI] [PubMed] [Google Scholar]
- 22.Safa B, Shores JT, Ingari JV, et al. Recovery of motor function after mixed and motor nerve repair with processed nerve allograft. Plast Reconstr Surg Glob Open. 2019;7:e2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelissen AJM, Beugels J, Lataster A, et al. Comparing the sensation of common donor site regions for autologous breast reconstruction to that of a healthy breast. J Plast Reconstr Aesthet Surg. 2018;71:327–335. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel AJ, Menn ZK, Eldor L, et al. Breast reinnervation: DIEP neurotization using the third anterior intercostal nerve. Plast Reconstr Surg Glob Open. 2013;1:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visconti G, Tomaselli F, Monda A, et al. Deep inferior epigastric artery perforator flap donor-site closure with cannula-assisted, limited undermining, and progressive high-tension sutures versus standard abdominoplasty: complications, sensitivity, and cosmetic outcomes. Plast Reconstr Surg. 2015;135:1–12. [DOI] [PubMed] [Google Scholar]
- 26.Ngaage LM, Kokosis G, Kachniarz B, et al. A two-step technique for neo-umbilicoplasty in the abdominal reconstructive population. Plast Reconstr Surg Glob Open. 2019;7:e2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahabedian MY, Momen B, Galdino G, et al. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475. [DOI] [PubMed] [Google Scholar]
- 28.Heller L, Feledy JA, Chang DW. Strategies and options for free TRAM flap breast reconstruction in patients with midline abdominal scars. Plast Reconstr Surg. 2005;116:753– 759. [DOI] [PubMed] [Google Scholar]
- 29.Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. 2012;129:16e–23e. [DOI] [PubMed] [Google Scholar]
- 30.Haddock NT, Greaney P, Otterburn D, et al. Predicting perforator location on preoperative imaging for the profunda artery perforator flap. Microsurgery. 2012;32:507–511. [DOI] [PubMed] [Google Scholar]
- 31.Haddock NT, Gassman A, Cho MJ, et al. 101 consecutive profunda artery perforator flaps in breast reconstruction: lessons learned with our early experience. Plast Reconstr Surg. 2017;140:229–239. [DOI] [PubMed] [Google Scholar]
- 32.Tuinder SMH, Beugels J, Lataster A, et al. The lateral thigh perforator flap for autologous breast reconstruction: a prospective analysis of 138 flaps. Plast Reconstr Surg. 2018;141:257–268. [DOI] [PubMed] [Google Scholar]
- 33.Tuinder S, Baetens T, De Haan MW, et al. Septocutaneous tensor fasciae latae perforator flap for breast reconstruction: radiological considerations and clinical cases. J Plast Reconstr Aesthet Surg. 2014;67:1248–1256. [DOI] [PubMed] [Google Scholar]
- 34.Hubmer MG, Schwaiger N, Windisch G, et al. The vascular anatomy of the tensor fasciae latae perforator flap. Plast Reconstr Surg. 2009;124:181–189. [DOI] [PubMed] [Google Scholar]
- 35.Maricevich MA, Bykowski MR, Schusterman MA, II, et al. Lateral thigh perforator flap for breast reconstruction: computed tomographic angiography analysis and clinical series. J Plast Reconstr Aesthet Surg. 2017;70:577–584. [DOI] [PubMed] [Google Scholar]
- 36.Vegas MR, Martin-Hervas C. The superolateral thigh flap: cadaver and computed tomographic angiography studies with a clinical series. Plast Reconstr Surg. 2013;131:310–322. [DOI] [PubMed] [Google Scholar]
- 37.Tessler O, Guste J, Bartow MJ, et al. Stacked lateral thigh perforator flap as a novel option for autologous breast reconstruction. Plast Reconstr Surg. 2019;143:1601–1604. [DOI] [PubMed] [Google Scholar]
- 38.Schoeller T, Huemer GM, Wechselberger G. The transverse musculocutaneous gracilis flap for breast reconstruction: guidelines for flap and patient selection. Plast Reconstr Surg. 2008;122:29–38. [DOI] [PubMed] [Google Scholar]
- 39.Saint-Cyr M, Wong C, Oni G, et al. Modifications to extend the transverse upper gracilis flap in breast reconstruction: clinical series and results. Plast Reconstr Surg. 2012;129:24e–36e. [DOI] [PubMed] [Google Scholar]
- 40.Bodin F, Dissaux C, Dupret-Bories A, et al. The transverse musculo-cutaneous gracilis flap for breast reconstruction: how to avoid complications. Microsurgery. 2016;36:42–48. [DOI] [PubMed] [Google Scholar]
- 41.Wechselberger G, Traintinger H, Larcher L, et al. Clinical applications of the transverse musculocutaneous gracilis flap for secondary breast reconstruction after simple mastectomy. Plast Reconstr Surg. 2016;137:19–28. [DOI] [PubMed] [Google Scholar]
- 42.McKane BW, Korn PT. The fleur-de-lis upper gracilis flap for breast reconstruction: flap design and outcome. Ann Plast Surg. 2012;69:383–386. [DOI] [PubMed] [Google Scholar]
- 43.Grabinski VF, Myckatyn TM, Lee CN, et al. Importance of shared decision-making for vulnerable populations: examples from postmastectomy breast reconstruction. Health Equity. 2018;2:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayo JL, Allen RJ, Sadeghi A. Four-flap breast reconstruction: bilateral stacked DIEP and PAP flaps. Plast Reconstr Surg Glob Open. 2015;3:e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal JP, Gottlieb LJ. Double pedicle deep inferior epigastric perforator/muscle-sparing TRAM flaps for unilateral breast reconstruction. Ann Plast Surg. 2007;58:359–363. [DOI] [PubMed] [Google Scholar]
- 46.Russe E, Kholosy H, Weitgasser L, et al. Autologous fat grafting for enhancement of breast reconstruction with a transverse myocutaneous gracilis flap: a cohort study. J Plast Reconstr Aesthet Surg. 2018;71:1557–1562. [DOI] [PubMed] [Google Scholar]
- 47.Losken A, Pinell XA, Sikoro K, et al. Autologous fat grafting in secondary breast reconstruction. Ann Plast Surg. 2011;66:518–522. [DOI] [PubMed] [Google Scholar]
- 48.Kaoutzanis C, Xin M, Ballard TN, et al. autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270–275. [DOI] [PubMed] [Google Scholar]
- 49.Batista BN, Mandujano CC, Liu J, et al. Lipofilling of the breast does not increase the risk of recurrence of breast cancer: a matched controlled study. Plast Reconstr Surg. 2016;138:1068e–1069e. [DOI] [PubMed] [Google Scholar]
- 50.Delay E, Garson S, Tousson G, et al. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29:360–376. [DOI] [PubMed] [Google Scholar]
- 51.Bach AD, Morgenstern IH, Horch RE. Secondary “hybrid reconstruction” concept with silicone implants after autologous breast reconstruction - is it safe and reasonable? Med Sci Monit. 2020;26:e921329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walters JA, III, Sato EA, Martinez CA, et al. Delayed mammoplasty with Silicone gel implants following DIEP flap breast reconstruction. Plast Reconstr Surg Glob Open. 2015;3:e540. [DOI] [PMC free article] [PubMed] [Google Scholar]


