Abstract
Objective
This study aims to investigate whether there was a difference between the levels of safety in terms of the postoperative residual liver volume in living transplant donors with normal liver anatomy and beaver tail liver.
Materials and Methods
Contrast-enhanced abdominal computed tomography (CT) images of 158 volunteers were retrospectively scanned. They were divided into 2 groups; with and without beaver tail liver. The total and left lobe volumes of the liver in all the candidates were calculated. The data were evaluated using the Mann-Whitney U test. Statistical values of p<0.05 were considered significant.
Results
The median value of the total liver volume was 1.252 mL and that of the left lobe percentage was 38% in the beaver tail group and 1.375 mL and 35%, respectively, in the normal liver group. A significant difference was observed in terms of the total liver volume and left lobe volume percentages of the 2 groups (p=0.012 and p<0.001, respectively).
Conclusion
The percentage of the left lobe in the beaver tail group was significantly higher, which indicates that liver transplantation donors with the beaver tail feature may be safer in terms of residual liver volume.
Keywords: Liver, anatomic variation, liver transplantation, living donor
Introduction
Liver transplantation is an important treatment option for patients with acute or chronic liver failure and primary hepatic malignancy. Transplantation can be performed from a cadaver or living donor. Adult living donor liver transplantation was started in 1998 owing to cadaveric donors being insufficient to meet the increasing need for organ transplants [1]. In living donor liver transplantation, unlike cadaveric transplants, the donor candidate should be specifically evaluated to minimize donor morbidity and mortality. The total right and left lobe volumes of the liver should be measured as accurately as possible, not only to ensure sufficient graft volume for the recipient but also to leave sufficient liver remnant volume for the donor. Post-transplantation liver failure in right lobe donors has been reported to be approximately 10%, and the accurate evaluation of the donor is important in preventing postoperative liver failure [2, 3]. The liver remnant volume should be at least 30% after transplantation [2].
Beaver tail liver (BTL) is also known as the sliver of liver where left border of the left liver lobe extends over the spleen and sometimes encloses the spleen. The term is a reference to a broad and thin tail of a beaver [4].
In our study, we examined the levels of safety in terms of liver remnant volume in donor candidates with a normal liver structure and those with a beaver tail feature.
Material and Methods
Patient Selection
This study was approved by the local ethics committee (B.30.2.ATA.0.01.00/328). All the donors were informed about the examination and procedure, and their written consent was obtained. A total of 170 volunteers aged between 18 and 45 years who registered with the radiology department of our hospital between July 2018 and January 2020 to be transplant donors were included in the study. A routine laboratory evaluation, hemogram analysis, liver ultrasonography, and triphasic abdominal computed tomography (CT) were performed on the volunteers. After the laboratory and radiological evaluation, 8 patients with fatty liver, 1 patient with hemangioma in the liver parenchyma, and 3 patients with widespread atherosclerosis in vascular structures were excluded from the study. The data of the remaining 158 volunteers were evaluated.
Computed Tomography Scan and Volume Calculation
In this study, a 320-row multi-detector CT device (Aquillion ONE Vision; Toshiba Medical Systems Corporation, Otawara, Japan) was used for liver imaging. All the CT scans were undertaken using the parameters recommended by the manufacturer (slice thickness 0.5 mm; rotation time 0.5 s; and scan interval 240 mm [480 slices, 0.5 mm]). Contrast agent (300 mg/mL iodexol) was administered at a dose of 1.5 mL/kg at a rate of 3.5 mL/sec with a pressure injector. Triphasic images were obtained in the arterial, portal, and hepatic vein phases. The images were evaluated by a single radiologist with 10 years of experience using the radiological workstation (Syngo Via Console, software version 2.1, Siemens AG Medical Solutions, Erlangen, Germany). The volumetric measurements of the liver were performed using special software (Myrian Pro, Intrasense, Montpellier, France).
Image Evaluation
If the left lobe of the liver crossed the left midclavicular line and extended to touch the spleen, it was accepted as a beaver tail liver. In patients with BTL and normal liver (NL), the total and left lobe liver volumes were measured, and the left lobe percentage was calculated according to the ratio of the left lobe volume to the total liver volume. Measurement images and values of both groups are shown in Figures 1 and 2.
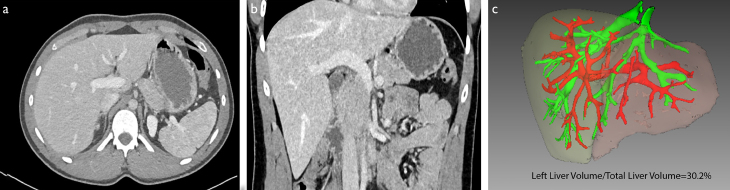
Figure 1. a–c.
Axial (a) and coronal (b) computed tomography images of a patient with a normal liver. In the 3D volumetric evaluation(c), the ratio of the left lobe volume to the total liver volume was calculated as 30.2%
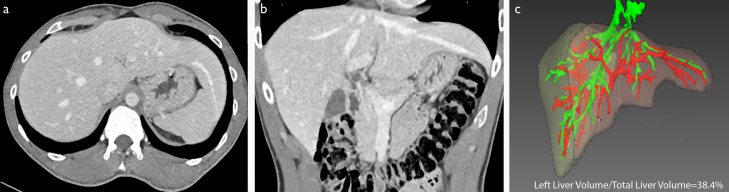
Figure 2. a–c.
Axial (a) and coronal (b) computed tomography images of a case in which the left lobe of the liver extends to the left hypochondrium. In the 3D volumetric evaluation (c), ratio of the left lobe volume to the total liver volume was calculated as 38.4%
Statistical Analysis
Statistical evaluation was performed using an R statistical package (R studio, Vienna Austria). The Shapiro-Wilk test was used to determine whether the data was parametric. In the patients with BTL, the total and left lobe volumes and left lobe volume percentages were compared with the Mann-Whitney U test. The demographic data between the groups were compared with the t-test for age and the chi-squared test for sex. A p-value <0.05 was accepted for statistical significance.
Results
The mean age of the 158 volunteers evaluated was 28±8.5 years. The number of patients with BTL was 52 (32.9%), and those with NL was 106. The mean age was 28.5±7.9 years in the BTL group and 29.9±8.5 years in the NL group (p=0.3016). The number of female volunteers was 65 (41.1%), of whom 25 had BTL and 40 had NL. There were 27 (51.9%) men in the BTL group and 66 (62.2%) in the NL group (p=0.3882). Although there was no significant difference between the left lobe volumes of the 2 groups (p=0.8311), a significant difference was observed in terms of the total liver volume and the left lobe volume percentage (p=0.0116 and p<0.0001, respectively). There was also a statistically significant difference regarding the number of patients who had their left lobe percentage lower than 35% (p=0.044). Demographics and study are summarized in Table 1.
Table 1.
Study and demographic data of the study groups with corresponding p values
| BTL group (n=52) | NL group (n=106) | p value | |
|---|---|---|---|
| Sex, male (%) | 27 (51.9) | 66 (62.2) | 0.3882 |
| Age | 28.5±7.9 | 29.9±8.5 | 0.3016 |
| Median total liver volume (mL) (95% CI for the median) | 1.252 mL (1.181–1.316) | 1.375 mL (1.327–1.424) | 0.012 |
| Median left lobe volume (mL) (95% CI for the median) | 463 mL (440–496) | 470 mL (448–492) | 0.8311 |
| Median left lobe percentage (95% CI for the median) | 38% (36.5–39.5) | 35% (34–35) | <0.001 |
| Number of patients with left lobe percentage lower than 35 (%) | 8 (15.3) | 34 (32.1) | 0.041 |
BTL: beaver tail liver, NL: normal liver, CI: confidence interval
Discussion
There are many studies in the literature on volumetric evaluations in liver transplantation [5, 6]. In these studies, especially the effects of age, sex, and vascular variations on the total and segmental volumes of the liver have been discussed. However, we did not find any study examining the effect of BTL on the total and liver remnant volumes. In this study, we examined whether there was a difference between living transplant donors with BTL and those with NL in terms of the safety of procedure correlating with postoperative liver remnant volume. We determined that in individuals with a liver extending to the left hypochondrium, the postoperative remnant volume was in a safe range.
Various lobar anomalies of the liver were described in the literature. BTL is one of those anomalies in which the left border of the left liver lobe extends over the spleen and sometimes wraps it [4]. Finding it is incidental and might be encountered during abdominal imaging. Although it does not pose any significant clinical symptom, this appearance might be misdiagnosed as a splenic subcapsular hematoma or perisplenic fluid collection, as the two organs have a similar density in CT and echogenicity in ultrasound imaging, particularly among persons with abdominal trauma [7–9].
As donor hepatectomy is an important surgery for healthy individuals, donor safety is considered as the most important factor in the process [10]. Abdalla et al. [11] have measured the total and segmental volumes of the liver using CT images in liver transplant candidates and found that the left lobe contributed 25% or less to the total liver volume in more than 10% of patients. Considering that the liver remnant volume should be at least 30% after transplantation, any value below this limit has life-threatening consequences for a right lobe donor. If the left liver lobe has a larger volume than the total liver volume, it is more likely to be sufficient as the remnant liver for the donor [12]. In our study, both groups were within safe limits in terms of the liver remnant volume.
In a study conducted by Shi et al. [13], they have reported that when the liver remnant volume was less than 35% of the standard liver volume, the remnant volume had a significant effect on delayed recovery of liver function and prolonged stay in intensive care. In our study, this ratio was below 35% in approximately one-third of the patients with NL. In the literature, studies have been conducted on different populations to investigate how different anatomical data can affect the liver volume [14–20]. Considering that 3D software providing preoperative volumetric evaluation is not available in every center, the volume of the liver was attempted to be evaluated with various formulations without the use of such special software [21]. Studies in the literature show that there are differences in the liver volume between different populations and individuals with different anatomical data [11, 14, 15, 20]. Vauthey et al. [20] have found a significant difference between the liver volumes of the patients from East and West USA. Similarly, Abdalla et al. [11] investigated the liver volume and segmentation in a Western population and showed the effect of their anatomical data on the liver volume. The authors determined significant differences between the left lobe volume percentages within the same population (33%±7% [17%–49%]). This shows that the risk of the liver remnant volume falling below 30% depends on anatomic, population-based, and physical variations; and no transplant decision should be made without performing a volumetric evaluation. Our study presented BTL as an important marker showing that transplantation was safe if the left lobe of the liver crossed the mid-clavicular line and extended into the left hypochondrium in patients where a 3D volumetric evaluation could not be performed.
Our study had some limitations. Our study population was relatively small, and larger studies are required to assesses the prevalence of the BTL and support our findings. BTL is described as the left lobe of the liver touching the spleen and wrapping it; however, there is no classification that assesses the degree of this contact and enclosing. Our study did not analyze this, and this classification might benefit the donor selection.
In conclusion, choosing living donors with the beaver tail feature in liver transplantation is safer in terms of the left lobe to liver volume percentage. It might also have a positive effect on the healing process of the donor after transplantation and reducing complications.
Main Points
Left lobe volume percentage is important in the development of postoperative complications in right lobe liver donor candidates.
Left lobe volume percentage is higher in BTL than in NL.
BTL might also have a positive effect on the healing process of the donor after transplantation and reducing complications.
Acknowledgements
I would like to express my gratitude to Dr. Ahmet Yalcin and Dr. Gokhan Polat (Ataturk University School of Medicine, Department of Radiology), for their support for statistical calculations and language editing.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Clinical Trials Ethics Committee of Ataturk University (B.30.2.ATA.0.01.00/328).
Informed Consent: Informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Conflict of Interest: The author has no conflict of interest to declare.
Financial Disclosure: The author declared that this study has received no financial support.
References
- 1.Brown RS, Jr, Russo MW, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–25. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 2.Mohapatra N, Bharathy KGS, Sinha PK, et al. Three-Dimensional Volumetric Assessment of Graft Volume in Living Donor Liver Transplantation: Does It Minimise Errors of Estimation? J Clin Exp Hepatol. 2020;10:1–8. doi: 10.1016/j.jceh.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiroshige S, Shimada M, Harada N, et al. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003;75:1561–4. doi: 10.1097/01.TP.0000053755.08825.12. [DOI] [PubMed] [Google Scholar]
- 4.Atalar MH, Karakus K. Beaver tail liver. Abdom Radiol (NY) 2018;43:1851–52. doi: 10.1007/s00261-017-1395-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhijun Z, Wei G, Lin W, et al. Middle hepatic vein allocation in adult right lobe living donor liver transplantation. Clin Transplant. 2014;28:1194–201. doi: 10.1111/ctr.12362. [DOI] [PubMed] [Google Scholar]
- 6.Tsang LL, Chen CL, Huang TL, et al. Preoperative imaging evaluation of potential living liver donors: reasons for exclusion from donation in adult living donor liver transplantation. Transplant Proc. 2008;40:2460–2. doi: 10.1016/j.transproceed.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 7.Glennison M, Salloum C, Lim C, et al. Accessory liver lobes: anatomical description and clinical implications. J Visc Surg. 2014;151:451–55. doi: 10.1016/j.jviscsurg.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Jones R, Tabbut M, Gramer D. Elongated left lobe of the liver mimicking a subcapsular hematoma of the spleen on the focused assessment with sonography for trauma exam. Am J Emerg Med. 2014;32:814. doi: 10.1016/j.ajem.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 9.Cholankeril JV, Zamora BO, Ketyer S. Left lobe of the liver draping around the spleen: a pitfall in computed tomography diagnosis of perisplenic hematoma. J Comput Tomogr. 1984;8:261–7. doi: 10.1016/0149-936X(84)90074-2. [DOI] [PubMed] [Google Scholar]
- 10.Rao PP, Routh D, Naidu CS, et al. Donor outcome in live-related liver transplantation. Med J Armed Forces India. 2014;70:100–4. doi: 10.1016/j.mjafi.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–10. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Chan CS, Cheunga TT, Chan CYA, et al. New insights after the first 1000 liver transplantations at The University of Hong Kong. Asian J Surg. 2016;39:202–10. doi: 10.1016/j.asjsur.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Shi ZR, Yan LN, Du CY. Donor safety and remnant liver volume in living donor liver transplantation. World J Gastroenterol. 2012;18:7327–32. doi: 10.3748/wjg.v18.i48.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altunkaynak BZ, Altunkaynak ME. Relationship of body weight and volume of liver. A morphometrical and stereological study. Saudi Med J. 2007;28:891–5. [PubMed] [Google Scholar]
- 15.Kokudo T, Hasegawa K, Uldry E, et al. A new formula for calculating standard liver volume for living donor liver transplantation without using body weight. J Hepatol. 2015;63:848–54. doi: 10.1016/j.jhep.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Tongyoo A, Pomfret EA, Pomposelli JJ. Accurate estimation of living donor right hemi-liver volume from portal vein diameter measurement and standard liver volume calculation. Am J Transplant. 2012;12:1229–39. doi: 10.1111/j.1600-6143.2011.03909.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi SH, Kwon JH, Kim KW, et al. Measurement of liver volumes by portal vein flow by Doppler ultrasound in living donor liver transplantation. Clin Transplant. 2017;31 doi: 10.1111/ctr.13050. [DOI] [PubMed] [Google Scholar]
- 18.Kayashima H, Shirabe K, Morita K, et al. Liver regeneration and venous collateral formation in the right lobe living-donor remnant: segmental volumetric analysis and three-dimensional visualization. Transplantation. 2013;95:353–60. doi: 10.1097/TP.0b013e31827147d8. [DOI] [PubMed] [Google Scholar]
- 19.Hwang S, Lee SG, Choi ST, et al. Hepatic vein anatomy of the medial segment for living donor liver transplantation using extended right lobe graft. Liver Transpl. 2005;11:449–55. doi: 10.1002/lt.20387. [DOI] [PubMed] [Google Scholar]
- 20.Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–40. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 21.Lee WC, Lee CS, Soong RS, et al. Split liver transplantation in adults: preoperative estimation of the weight of right and left hemiliver grafts. Liver Transpl. 2011;17:93–4. doi: 10.1002/lt.22213. [DOI] [PubMed] [Google Scholar]