Abstract
Objective
After chemotherapy, cancer survivors suffer from acquired immunological defects and become vulnerable to vaccine-preventable diseases. There are no universally approved revaccination guidelines for non-transplanted oncology patients. This study aimed to share our experience of revaccination in childhood cancer survivors to plan future vaccination schedules.
Materials and Methods
This retrospective study was conducted in a Pediatric Oncology Department of a university-affiliated hospital. Patients who were diagnosed with malignancy other than leukemia constituted the study population. Patients were directed for revaccination 6 months after the cessation of treatment. Revaccination was performed according to patients’ vaccination status before chemotherapy and seronegativity.
Results
Of the 64 patients in the study, 44 (68.75%) were boys. The mean age at the time of diagnosis and at start of vaccination was 8.8±5.3 years and 10.6±5.1 years, respectively. Hodgkin’s lymphoma was the most common diagnosis. The vaccination schedule of 7 patients was interrupted because of chemotherapy; after completing the missing vaccine doses, the serology of 2 patients was negative for at least 2 antigens. The vaccination schedule of 57 patients was completed before beginning chemotherapy and 52 of them were seronegative for at least 1 antigen. No adverse reactions or life-threatening infections were observed because of vaccinations.
Conclusion
There are different approaches when vaccinating the oncology patients after chemotherapy. Watching out for the four touchstones mentioned in our study will protect the patient and do no harm. More studies are needed to constitute universal and standardized revaccination guidelines for these patients.
Keywords: Vaccination, chemotherapy, pediatric oncology
Introduction
Survival rates have significantly improved in pediatric malignant diseases. Acquired immunological defects occur after chemotherapy in both cell-mediated and humoral immunity, which result in decreased measurable vaccine protection [1]. In these patients, recovery of immune functions has been shown to occur 6–12 months after the completion of chemotherapy [2, 3]. This acquired immunodeficiency poses a risk for life-threatening vaccine-preventable infections for the survivors [4]. Unfortunately, there are no universally approved revaccination guidelines for non-transplanted childhood cancer survivors. Besides, compliance of revaccination in oncology patients has been reported to be poor in clinical settings [5].
Revaccination times after chemotherapy are 3 and 6 months [6, 7]. Furthermore, it is recommended that the patient should be in continuous remission for at least 1 year for varicella vaccinations [8].
In our country, routine vaccination schedules for children include those for diphtheria, pertussis, tetanus, inactivated and oral polio, Hemophilus influenzae type B (Hib) (before 5 years of age), conjugated pneumococcus (before 5 years of age), hepatitis B, hepatitis A, varicella, measles, mumps, rubella, and tuberculosis (BCG) antigens. For immunocompromised patients, BCG and oral polio vaccines are not administered, but meningococcus (A, C, Y, and W), Hib (after 5 years), conjugated (after 5 years) and polysaccharide pneumococcus, and seasonal influenza vaccines are recommended in addition to the routine vaccination schedules [9]. However, there are no national vaccination guidelines for revaccination of children after chemotherapy.
In this study, we share our experience of revaccination in childhood cancer survivors to plan future vaccination schedules.
Materials and Methods
This retrospective study was conducted between March 2016 and May 2019 in the Pediatric Oncology Department of a university-affiliated hospital in Istanbul, Turkey. Patients who were diagnosed with malignancy other than leukemia constituted the study population. Clinical data of each patient were retrospectively reviewed from the patient’s medical records. This study was approved by the hospital ethics committee (48670771-514.10) and carried out according to the Declaration of Helsinki protocol.
Patients who died during the therapy (n=3), moved to another city for treatment (n=2), did not come in for their vaccinations (n=5), or had a disease relapse (n=12) were excluded from the study. Patients with bone marrow or solid organ transplant were not recruited in the study; only patients with complete data were included. None of the patients received anti-B cell antibody therapy.
In the routine practice of the clinic, 6 months after cessation of treatment, patients were directed to the well-child outpatient clinic for vaccinations. If the vaccination was interrupted because of the treatment, it continued from the point where it had been halted. Only varicella vaccination was given after 1 year of continuous remission. Moreover, meningococcus, Hib, conjugated and polysaccharide pneumococcus (8 weeks after conjugated pneumococcus vaccine), and seasonal influenza (between October and February) vaccines were administered. Furthermore, 1 month after completing the vaccination schedule, serology was tested for measles, mumps, rubella, varicella, hepatitis A, and hepatitis B. If the patient was seronegative for any of these antigens, revaccination was carried out.
For patients who completed their vaccination schedule before chemotherapy, the serologies of measles, mumps, rubella, hepatitis B, hepatitis A, and varicella were checked routinely. If the patients were seronegative for any of these antigens, the vaccine was administered. Moreover, booster doses for diphtheria, pertussis, tetanus, inactivated polio, Hib, conjugated and polysaccharide pneumococcus (8 weeks after the conjugated pneumococcus vaccine), meningococcus, and seasonal influenza (between October and February) vaccines were administered to all the patients. In addition, 1 month after the last dose of revaccination, the antibody responses were checked. Revaccination was performed for seronegative antigens.
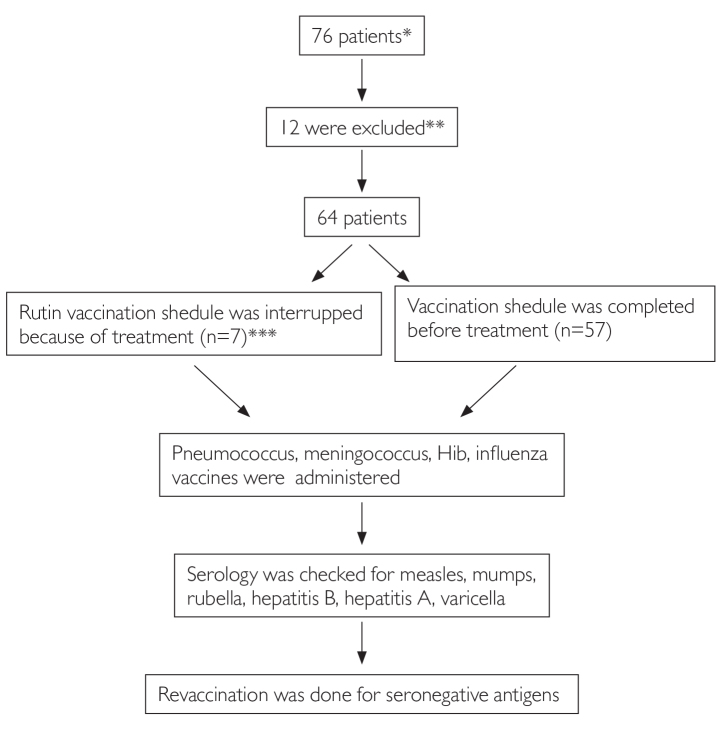
Assessment of patients and revaccination practice is shown in Figure 1.
Figure 1.
Assessment of patients and revaccination practice
*Malignancy other than leukemia
**Missing, moving to another city, not attended revaccination, disease relapsed
***Vaccination was continued from the point it was paused 6 months after the end of treatment
**** Serology was checked, vaccines were administered for seronegative antigens
Anti-hepatitis A IgG and anti-Hbs antibodies were tested with microparticles immunoassay. Rubella IgG, measles IgG, mumps IgG, and varicella zoster IgG were detected using the chemiluminescence method. The results were seropositive or seronegative according to the laboratory test manufacturer’s guidelines.
Results
Of the 64 patients, 44 (68.75%) were boys. The mean age at the time of cancer diagnosis and at the beginning of revaccination was 8.8±5.3 (0.1–17) years and 10.6±5.1 (2–18) years, respectively.
Hodgkin’s lymphoma and non-Hodgkin’s lymphoma were the most common diagnoses as seen in Table 1.
Table 1.
Demographic characteristics of patients
| n | % | |
|---|---|---|
| Sex | ||
| Male | 44 | 68.8 |
| Female | 20 | 31.2 |
| Age at diagnosis Mean+SD | 8.8±5.3 years | |
| Age before vaccination Mean+SD | 10.6±5.1 years | |
| Malignancy types (64) | ||
| Hodgkin’s lymphoma | 16 | 25 |
| Non-Hodgkin’s lymphoma | 15 | 23.4 |
| Bone tumors | 8 | 12.5 |
| Osteosarcoma (6) | ||
| Ewing sarcoma (1) | ||
| Synovial sarcoma (1) | ||
| Brain tumor | 6 | 9.3 |
| Wilms’ tumor | 5 | 7.8 |
| Neuroblastoma | 5 | 7.8 |
| Nasopharyngeal carcinoma | 4 | 6.4 |
| Others | 5 | 7.8 |
| Hepatoblastoma (1) | ||
| Rhabdomyosarcoma (1) | ||
| Testis tumor (1) | ||
| Ovarian germ cell tumor (1) | ||
| Langerhans cell histocytosis (1) | ||
| Total | ||
Serologies were checked after the termination of vaccinations for 7 patients whose vaccinations were resumed. One patient’s mumps and varicella serologies were negative, and another’s measles, mumps, varicella, and hepatitis A serologies were negative. These patients were revaccinated, and their serologies were positive after the booster doses.
Among 57 patients, only 5 were seropositive for all the 6 antigens. Of 52 (91.2%) patients, 29 (49.2%) were seronegative for measles, 21 (36.8%) for rubella, 29 (49.2%) for mumps, 21 (36.8%) for varicella, 38 (66.6%) for hepatitis A, and 29 (49.2%) for hepatitis B. We administered the vaccinations for seronegative antigens for each patient. As we could not test for diphtheria, pertussis, tetanus, polio, Hib, meningococcus, and pneumococcus, booster vaccinations for these antigens were administered to all the patients.
Vaccine serologies were rechecked in 52 patients after the end of the booster doses. Negative serologies were found in 4 patients for mumps, 2 patients for hepatitis A, and 1 patient each for rubella, measles, hepatitis B, and varicella. One more dose of these vaccines was administered, and the serologies were checked again. Furthermore, 5 patients did not consent for serological testing after the last vaccination. No adverse reactions or life-threatening infections were observed during the whole series of vaccinations.
Discussion
For oncology patients, as long as there are no universal revaccination guidelines, every center applies their own schedule according to the country’s vaccination practices. Most guidelines are based on the data from healthy children and the experience of the center [10]. In this study, we share our experience considering the 4 touchstones when revaccinating these patients.
The first question to be discussed is: “Does this special disease and treatment make any vaccine a contradiction”?
Chemotherapy causes immunosuppression mostly during the induction and consolidation phases. After cessation of treatment, the immune system begins to recover within 3 months or more [11]. There are no contradictions for inactivated or recombinant vaccines for these patients. However, live vaccines can cause vaccine-derived infections for a longer period after the chemotherapy in addition to the possible insufficient immune responses. Oral polio, live attenuated influenza, oral typhoid, yellow fever, and BCG vaccines are contraindicated for cancer patients after treatment [6, 12]. It is better to do Measles-mumps-rubella (MMR) vaccinations 6 months after completion of chemotherapy and varicella 1 year after chemotherapy. In light of the available literature, all the patients were revaccinated after chemotherapy [13–15].
Second, we have to discuss: “When should the patient be vaccinated”?
Although immune recovery has been shown to begin a few months after the discontinuation of chemotherapy, it takes 6–12 months for the immune system to fully recover [2, 3]. The immune reconstitution depends on the nature of the disease, dosing of the treatment, and the age of the patient [16]. After chemotherapy, B-cells and CD8+ T-cells recover after the 3rd month, it takes 6–12 months for CD4+ T-cells to recover [17, 18].
Although early revaccination shortens the patient’s unprotected period, in the first 6 months after the therapy, the host may not produce sufficient immunogenicity against the vaccines [2, 13, 19]. Studies generally suggest that it is better to do MMR vaccinations 6 months after and varicella 1 year after the completion of chemotherapy [8, 15].
Our study population was vaccinated with at least a 6-month gap from the cessation of treatment for inactivated/recombinant vaccines and 1 year after for varicella vaccine, consistent with the literature. In the presence of risk for outbreaks of any infection, each patient was discussed separately [20].
The third question was: “Which schedule should be used for revaccination? Should we continue from the point where it was stopped or does the patient need any booster vaccination”?
There are different approaches to this topic. According to Lillian Sung et al. [20], if the vaccination schedule was not completed, it should be continued from the point at which the child was last vaccinated. According to Espossito et al. [19], the best way is to test the residual immunity and then choose to vaccinate all of the schedule or only a booster. Furthermore, Ruggiero et al. [21] recommended to continue the routine schedule of vaccination 6 months after chemotherapy and then test the residual seroprotectivity. For the patients who did not complete the vaccination schedule, vaccination should be continued from the point where it was stopped [4, 21]. After completing the missed doses, serology was checked, and the patients were revaccinated for the seronegative antigens.
For the patients who completed the vaccination before chemotherapy, 2 options are recommended. The first is administering a booster dose for each antigen and the second is checking the serology and making a vaccination plan for each patient [20, 22, 23]. In our study population, every patient was checked for serology 6 months after the end of chemotherapy. In total, 52 (91.2%) patients were seronegative for at least 1 antigen, and each patient was revaccinated according to the serological findings. Booster doses of the vaccines, which could not be checked for serology, were administered; 1 month after completing the vaccination schedules, the serology was checked again. We cannot argue that this is the best way, but it is more comfortable when revaccinating the vulnerable host.
The last question is, “Does this special disease make any infection riskier? Does the patient need any extra vaccines”?
For cancer patients, both the disease and the chemotherapy cause immune suppression. Furthermore, these patients are at a high risk of invasive pneumococcal and Hemophilus type B infections. It is recommended that the patients should receive both conjugated and polysaccharide pneumococcus, Hib, meningococcal, and inactivated influenza vaccines annually [24–27].
In our country’s vaccination schedule, Hib and pneumococcus vaccines are administered only to children under 5 years of age, and meningococcus (A, C, Y, and W) and influenza vaccines are not administered routinely [9]. However, for immunocompromised hosts, these vaccines are offered regardless of age. In our study population, 1 dose of Hib, meningococcus, inactivated influenza, and conjugated and polysaccharide pneumococcus (8 weeks after the conjugated vaccine is administered) vaccines were administered appropriately.
There were some limitations to our study. Because the patients in our study were vulnerable to vaccine-preventable infections, seropositivity was checked for each patient, which may not be cost effective. Every center does not have the opportunity to test the seropositivity for each antigen. It may be simpler or more effective to administer booster doses of vaccines and check the seropositivity later. Each center has the right to implement its own schedule.
In our study, booster doses for diphtheria, pertussis, tetanus, polio, Hib, and pneumococcus antigens were administered, but the serology was not checked. We cannot say that the patients are protected against these antigens as long as we do not test the serology after completing the vaccination.
When revaccinating the pediatric oncology patients, there are different host- and disease-related variables to consider. It is difficult to say that one is better or the other is more effective. The vaccination schedule of the country should be adopted for the specific population. We think that revaccination of patients taking the 4 touchstones mentioned above into consideration will give the best protection and cause the least harm for the children who survived cancer.
In conclusion, there are different revaccination applications for non-transplanted oncology patients. After chemotherapy, most of the patients were seronegative for the antigens, which they had been vaccinated for before; hence, checking the serology after chemotherapy is useful. More studies are needed to constitute standardized, applicable, and simple revaccination schedules for these patients.
Main Points
Checking the serology of vaccine preventable diseases after chemotherapy is useful.
After chemotherapy, most of the patients were seronegative for the antigens which they had been vaccinated for before.
More studies are needed to constitute standardized, applicable, and simple revaccination schedules for these patien.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Okmeydani Training and Research Hospital (48670771-514.10).
Informed Consent: Informed consents were obtained from all participants.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - P.Y., H.S.S.; Design - P.Y., S.O.; Supervision- H.S.S., S.O.; Resources- P.Y., H.S.S.; Data Collection and/or Processing - P.Y., S.O.; Analysis and/or Interpretation - H.S.S., S.O.; Literature Search- P.Y., H.S.S.; Writing Manuscript - P.Y., H.S.S.; Critical Review - S.O., P.Y., H.S.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia, Pa; USA: Lippincott; 2011. Principles of multimodal therapy. [Google Scholar]
- 2.Zignol M, Peracchi M, Tridello G, et al. Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer. 2004;101:635–41. doi: 10.1002/cncr.20384. [DOI] [PubMed] [Google Scholar]
- 3.Ek T, Mellander L, Andersson B, Abrahamsson J. Immune reconstitution after childhood acute lymphoblastic leukemia in most severely affected in the high-risk group. Pediatr Blood Cancer. 2005;44:461–8. doi: 10.1002/pbc.20255. [DOI] [PubMed] [Google Scholar]
- 4.Shetty AK, Winter MA. Immunization of children receiving immunosuppressive therapy for cancer or hematopoietic stem cell transplantation. Ochsner J. 2012;12:228–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford NW, Health JA, Ashley D, Downie P, Buttery JP. Survivors of childhood cancer: an Australian audit of vaccination status after treatment. Pediatr Blood Cancer. 2010;54:128–33. doi: 10.1002/pbc.22256. [DOI] [PubMed] [Google Scholar]
- 6.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–18. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 7.Han JH, Harmoney KM, Torrez J, et al. Dynamic re-immunization of off-treatment childhood cancer survivors: An Implementation feasibility study. Plos One. 2018;13:e0191804. doi: 10.1371/journal.pone.0191804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Succi RC, Farhat CK. Vaccination in special situations. J Pediatr (Rio J) 2006;82(3 Suppl):S91–100. doi: 10.1590/S0021-75572006000400011. [DOI] [PubMed] [Google Scholar]
- 9. [Date of access: 10.3.2019]. https://dosyasb.saglik.gov.tr/Eklenti/1117.gbpgenelge2008pdf.pdf?0.
- 10.Avinash KS, Winter MA. Immunization of Children Receiving Immunosuppressive Therapy for Cancer or Hematopoietic Stem Cell Transplantation. Ochsner J. 2012;12:228–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Ridgeway D, Wolff LJ. Active immunization of children with leukemia and other malignancies. Leuk Lymphoma. 1993;9:177–92. doi: 10.3109/10428199309147369. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines. Morbidity and Mortality Weekly Report. 2010;59:1–62. [Google Scholar]
- 13.Skinner R, Davies EG, Cant AI, Finn A, Foot A. Royal College of Paediatrics and Child Health (RCPCH) best practice statement on İmmunisation in the immunocompromised child. Int Jour of Infec Dis. 2002;6:58–9. doi: 10.1016/S1201-9712(02)90303-6. [DOI] [Google Scholar]
- 14.Garonzi C, Balter R, Tridello G, et al. The Impact of Chemotherapy after Pediatric Malignancy on Humoral Immunity to Vaccine-Preventable Diseases. Mediterr J Hematol Infect Dis. 2020;12:e2020014. doi: 10.4084/mjhid.2020.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics. Poliovirus infections. In: Pickering LK, Baker CJ, Kimberlin DW, et al., editors. Red Book 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009. pp. 541–45. [Google Scholar]
- 16.Lernbecher T, Foster C, Vazquez N, et al. Therapy induced alterations in host defense in children receiving therapy for cancer. J Pediatr Hematol Oncol. 1997;19:399–417. doi: 10.1097/00043426-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 18.Mackall CL, Fleisher TA, Brown MR, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–07. doi: 10.1182/blood.V89.10.3700. [DOI] [PubMed] [Google Scholar]
- 19.Recommendations of the Advisory Committee on Immunisation Practices (ACIP): the use of vaccines and immunoglobulins in persons with altered immunocompetence. MMWR Recomm Rep. 1993;42:1–18. [PubMed] [Google Scholar]
- 20.Esposito S, Cecinati V, Brescia L, Principi N. Vaccinations in children with cancer. Vaccine. 2010;28:3278–84. doi: 10.1016/j.vaccine.2010.02.096. [DOI] [PubMed] [Google Scholar]
- 21.Sung L, Heurter H, Zokvic K, et al. Practical vaccination guidelines for children with cancer. Paediatr Child Health. 2001;6:379–83. doi: 10.1093/pch/6.6.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruggiero A, Battista A, Coccia P, Attina G, Riccardi R. How to manage vaccinations in children with cancer. Pediatr Blood Cancer. 2011;57:1104–8. doi: 10.1002/pbc.23333. [DOI] [PubMed] [Google Scholar]
- 23.Ariza-Heradia E, Chemaly R. Practical review of immunizations in adult patients with cancer. Hum Vaccin Immunother. 2015;11:2606–14. doi: 10.1080/21645515.2015.1062189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehrnbecher T, Schubert R, Behl M, et al. Impaired pneumococcal immunity in children after treatment for acute lymphoblastic leukemia. Br J Haematol. 2009;147:700–5. doi: 10.1111/j.1365-2141.2009.07903.x. [DOI] [PubMed] [Google Scholar]
- 25.Feldman S, Gigliotti F, Shenep JL, et al. Risk of Haemophilus influenza type b disease in children with cancer and the response of immunocompromised leukemic children to a conjugated vaccine. J Infect Dis. 1990;161:929–31. doi: 10.1093/infdis/161.5.926. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease C, Prevention. Recommendation of the advisory committee on immunization practices (ACIP) for use of the quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mort Wkly Rep. 2011;60:1391–2. [PubMed] [Google Scholar]
- 27.Loulergue P, Mir O, Alexandre J, Ropert S, Goldwasser F, Launay O. Low influenza vaccination rate among patients receiving chemotherapy for cancer. Ann Oncol. 2008;19:1658. doi: 10.1093/annonc/mdn531. [DOI] [PubMed] [Google Scholar]